Chemistry Homework: Analyzing Dissociation Energy and Molecular Modes
VerifiedAdded on 2020/04/15
|4
|437
|606
Homework Assignment
AI Summary
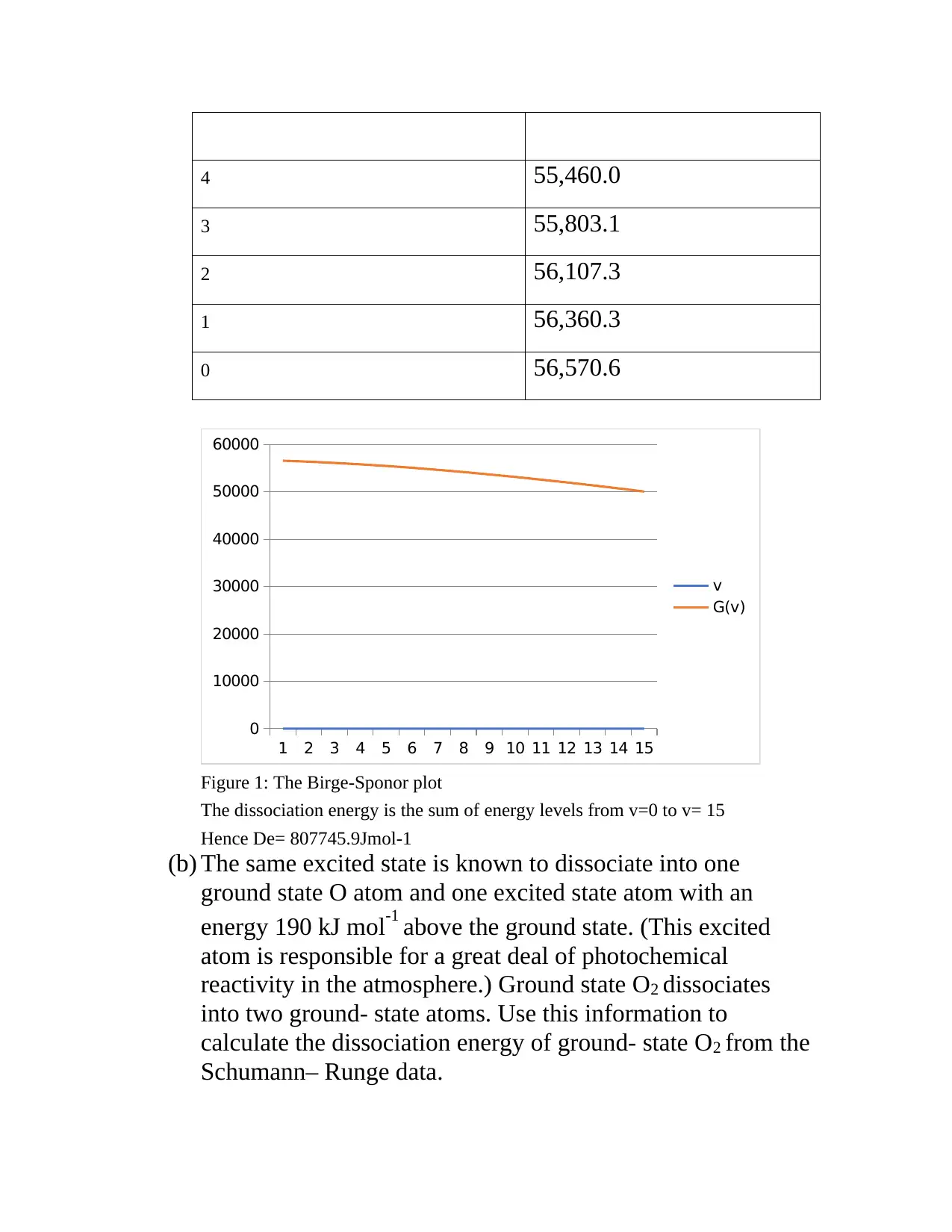
This document presents solutions to a chemistry assignment focusing on two main problems. The first problem involves calculating the dissociation energy of the upper electronic state of O2 using the Schumann–Runge band data and constructing a Birge–Sponer plot. The solution details the process of arranging the wavenumber data and determining the dissociation energy. Furthermore, it uses additional information about the excited state and ground state dissociation to calculate the ground-state O2 dissociation energy. The second problem addresses a planar, T-shaped molecule of C2v symmetry, asking for the number and irreducible representations of its vibrational modes. The solution indicates that there are six vibrational modes, which can be reduced to four due to double degeneracy, providing a structural representation of the molecule and citing relevant references.
1 out of 4



![[object Object]](/_next/static/media/star-bottom.7253800d.svg)