SCH4U Chemistry: Investigating Temperature's Impact on Reaction Rate
VerifiedAdded on 2023/06/11
|8
|1700
|305
Report
AI Summary
This SCH4U lab report investigates the effects of temperature on the rate of a chemical reaction between baking soda and vinegar. The experiment aims to identify and control variables to demonstrate how temperature changes affect the reaction speed and explain the molecular-level reasons behind this effect. The hypothesis predicts that increasing temperature will increase the reaction rate. The procedure involves reacting baking soda with vinegar at different temperatures (room temperature, heated, and 50 degrees Celsius) and recording the time taken for the reaction to complete. Observations include effervescence of hydrogen gas and a slight temperature increase during the reaction. The results show that higher temperatures lead to faster reaction rates, explained by increased kinetic energy and collision frequency of molecules. The report also discusses potential errors, such as timing inaccuracies and temperature control issues. It concludes that the experiment successfully demonstrated the relationship between temperature and reaction rate, supporting the initial hypothesis. Desklib provides access to similar reports and study resources for students.

EFFECTS OF TEMPERATURE ON THE RATE OF A CHEMICAL REACTION.
SCH4U
Factors Affecting the Rates of Chemical Reactions Lab Report.
[Author Name(s), First M. Last, Omit Titles and Degrees]
[Institutional Affiliation(s)]
Author Note
[Include any grant/funding information and a complete correspondence address.]
SCH4U
Factors Affecting the Rates of Chemical Reactions Lab Report.
[Author Name(s), First M. Last, Omit Titles and Degrees]
[Institutional Affiliation(s)]
Author Note
[Include any grant/funding information and a complete correspondence address.]
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Rate of reaction between Baking soda and vinegar. 2
Factors Affecting the Rates of Chemical Reactions Lab Report.
Introduction
According to Chemistry Libretexts (2016), the rate of a chemical reaction is the amount
of reactant reacted per unit time or the amount of product formed per unit time. Depending on
the time interval between measurements, the rates are called initial rate, average rate and
instantaneous rate. The rate at which the chemical reaction occurs is governed by various factors:
concentration, temperature, catalysts, reaction medium and pressure (Vutturi, n.d.).
An increase in temperature in a reaction, consequently leads to an increase in the rate of a
reaction. This is as a result of the increase in the kinetic energy of the molecules. Temperature
increase causes an increase in the excitation in the molecules of the reactants. This increases their
vibrations and kinetic energy and hence the probability of collisions is increased. As a result of
the increased number of collisions, the rate of the reaction increases and the reaction proceeds
faster.
The objectives of the experiment include to identify and control variables in order to
design an experiment that shows how temperature change affects the rate of a chemical reaction
between baking soda and vinegar and also to explain why the temperature affects the speed of
the reaction in a molecular level.
Variables.
In the experiment, time taken for the reaction to come to completion is the dependent
variable as it is affected by alterations of the temperature of the water. Temperature is the
independent variable as it is the factor that is changed in the experiment leading to time
difference in the time taken for the sodium hydrogen carbonate (baking soda) to react with
Factors Affecting the Rates of Chemical Reactions Lab Report.
Introduction
According to Chemistry Libretexts (2016), the rate of a chemical reaction is the amount
of reactant reacted per unit time or the amount of product formed per unit time. Depending on
the time interval between measurements, the rates are called initial rate, average rate and
instantaneous rate. The rate at which the chemical reaction occurs is governed by various factors:
concentration, temperature, catalysts, reaction medium and pressure (Vutturi, n.d.).
An increase in temperature in a reaction, consequently leads to an increase in the rate of a
reaction. This is as a result of the increase in the kinetic energy of the molecules. Temperature
increase causes an increase in the excitation in the molecules of the reactants. This increases their
vibrations and kinetic energy and hence the probability of collisions is increased. As a result of
the increased number of collisions, the rate of the reaction increases and the reaction proceeds
faster.
The objectives of the experiment include to identify and control variables in order to
design an experiment that shows how temperature change affects the rate of a chemical reaction
between baking soda and vinegar and also to explain why the temperature affects the speed of
the reaction in a molecular level.
Variables.
In the experiment, time taken for the reaction to come to completion is the dependent
variable as it is affected by alterations of the temperature of the water. Temperature is the
independent variable as it is the factor that is changed in the experiment leading to time
difference in the time taken for the sodium hydrogen carbonate (baking soda) to react with

Rate of reaction between Baking soda and vinegar. 3
vinegar. Concentration, catalysts, mass of the baking soda which is 1gram, the fine baking soda
crystals, volume of vinegar, 100ml and the medium in which the reaction is taking place are kept
constant as they also affect the rate of chemical reactions. These factors are the variables that are
held constant for the experiment to be successful.
Question of the Experiment.
How does the temperature of vinegar change affect the speed at which sodium hydrogen
carbonate (baking soda) reacts with vinegar?
Hypothesis.
The prediction for the experiment was that an increase in temperature would increase the
rate at which the reaction takes place with vinegar and baking soda as the vinegar`s temperature
is increased.
Materials Used for the Experiment.
Vinegar
Hot surface
Sodium hydrogen carbonate (Baking Soda)
Camera film containers
Stopwatch
Thermometer
Measuring cylinder
Analytical Weigh Balance
Procedure.
vinegar. Concentration, catalysts, mass of the baking soda which is 1gram, the fine baking soda
crystals, volume of vinegar, 100ml and the medium in which the reaction is taking place are kept
constant as they also affect the rate of chemical reactions. These factors are the variables that are
held constant for the experiment to be successful.
Question of the Experiment.
How does the temperature of vinegar change affect the speed at which sodium hydrogen
carbonate (baking soda) reacts with vinegar?
Hypothesis.
The prediction for the experiment was that an increase in temperature would increase the
rate at which the reaction takes place with vinegar and baking soda as the vinegar`s temperature
is increased.
Materials Used for the Experiment.
Vinegar
Hot surface
Sodium hydrogen carbonate (Baking Soda)
Camera film containers
Stopwatch
Thermometer
Measuring cylinder
Analytical Weigh Balance
Procedure.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Rate of reaction between Baking soda and vinegar. 4
250ml of hot water was filled in the camera film container and the temperature of
the water was measured and recorded.
Thereafter, 1 gram of sodium hydrogen carbonate (baking soda) was added to the
vinegar at room temperature and the time taken for hydrogen gas to be completely
formed is recorded.
The experiment was repeated with vinegar that had been heated in order to
increase its temperature with the time taken for the reaction to come to
completion after effervescence stops is recorded.
The reaction was redone with vinegar heated till 50 degrees Celsius, with the time
taken for effervescence to stop for each reaction being recorded.
Experimental Observations.
There is effervescence of a colorless gas that burns with a pop sound, indicating that the
gas is hydrogen. The vinegar retains its colorless solution even after the sodium hydrogen
carbonate dissolves, indicating that sodium ions does not impart color on solutions, since
they are colorless ions. In addition to that, there is a slight rise in temperature after the
sodium hydrogen carbonate dissolves indicating that the reaction is exothermic.
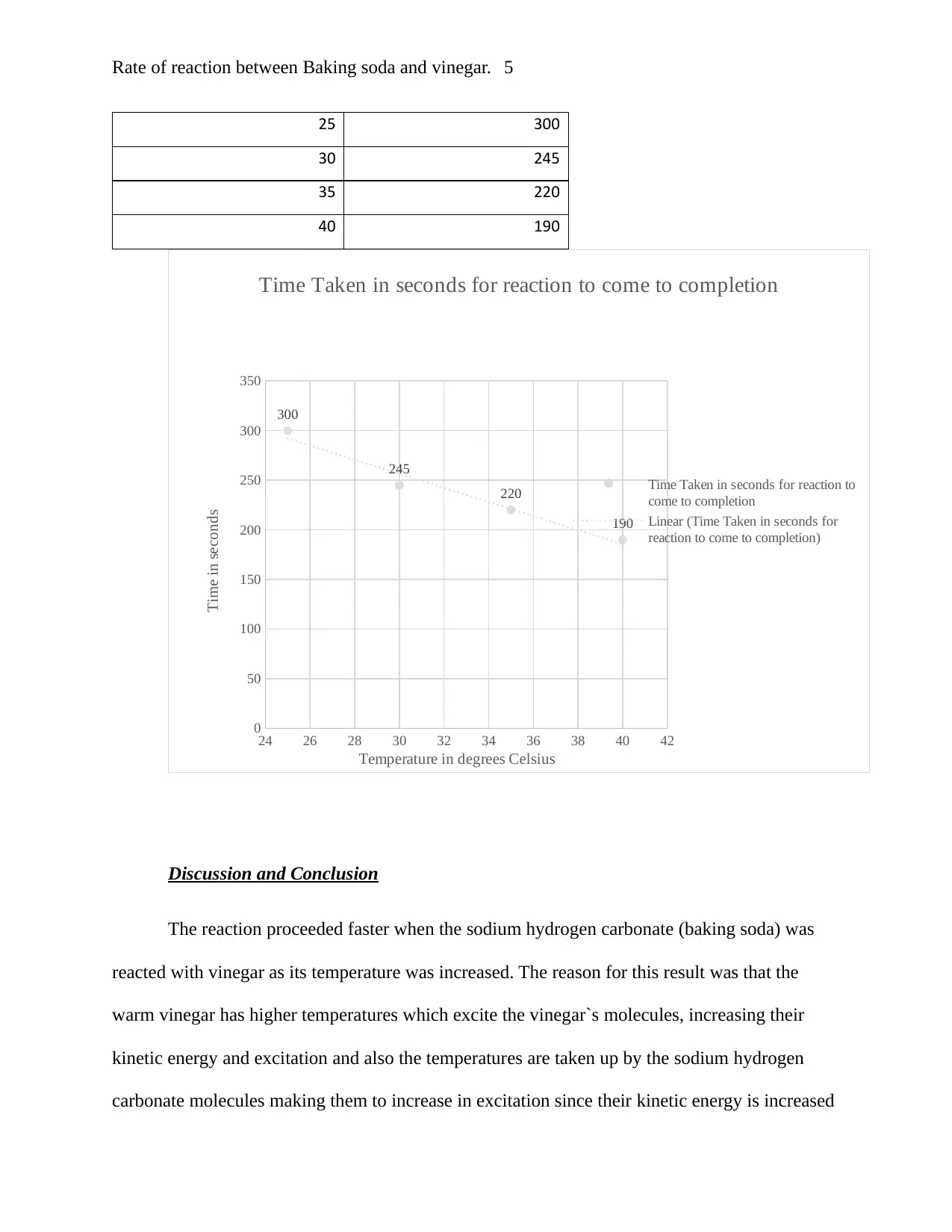
Results.
The data shows the time it took for the sodium hydrogen carbonate (baking soda) to
completely react with vinegar. A graph of the results is also provided.
Vinegar at room temperature
Temperatures in degrees
Celsius.
Time Taken in seconds for
reaction to come to
completion
250ml of hot water was filled in the camera film container and the temperature of
the water was measured and recorded.
Thereafter, 1 gram of sodium hydrogen carbonate (baking soda) was added to the
vinegar at room temperature and the time taken for hydrogen gas to be completely
formed is recorded.
The experiment was repeated with vinegar that had been heated in order to
increase its temperature with the time taken for the reaction to come to
completion after effervescence stops is recorded.
The reaction was redone with vinegar heated till 50 degrees Celsius, with the time
taken for effervescence to stop for each reaction being recorded.
Experimental Observations.
There is effervescence of a colorless gas that burns with a pop sound, indicating that the
gas is hydrogen. The vinegar retains its colorless solution even after the sodium hydrogen
carbonate dissolves, indicating that sodium ions does not impart color on solutions, since
they are colorless ions. In addition to that, there is a slight rise in temperature after the
sodium hydrogen carbonate dissolves indicating that the reaction is exothermic.
Results.
The data shows the time it took for the sodium hydrogen carbonate (baking soda) to
completely react with vinegar. A graph of the results is also provided.
Vinegar at room temperature
Temperatures in degrees
Celsius.
Time Taken in seconds for
reaction to come to
completion
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Rate of reaction between Baking soda and vinegar. 5
25 300
30 245
35 220
40 190
24 26 28 30 32 34 36 38 40 42
0
50
100
150
200
250
300
350
300
245
220
190
Time Taken in seconds for reaction to come to completion
Time Taken in seconds for reaction to
come to completion
Linear (Time Taken in seconds for
reaction to come to completion)
Temperature in degrees Celsius
Time in seconds
Discussion and Conclusion
The reaction proceeded faster when the sodium hydrogen carbonate (baking soda) was
reacted with vinegar as its temperature was increased. The reason for this result was that the
warm vinegar has higher temperatures which excite the vinegar`s molecules, increasing their
kinetic energy and excitation and also the temperatures are taken up by the sodium hydrogen
carbonate molecules making them to increase in excitation since their kinetic energy is increased
25 300
30 245
35 220
40 190
24 26 28 30 32 34 36 38 40 42
0
50
100
150
200
250
300
350
300
245
220
190
Time Taken in seconds for reaction to come to completion
Time Taken in seconds for reaction to
come to completion
Linear (Time Taken in seconds for
reaction to come to completion)
Temperature in degrees Celsius
Time in seconds
Discussion and Conclusion
The reaction proceeded faster when the sodium hydrogen carbonate (baking soda) was
reacted with vinegar as its temperature was increased. The reason for this result was that the
warm vinegar has higher temperatures which excite the vinegar`s molecules, increasing their
kinetic energy and excitation and also the temperatures are taken up by the sodium hydrogen
carbonate molecules making them to increase in excitation since their kinetic energy is increased

Rate of reaction between Baking soda and vinegar. 6
and hence, the molecules collide more, thus increasing the rate of the reaction and the time taken
for effervescence to stop is shorter (Clark, 2013). Consequently, the increase in the kinetic
energy of the particles increases the rate of collision of the particles in the reaction therefore,
increasing the rate of the reaction. Moreover, the increased temperatures from the warm vinegar
lower the attractive forces between the molecules of the sodium hydrogen carbonate (baking
soda) therefore decreasing rigidity between the molecules and increasing movements between
the molecules of the sodium hydrogen carbonate and vinegar. Hence, there are more collisions
and thus, the reaction proceeds at a faster rate, taking a short period of time before coming to
completion. The vinegar at room temperature on the other hand has low temperatures. These low
temperatures make both the sodium hydrogen carbonate and vinegar molecules to lose their
excitement and hence, they have a reduced kinetic energy. The particles therefore have a
decreased collision and hence the rate of the reaction proceeds at a slower and therefore takes a
longer time for the reaction to come to completion (Veerendra, 2017). Furthermore, the reduced
temperature strengthens attractive forces between the molecules of the sodium hydrogen
carbonate (baking soda) hence, reducing the movement between the sodium hydrogen carbonate
molecules and vinegar molecules due to the increased rigidity that decreases the collisions and
hence, decreases the rate of the reaction.
The experiment is prone to errors with the recording of time since the sodium hydrogen
carbonate dissolves very fast in vinegar. However, if done accurately there may be minimal
errors that may not affect the results obtained to a great extent. Handling of the stopwatch in
order to measure the time taken for the experiment to occur may be a challenge to most students
(Johnson, 2009). This is because the student might start the stopwatch before the effervescence
is completely over. This gives errors of time which is the dependent variable supposed to be used
and hence, the molecules collide more, thus increasing the rate of the reaction and the time taken
for effervescence to stop is shorter (Clark, 2013). Consequently, the increase in the kinetic
energy of the particles increases the rate of collision of the particles in the reaction therefore,
increasing the rate of the reaction. Moreover, the increased temperatures from the warm vinegar
lower the attractive forces between the molecules of the sodium hydrogen carbonate (baking
soda) therefore decreasing rigidity between the molecules and increasing movements between
the molecules of the sodium hydrogen carbonate and vinegar. Hence, there are more collisions
and thus, the reaction proceeds at a faster rate, taking a short period of time before coming to
completion. The vinegar at room temperature on the other hand has low temperatures. These low
temperatures make both the sodium hydrogen carbonate and vinegar molecules to lose their
excitement and hence, they have a reduced kinetic energy. The particles therefore have a
decreased collision and hence the rate of the reaction proceeds at a slower and therefore takes a
longer time for the reaction to come to completion (Veerendra, 2017). Furthermore, the reduced
temperature strengthens attractive forces between the molecules of the sodium hydrogen
carbonate (baking soda) hence, reducing the movement between the sodium hydrogen carbonate
molecules and vinegar molecules due to the increased rigidity that decreases the collisions and
hence, decreases the rate of the reaction.
The experiment is prone to errors with the recording of time since the sodium hydrogen
carbonate dissolves very fast in vinegar. However, if done accurately there may be minimal
errors that may not affect the results obtained to a great extent. Handling of the stopwatch in
order to measure the time taken for the experiment to occur may be a challenge to most students
(Johnson, 2009). This is because the student might start the stopwatch before the effervescence
is completely over. This gives errors of time which is the dependent variable supposed to be used
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Rate of reaction between Baking soda and vinegar. 7
for giving deductions from the results. Therefore, the handling of the stopwatch during the
experiment is crucial in order for one to get accurate results. In addition to that the container
holding the vinegar with the varying temperatures should be lagged. Lagging is done in order to
prevent the vinegar from either losing or gaining external temperatures from the surrounding that
would alter the results of the experiment. Another error would arise from using different amounts
of sodium hydrogen carbonate (baking soda) to dissolve since this would alter the concentration
as this is a factor that should be kept constant in the experiment because concentration also alters
the rate of a chemical reaction. The experimental results for the reaction show that the results had
some errors resulting from either of the mentioned points. This explains the reason why the line
graph`s behavior.
The experiment was however a success since the control variables were identified as time
and temperature and it was found out how the temperature affects the rate of the reaction in the
experiment. Moreover, the hypothesis was proven true that temperature increase increases the
rate of the chemical reaction.
References.
for giving deductions from the results. Therefore, the handling of the stopwatch during the
experiment is crucial in order for one to get accurate results. In addition to that the container
holding the vinegar with the varying temperatures should be lagged. Lagging is done in order to
prevent the vinegar from either losing or gaining external temperatures from the surrounding that
would alter the results of the experiment. Another error would arise from using different amounts
of sodium hydrogen carbonate (baking soda) to dissolve since this would alter the concentration
as this is a factor that should be kept constant in the experiment because concentration also alters
the rate of a chemical reaction. The experimental results for the reaction show that the results had
some errors resulting from either of the mentioned points. This explains the reason why the line
graph`s behavior.
The experiment was however a success since the control variables were identified as time
and temperature and it was found out how the temperature affects the rate of the reaction in the
experiment. Moreover, the hypothesis was proven true that temperature increase increases the
rate of the chemical reaction.
References.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Rate of reaction between Baking soda and vinegar. 8
Chemistry Libretexts, (2016). Reaction Rates, Retrieved from
https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Kinetics/
Reaction_Rates/Reaction_Rate Retrieved 2 June, 2018.
Vutturi, A. (n.d). Factors Affecting Rate of Chemical Reactions. Retrieved from
http://www.adichemistry.com/physical/kinetics/factors/factors-affecting-rate-reaction.html
Retrieved 2 June, 2018.
Clark, J. (2013). The Effect of Temperature on Reaction Rates. Retrieved from
http://chemguide.co.uk/physical/basicrates/temperature.html Retrieved 2 June, 2018.
Veerendra. (2017). How Does the Temperature Affect the Rate of a Chemical Reaction?
Retrieved from https://www.aplustopper.com/temperature-affect-rate-chemical-reaction/
Retrieved 2 June, 2018.
Johnson, S. (2009). Rates of Reaction Experiment. Retrieved from
http://www.theibguide.com/Content/rates_of_reaction_experiment_v.1.02.pd Retrieved 2 June,
2018.
Chemistry Libretexts, (2016). Reaction Rates, Retrieved from
https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Kinetics/
Reaction_Rates/Reaction_Rate Retrieved 2 June, 2018.
Vutturi, A. (n.d). Factors Affecting Rate of Chemical Reactions. Retrieved from
http://www.adichemistry.com/physical/kinetics/factors/factors-affecting-rate-reaction.html
Retrieved 2 June, 2018.
Clark, J. (2013). The Effect of Temperature on Reaction Rates. Retrieved from
http://chemguide.co.uk/physical/basicrates/temperature.html Retrieved 2 June, 2018.
Veerendra. (2017). How Does the Temperature Affect the Rate of a Chemical Reaction?
Retrieved from https://www.aplustopper.com/temperature-affect-rate-chemical-reaction/
Retrieved 2 June, 2018.
Johnson, S. (2009). Rates of Reaction Experiment. Retrieved from
http://www.theibguide.com/Content/rates_of_reaction_experiment_v.1.02.pd Retrieved 2 June,
2018.
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





