Pressure Ulcer Prevention in Hospitals: Silicone Foam Dressings Review
VerifiedAdded on 2022/08/23
|6
|3919
|113
Report
AI Summary
This open access review article examines the efficacy of silicone foam dressings in preventing pressure ulcers within the hospital setting. The study, conducted by researchers from the University of Central Florida College of Medicine, analyzes existing literature to compare the effectiveness of silicone foam dressings against standard preventative protocols, such as barrier creams and hydrocolloid dressings. The review encompasses several studies, including those focusing on patients undergoing intubation, cast immobilization, and cardiac surgery, and evaluates the impact of silicone foam dressings on the incidence of Stage I pressure ulcers. The findings suggest that silicone foam dressings, particularly Mepilex, demonstrate promise and may be superior to standard care, although further research is needed to confirm these benefits fully. The article highlights the importance of patient care, the significant healthcare costs associated with pressure ulcers, and the potential for silicone foam dressings to improve patient outcomes and streamline care processes.

Received 04/15/2016
Review began 05/17/2016
Review ended 07/30/2016
Published 08/08/2016
© Copyright 2016
Truong et al. This is an open access
article distributed under the terms of
the Creative Commons Attribution
License CC-BY 3.0., which permits
unrestricted use, distribution, and
reproduction in any medium,
provided the original author and
source are credited.
Pressure Ulcer Prevention in the Hospita
Setting Using Silicone Foam Dressings
Bao Truong , Eileen Grigson, Maulik Patel , Xinwei Liu
1. University of Central Florida College of Medicine
Corresponding author: Bao Truong, baotruong@knights.ucf.edu
Disclosures can be found in Additional Information at the end of the article
Abstract
Patient care is of the utmost importance in the hospital setting. Bedrest and immobility durin
hospitalization, especially in the surgical and intensive care setting, place the patient at high
risk for pressure ulcers. It is very important to prevent or notice a pressure ulcer forming due
the significant health care costs involved and patient health associated with them. Various
measures are in place to prevent patients from getting pressure ulcers, but a newer materia
silicone foam dressings, has been introduced as an alternative solution for the prevention of
these ulcers. We review the current literature to examine whether the standard protocol or
silicone material is superior to the prevention of pressure ulcer formation. We conclude that
silicone foam dressings, when used as prophylactic treatment, seems very promising and m
even be superior to the standard care of prevention. However, there were limitations to som
studies and further research is needed to confirm the role of silicone foam dressings.
Categories: Preventive Medicine
Keywords: pressure ulcers, silicone dressings, preventive medicine, hospital acquired infection,
pressure sores, decubitus ulcer
Introduction And Background
Pressure ulcers, also called pressure sores or bedsores, are a burden to healthcare and have
significant cost of morbidity. In 2013, a United States Medicare study reported the incidence
hospital-acquired pressure ulcers to be 4.5% in hospitalized patients, with an estimated 11
billion dollars for the cost of pressure ulcer care [1-2].
Pressure ulcers are due to a multitude of factors that contribute to tissue vulnerability and
breakdown. In 2007, the National Pressure Ulcer Advisory Panel (NPUAP) established a stagin
system for categorizing pressure ulcer injuries. Pressure ulcers are often formed where skin
covers bony areas and common sites are the back of the head and ears, the shoulders, the
elbows, the lower back and buttocks, the hips, the inner knees, and the heels. Although pres
ulcers can develop over the course of 24 hours, they may not present until a week later. Sta
pressure ulcers present with intact, erythematous skin that does not blanch. Stage II pressur
ulcers can appear as a fluid-filled blister, which represents breakage of the epidermis and m
involve the underlying dermis. Stage III pressure ulcers present with necrotic tissue and exte
into the subcutaneous tissue. Finally, Stage IV pressure ulcers extend deep into the bone or
muscle with full thickness tissue loss [3].
The incidence and prevalence of pressure ulcers vary greatly, depending on the setting.
Patients hospitalized in the intensive care unit (ICU) or immobilized after major surgery are a
higher risk [4]. Vigilant patient care teams educated in pressure ulcer care can identify the s
1 1 1 1
Open Access Review
Article DOI: 10.7759/cureus.730
How to cite this article
Truong B, Grigson E, Patel M, et al. (August 08, 2016) Pressure Ulcer Prevention in the Hospital Setting
Using Silicone Foam Dressings. Cureus 8(8): e730. DOI 10.7759/cureus.730
Review began 05/17/2016
Review ended 07/30/2016
Published 08/08/2016
© Copyright 2016
Truong et al. This is an open access
article distributed under the terms of
the Creative Commons Attribution
License CC-BY 3.0., which permits
unrestricted use, distribution, and
reproduction in any medium,
provided the original author and
source are credited.
Pressure Ulcer Prevention in the Hospita
Setting Using Silicone Foam Dressings
Bao Truong , Eileen Grigson, Maulik Patel , Xinwei Liu
1. University of Central Florida College of Medicine
Corresponding author: Bao Truong, baotruong@knights.ucf.edu
Disclosures can be found in Additional Information at the end of the article
Abstract
Patient care is of the utmost importance in the hospital setting. Bedrest and immobility durin
hospitalization, especially in the surgical and intensive care setting, place the patient at high
risk for pressure ulcers. It is very important to prevent or notice a pressure ulcer forming due
the significant health care costs involved and patient health associated with them. Various
measures are in place to prevent patients from getting pressure ulcers, but a newer materia
silicone foam dressings, has been introduced as an alternative solution for the prevention of
these ulcers. We review the current literature to examine whether the standard protocol or
silicone material is superior to the prevention of pressure ulcer formation. We conclude that
silicone foam dressings, when used as prophylactic treatment, seems very promising and m
even be superior to the standard care of prevention. However, there were limitations to som
studies and further research is needed to confirm the role of silicone foam dressings.
Categories: Preventive Medicine
Keywords: pressure ulcers, silicone dressings, preventive medicine, hospital acquired infection,
pressure sores, decubitus ulcer
Introduction And Background
Pressure ulcers, also called pressure sores or bedsores, are a burden to healthcare and have
significant cost of morbidity. In 2013, a United States Medicare study reported the incidence
hospital-acquired pressure ulcers to be 4.5% in hospitalized patients, with an estimated 11
billion dollars for the cost of pressure ulcer care [1-2].
Pressure ulcers are due to a multitude of factors that contribute to tissue vulnerability and
breakdown. In 2007, the National Pressure Ulcer Advisory Panel (NPUAP) established a stagin
system for categorizing pressure ulcer injuries. Pressure ulcers are often formed where skin
covers bony areas and common sites are the back of the head and ears, the shoulders, the
elbows, the lower back and buttocks, the hips, the inner knees, and the heels. Although pres
ulcers can develop over the course of 24 hours, they may not present until a week later. Sta
pressure ulcers present with intact, erythematous skin that does not blanch. Stage II pressur
ulcers can appear as a fluid-filled blister, which represents breakage of the epidermis and m
involve the underlying dermis. Stage III pressure ulcers present with necrotic tissue and exte
into the subcutaneous tissue. Finally, Stage IV pressure ulcers extend deep into the bone or
muscle with full thickness tissue loss [3].
The incidence and prevalence of pressure ulcers vary greatly, depending on the setting.
Patients hospitalized in the intensive care unit (ICU) or immobilized after major surgery are a
higher risk [4]. Vigilant patient care teams educated in pressure ulcer care can identify the s
1 1 1 1
Open Access Review
Article DOI: 10.7759/cureus.730
How to cite this article
Truong B, Grigson E, Patel M, et al. (August 08, 2016) Pressure Ulcer Prevention in the Hospital Setting
Using Silicone Foam Dressings. Cureus 8(8): e730. DOI 10.7759/cureus.730
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

of tissue breakdown and are aware of the numerous factors that place patients at risk of
pressure ulcer formation, including reduced mobility, nutritional status, urine or fecal
incontinence, medications that cause reduced sensation and immobility, instruments and to
that create mechanical pressure against the body, and conditions that decrease tissue
oxygenation [3].
Barrier creams used in the prevention of pressure ulcers form a protective layer that keeps a
excessive moisture due to incontinence, perspiration, or wound drainage and aid in
maintaining the integrity of the skin [5]. These creams include Calmoseptine® (Calmoseptin
Inc., Huntington Beach, CA), Lantiseptic® (Santus, Duluth, GA), Silvadene® (Pfizer, Inc., New
York, NY), and others that use silver sulfadiazine, zinc oxide, or lanolin as active ingredients
prevent infection. A hydrocolloid dressing normally used in wound care, such as
Tegaderm® (3M Center, St. Paul, MN), is also frequently used in combination with barrier
creams and contains an adhesive compound in combination with a water-resistant outer lay
to prevent additional moisture exposure [6]. Silicone foam dressings, such as
Mepilex® (Mölnlycke Health Care, Gothenburg, Sweden), are soft silicone multi-layered foam
dressings that contain silver, which acts as an antibiotic agent, and an adhesive material
different from the regularly used hydrocolloids that result in less skin abrasion when it is tim
to remove and replace the dressing [7].
Currently, standard protocols for care in pressure ulcer prevention vary between hospital
systems with different algorithms for inpatient and outpatient situations but include the use
low-pressure beds, positioning and turning, and barrier cream with a hydrocolloid layering
placed over the area of application in areas at risk of pressure formation, such as the sacrum
heels, and buttocks [3]. Although the standard care has been effective, a newer material,
silicone foam dressing, has been introduced as an alternative for pressure ulcer prevention w
potentially greater cost and health benefits for hospitals and patients, respectively [8]. If the
silicone foam dressings are, indeed, better at treating and preventing sore formation in
immobilized patients, then nurses should also benefit in their role as patient caregivers by
being able to provide greater and more efficient care to their patients.
This literature review will examine whether immobilized patients in the hospital setting who
are given silicone foam dressings compared to the standard protocol, which utilizes barrier
creams under a hydrocolloid layering for the prevention of pressure ulcer, have an effect on
incidence of Stage I pressure ulcer formation.
Review
A literature review was conducted to determine the effectiveness of standard protocols for
pressure ulcer care versus a newer silicone foam dressing. PubMed searches were performe
using the phrases “silicone foam dressing” and “barrier creams” in the English language wit
the modifier of articles published in the last seven years. Articles were then screened for
relevance and excluded if the studies were not primarily focused on pressure ulcer preventio
This search process yielded five quantitative research articles focusing on the usage and
effectiveness of silicone foam dressings.
This review covers the five separate studies at various institutions detailing the utility and
benefits of using silicone foam dressing as an alternative to the standard care of pressure ul
prevention (Table 1). Huang, et al. sought to determine if there was any way to reduce the
incidence of nasal pressure ulcers that arise as a complication of nasotracheal intubation
during oral and maxillofacial surgery [6]. By using an initial animal model to test the clinical
application of silicone foam material as a means of reducing pressure on the nasal area, Hua
et al. believed that the use of the cushioning material would aid in protection during intubati
as opposed to intubation without additional cushion protection. Eighteen patients were
2016 Truong et al. Cureus 8(8): e730. DOI 10.7759/cureus.730 2 of 6
pressure ulcer formation, including reduced mobility, nutritional status, urine or fecal
incontinence, medications that cause reduced sensation and immobility, instruments and to
that create mechanical pressure against the body, and conditions that decrease tissue
oxygenation [3].
Barrier creams used in the prevention of pressure ulcers form a protective layer that keeps a
excessive moisture due to incontinence, perspiration, or wound drainage and aid in
maintaining the integrity of the skin [5]. These creams include Calmoseptine® (Calmoseptin
Inc., Huntington Beach, CA), Lantiseptic® (Santus, Duluth, GA), Silvadene® (Pfizer, Inc., New
York, NY), and others that use silver sulfadiazine, zinc oxide, or lanolin as active ingredients
prevent infection. A hydrocolloid dressing normally used in wound care, such as
Tegaderm® (3M Center, St. Paul, MN), is also frequently used in combination with barrier
creams and contains an adhesive compound in combination with a water-resistant outer lay
to prevent additional moisture exposure [6]. Silicone foam dressings, such as
Mepilex® (Mölnlycke Health Care, Gothenburg, Sweden), are soft silicone multi-layered foam
dressings that contain silver, which acts as an antibiotic agent, and an adhesive material
different from the regularly used hydrocolloids that result in less skin abrasion when it is tim
to remove and replace the dressing [7].
Currently, standard protocols for care in pressure ulcer prevention vary between hospital
systems with different algorithms for inpatient and outpatient situations but include the use
low-pressure beds, positioning and turning, and barrier cream with a hydrocolloid layering
placed over the area of application in areas at risk of pressure formation, such as the sacrum
heels, and buttocks [3]. Although the standard care has been effective, a newer material,
silicone foam dressing, has been introduced as an alternative for pressure ulcer prevention w
potentially greater cost and health benefits for hospitals and patients, respectively [8]. If the
silicone foam dressings are, indeed, better at treating and preventing sore formation in
immobilized patients, then nurses should also benefit in their role as patient caregivers by
being able to provide greater and more efficient care to their patients.
This literature review will examine whether immobilized patients in the hospital setting who
are given silicone foam dressings compared to the standard protocol, which utilizes barrier
creams under a hydrocolloid layering for the prevention of pressure ulcer, have an effect on
incidence of Stage I pressure ulcer formation.
Review
A literature review was conducted to determine the effectiveness of standard protocols for
pressure ulcer care versus a newer silicone foam dressing. PubMed searches were performe
using the phrases “silicone foam dressing” and “barrier creams” in the English language wit
the modifier of articles published in the last seven years. Articles were then screened for
relevance and excluded if the studies were not primarily focused on pressure ulcer preventio
This search process yielded five quantitative research articles focusing on the usage and
effectiveness of silicone foam dressings.
This review covers the five separate studies at various institutions detailing the utility and
benefits of using silicone foam dressing as an alternative to the standard care of pressure ul
prevention (Table 1). Huang, et al. sought to determine if there was any way to reduce the
incidence of nasal pressure ulcers that arise as a complication of nasotracheal intubation
during oral and maxillofacial surgery [6]. By using an initial animal model to test the clinical
application of silicone foam material as a means of reducing pressure on the nasal area, Hua
et al. believed that the use of the cushioning material would aid in protection during intubati
as opposed to intubation without additional cushion protection. Eighteen patients were
2016 Truong et al. Cureus 8(8): e730. DOI 10.7759/cureus.730 2 of 6
studied. The results showed a decrease in the size of nasal pressure sores between the cont
and comparison groups to which Huang, et al. attributed to the protective efficacy of the
silicone foam dressing used as lining during nasal intubation. It should be noted, however, th
only a small sample size of 18 participants was included in this study.
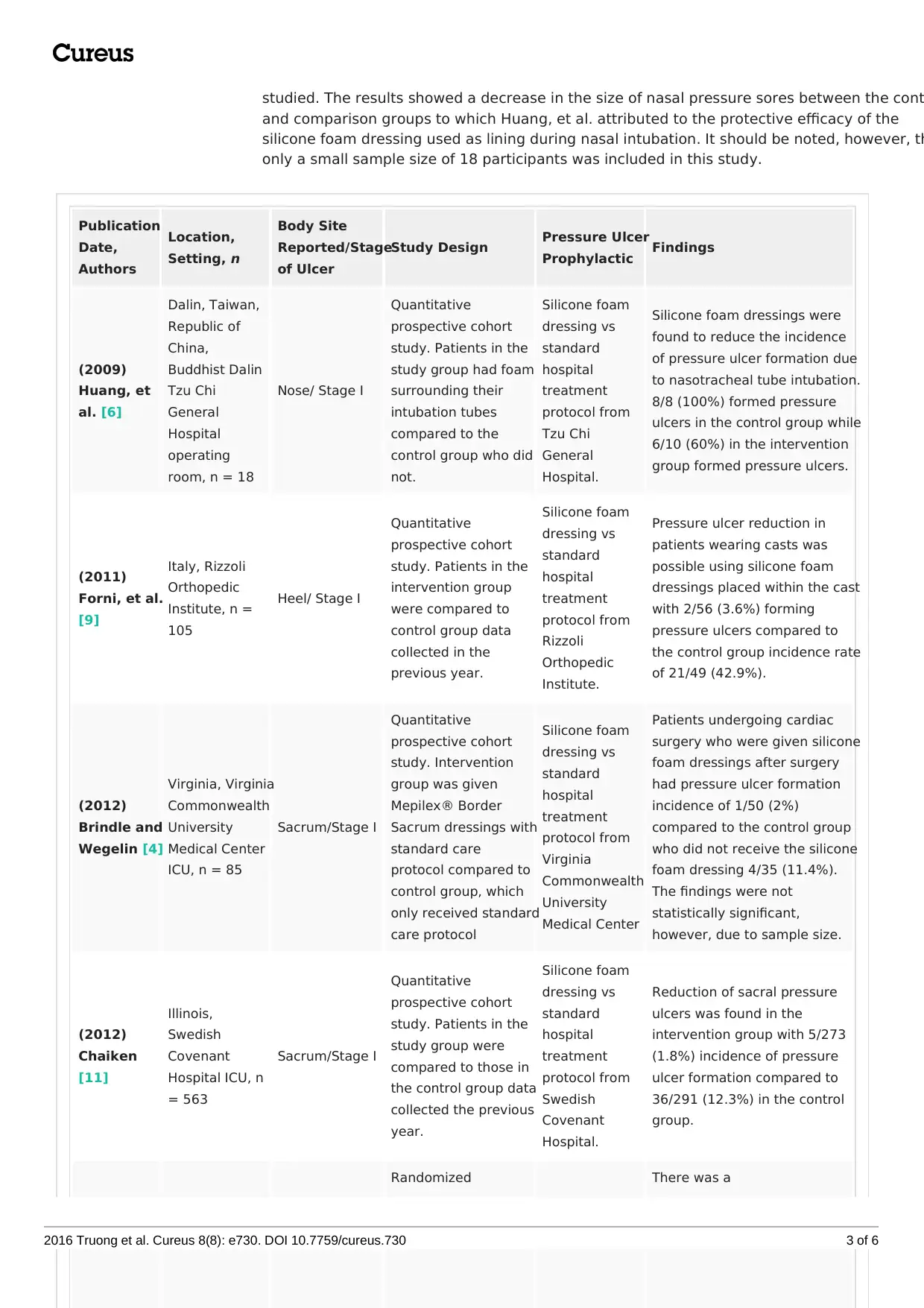
Publication
Date,
Authors
Location,
Setting, n
Body Site
Reported/Stage
of Ulcer
Study Design Pressure Ulcer
Prophylactic Findings
(2009)
Huang, et
al. [6]
Dalin, Taiwan,
Republic of
China,
Buddhist Dalin
Tzu Chi
General
Hospital
operating
room, n = 18
Nose/ Stage I
Quantitative
prospective cohort
study. Patients in the
study group had foam
surrounding their
intubation tubes
compared to the
control group who did
not.
Silicone foam
dressing vs
standard
hospital
treatment
protocol from
Tzu Chi
General
Hospital.
Silicone foam dressings were
found to reduce the incidence
of pressure ulcer formation due
to nasotracheal tube intubation.
8/8 (100%) formed pressure
ulcers in the control group while
6/10 (60%) in the intervention
group formed pressure ulcers.
(2011)
Forni, et al.
[9]
Italy, Rizzoli
Orthopedic
Institute, n =
105
Heel/ Stage I
Quantitative
prospective cohort
study. Patients in the
intervention group
were compared to
control group data
collected in the
previous year.
Silicone foam
dressing vs
standard
hospital
treatment
protocol from
Rizzoli
Orthopedic
Institute.
Pressure ulcer reduction in
patients wearing casts was
possible using silicone foam
dressings placed within the cast
with 2/56 (3.6%) forming
pressure ulcers compared to
the control group incidence rate
of 21/49 (42.9%).
(2012)
Brindle and
Wegelin [4]
Virginia, Virginia
Commonwealth
University
Medical Center
ICU, n = 85
Sacrum/Stage I
Quantitative
prospective cohort
study. Intervention
group was given
Mepilex® Border
Sacrum dressings with
standard care
protocol compared to
control group, which
only received standard
care protocol
Silicone foam
dressing vs
standard
hospital
treatment
protocol from
Virginia
Commonwealth
University
Medical Center
Patients undergoing cardiac
surgery who were given silicone
foam dressings after surgery
had pressure ulcer formation
incidence of 1/50 (2%)
compared to the control group
who did not receive the silicone
foam dressing 4/35 (11.4%).
The findings were not
statistically significant,
however, due to sample size.
(2012)
Chaiken
[11]
Illinois,
Swedish
Covenant
Hospital ICU, n
= 563
Sacrum/Stage I
Quantitative
prospective cohort
study. Patients in the
study group were
compared to those in
the control group data
collected the previous
year.
Silicone foam
dressing vs
standard
hospital
treatment
protocol from
Swedish
Covenant
Hospital.
Reduction of sacral pressure
ulcers was found in the
intervention group with 5/273
(1.8%) incidence of pressure
ulcer formation compared to
36/291 (12.3%) in the control
group.
Randomized There was a
2016 Truong et al. Cureus 8(8): e730. DOI 10.7759/cureus.730 3 of 6
and comparison groups to which Huang, et al. attributed to the protective efficacy of the
silicone foam dressing used as lining during nasal intubation. It should be noted, however, th
only a small sample size of 18 participants was included in this study.
Publication
Date,
Authors
Location,
Setting, n
Body Site
Reported/Stage
of Ulcer
Study Design Pressure Ulcer
Prophylactic Findings
(2009)
Huang, et
al. [6]
Dalin, Taiwan,
Republic of
China,
Buddhist Dalin
Tzu Chi
General
Hospital
operating
room, n = 18
Nose/ Stage I
Quantitative
prospective cohort
study. Patients in the
study group had foam
surrounding their
intubation tubes
compared to the
control group who did
not.
Silicone foam
dressing vs
standard
hospital
treatment
protocol from
Tzu Chi
General
Hospital.
Silicone foam dressings were
found to reduce the incidence
of pressure ulcer formation due
to nasotracheal tube intubation.
8/8 (100%) formed pressure
ulcers in the control group while
6/10 (60%) in the intervention
group formed pressure ulcers.
(2011)
Forni, et al.
[9]
Italy, Rizzoli
Orthopedic
Institute, n =
105
Heel/ Stage I
Quantitative
prospective cohort
study. Patients in the
intervention group
were compared to
control group data
collected in the
previous year.
Silicone foam
dressing vs
standard
hospital
treatment
protocol from
Rizzoli
Orthopedic
Institute.
Pressure ulcer reduction in
patients wearing casts was
possible using silicone foam
dressings placed within the cast
with 2/56 (3.6%) forming
pressure ulcers compared to
the control group incidence rate
of 21/49 (42.9%).
(2012)
Brindle and
Wegelin [4]
Virginia, Virginia
Commonwealth
University
Medical Center
ICU, n = 85
Sacrum/Stage I
Quantitative
prospective cohort
study. Intervention
group was given
Mepilex® Border
Sacrum dressings with
standard care
protocol compared to
control group, which
only received standard
care protocol
Silicone foam
dressing vs
standard
hospital
treatment
protocol from
Virginia
Commonwealth
University
Medical Center
Patients undergoing cardiac
surgery who were given silicone
foam dressings after surgery
had pressure ulcer formation
incidence of 1/50 (2%)
compared to the control group
who did not receive the silicone
foam dressing 4/35 (11.4%).
The findings were not
statistically significant,
however, due to sample size.
(2012)
Chaiken
[11]
Illinois,
Swedish
Covenant
Hospital ICU, n
= 563
Sacrum/Stage I
Quantitative
prospective cohort
study. Patients in the
study group were
compared to those in
the control group data
collected the previous
year.
Silicone foam
dressing vs
standard
hospital
treatment
protocol from
Swedish
Covenant
Hospital.
Reduction of sacral pressure
ulcers was found in the
intervention group with 5/273
(1.8%) incidence of pressure
ulcer formation compared to
36/291 (12.3%) in the control
group.
Randomized There was a
2016 Truong et al. Cureus 8(8): e730. DOI 10.7759/cureus.730 3 of 6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

(2015)
Santamaria,
et al. [12]
Australia, Royal
Melbourne
Hospital ICU n
= 313
Sacrum/
heel/Stage I
controlled trial with
the intervention group
receiving Mepilex®
Border Sacrum and
Mepilex® Heel
dressings. Both
groups received
standard prevention
strategies.
Silicone foam
dressing vs
standard
hospital
treatment
protocol from
Royal
Melbourne
Hospital.
significantly decreased
formation of pressure ulcers in
the intervention group in
comparison to the control
group who received traditional
wound dressing. 5/161 (3.1%)
developed pressure ulcers in
the intervention group vs 20/152
(13.1%) in the control group.
TABLE 1: Study Characterisitics
n = number
Forni, et al. investigated pressure ulcer prevention by using foam dressings in patients with
immobilization [9]. One hundred and fifty-six patients were included in this study, 85 in the
control group and 71 in the experimental group. Inclusion criteria included any patient
admitted to an Italian orthopedic research hospital for an orthopedic associated disease
requiring a plaster cast of their foot. The results provided substantial evidence of pressure u
prevention by applying foam within the plaster cast of patients with data that was statistical
significant for differences among the control and experimental groups. Forni, et al. discussed
the positive impact that foams can have as a deterrent against pressure ulcers in patients
requiring casts as well as its potential use in diabetics who are more prone to developing
pressure ulcers [9].
Cardiac surgery patients are one of the most at-risk patient populations in the incidence of
hospital-acquired pressure ulcers [10]. In 2012, Brindle and Wegelin hypothesized that using
Mepilex®, a silicone foam dressing, around the area of the sacrum in these patients would
decrease incidence rates of pressure ulcer formation in comparison to the hospital’s current
standard care, which consisted of the use of a skin protectant, Calmoseptine®, on high risk
areas [4]. Silicone foam dressing impregnated with silver as an antibiotic was the independe
variable in the study, while the dependent variable was the formation of pressure ulcers in t
area of the sacrum. A total of 85 patients were enrolled in this study who had met the criteri
for inclusion and exclusion. Eligibility for the study included patients who had undergone a
surgical procedure within a certain period of time, cardiac arrest on admission, in shock, or h
some sort of condition that required chronic bed rest. Statistical analyses for the variables
collected showed no significant differences when compared between the experimental versu
the control groups. Results showed that the incidence rate of pressure ulcer formation in
patients who were given Mepilex® silicone foam dressing was not considerably less than in
patients who were given the barrier cream. Brindle and Wegelin concluded in their paper tha
while using silicone foam dressings did produce a decreased incidence rate in pressure ulcer
the intervention group, it was not by much in comparison to the control group. They reasone
that the smaller sample size than what was originally planned, in addition to the standard ca
procedures and attentive efforts of the ICU nurses in patient treatment, may have played a p
in the findings of the study.
In 2012, Chaiken, in a similar study, sought to determine whether the incidence of pressure
ulcer formation would decrease in the ICU through the use of silicone foam dressing placed a
the area of the sacrum [11]. During the prospective experiment, Mepilex® Border Sacrum, a
type of silicone foam, was given to a group of 273 newly admitted patients in the ICU with
nurses instructed in changing the dressing twice a week while following the hospital's standa
2016 Truong et al. Cureus 8(8): e730. DOI 10.7759/cureus.730 4 of 6
Santamaria,
et al. [12]
Australia, Royal
Melbourne
Hospital ICU n
= 313
Sacrum/
heel/Stage I
controlled trial with
the intervention group
receiving Mepilex®
Border Sacrum and
Mepilex® Heel
dressings. Both
groups received
standard prevention
strategies.
Silicone foam
dressing vs
standard
hospital
treatment
protocol from
Royal
Melbourne
Hospital.
significantly decreased
formation of pressure ulcers in
the intervention group in
comparison to the control
group who received traditional
wound dressing. 5/161 (3.1%)
developed pressure ulcers in
the intervention group vs 20/152
(13.1%) in the control group.
TABLE 1: Study Characterisitics
n = number
Forni, et al. investigated pressure ulcer prevention by using foam dressings in patients with
immobilization [9]. One hundred and fifty-six patients were included in this study, 85 in the
control group and 71 in the experimental group. Inclusion criteria included any patient
admitted to an Italian orthopedic research hospital for an orthopedic associated disease
requiring a plaster cast of their foot. The results provided substantial evidence of pressure u
prevention by applying foam within the plaster cast of patients with data that was statistical
significant for differences among the control and experimental groups. Forni, et al. discussed
the positive impact that foams can have as a deterrent against pressure ulcers in patients
requiring casts as well as its potential use in diabetics who are more prone to developing
pressure ulcers [9].
Cardiac surgery patients are one of the most at-risk patient populations in the incidence of
hospital-acquired pressure ulcers [10]. In 2012, Brindle and Wegelin hypothesized that using
Mepilex®, a silicone foam dressing, around the area of the sacrum in these patients would
decrease incidence rates of pressure ulcer formation in comparison to the hospital’s current
standard care, which consisted of the use of a skin protectant, Calmoseptine®, on high risk
areas [4]. Silicone foam dressing impregnated with silver as an antibiotic was the independe
variable in the study, while the dependent variable was the formation of pressure ulcers in t
area of the sacrum. A total of 85 patients were enrolled in this study who had met the criteri
for inclusion and exclusion. Eligibility for the study included patients who had undergone a
surgical procedure within a certain period of time, cardiac arrest on admission, in shock, or h
some sort of condition that required chronic bed rest. Statistical analyses for the variables
collected showed no significant differences when compared between the experimental versu
the control groups. Results showed that the incidence rate of pressure ulcer formation in
patients who were given Mepilex® silicone foam dressing was not considerably less than in
patients who were given the barrier cream. Brindle and Wegelin concluded in their paper tha
while using silicone foam dressings did produce a decreased incidence rate in pressure ulcer
the intervention group, it was not by much in comparison to the control group. They reasone
that the smaller sample size than what was originally planned, in addition to the standard ca
procedures and attentive efforts of the ICU nurses in patient treatment, may have played a p
in the findings of the study.
In 2012, Chaiken, in a similar study, sought to determine whether the incidence of pressure
ulcer formation would decrease in the ICU through the use of silicone foam dressing placed a
the area of the sacrum [11]. During the prospective experiment, Mepilex® Border Sacrum, a
type of silicone foam, was given to a group of 273 newly admitted patients in the ICU with
nurses instructed in changing the dressing twice a week while following the hospital's standa
2016 Truong et al. Cureus 8(8): e730. DOI 10.7759/cureus.730 4 of 6
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

protocol for pressure ulcer prevention. The study took place over a six-month period and the
results showed a decrease in the incidence rates of pressure ulcers from 12.3% during the 3
month observation period in the comparison group to 1.8% in the experiment group. In
addition to the lower incidence rate of pressure ulcers, Chaiken further reported an average
expenditure of $6,653.00 for the six-month experiment period, which, in turn, saved the
hospital money by avoiding the higher cost of treatment associated with treating pressure
ulcers.
In 2013, Santamaria, et al. examined the effectiveness of soft silicone dressings of the sacru
and the heel of the body in the ICU setting in preventing pressure ulcers [12]. This randomiz
control study included 440 patients who were newly admitted to the emergency department
were subsequently transferred to the ICU. Findings were significant for decreased formation
pressure ulcers in the intervention group, as measured by the Australian Wound Managemen
Association (AWMA) four-point grading system, in comparison to the control group who
received a standard barrier cream with a hydrocolloid layering. Santamaria, et al. concluded
that use of silicone foam dressings in combination with thorough risk assessment and
evidence-based pressure ulcer prevention strategies had a substantial impact on the reducti
of pressure ulcer incidence.
Upon review of the articles, there is efficacy and utility of silicone foam dressings among oth
forms of barriers in the prevention of pressure ulcers. This literature review shows that
standard traditional methods were helpful in preventing pressure ulcers, but silicone foam
dressings were even more effective in reducing the incidence of pressure ulcer formation (3.
versus 42.9% in the study conducted by Forni, et al.) [9]. Not only should silicone foam
dressings be considered due to their effectiveness, Santamaria, et al. concluded in their stud
that they should be considered due to their economic savings to health care institutions
(average net cost of intervention compared to control, $52.87 versus $107.91) [12]. There w
still limitations, which were evident when reviewing the literature, even though each article
provided evidence regarding the effectiveness of silicone foam dressings. Research conduct
by Huang, et al. provided information regarding protection of pressure ulcer formation, but t
paper did not include the methods of data collection or methods of analyses [6]. The researc
Forni, et al. and Chaiken was limited by a lack of a parallel control within these respective
studies and used past patient data as a control instead [9, 11]. Brindle and Wegelin mention
that a major limitation of their study was indeed the duration of the experiment and sample
size [4].
Conclusions
We would like to recommend that future research would not only benefit from increasing the
sample size and diversifying the population but should strive to conduct trials over longer
periods of time in order to obtain adequate data and use the maximal amount of data it can
analysis. Additionally, the research may be expanded to other clinical areas where the incide
of pressure ulcer formation is high, such as patients with incontinence, recurrent pressure
ulcers, and the elderly hospitalized with altered mental status.
Proper skin care is very important in the prevention of these ulcers. Even though guidelines
in place for the prevention of pressure ulcers, the incidence is still very high in patients who
in the ICU or who have had major surgeries. Prevention is extremely important because not
only does the patient suffer from the pressure ulcer, but there is an economic impact related
them as the hospital may incur additional costs related to pressure ulcer management. In
addition to standard protocols, the use of silicone foam dressings as a barrier against irritatio
and constant pressure to the skin should be effectively utilized in pressure ulcer prevention.
2016 Truong et al. Cureus 8(8): e730. DOI 10.7759/cureus.730 5 of 6
results showed a decrease in the incidence rates of pressure ulcers from 12.3% during the 3
month observation period in the comparison group to 1.8% in the experiment group. In
addition to the lower incidence rate of pressure ulcers, Chaiken further reported an average
expenditure of $6,653.00 for the six-month experiment period, which, in turn, saved the
hospital money by avoiding the higher cost of treatment associated with treating pressure
ulcers.
In 2013, Santamaria, et al. examined the effectiveness of soft silicone dressings of the sacru
and the heel of the body in the ICU setting in preventing pressure ulcers [12]. This randomiz
control study included 440 patients who were newly admitted to the emergency department
were subsequently transferred to the ICU. Findings were significant for decreased formation
pressure ulcers in the intervention group, as measured by the Australian Wound Managemen
Association (AWMA) four-point grading system, in comparison to the control group who
received a standard barrier cream with a hydrocolloid layering. Santamaria, et al. concluded
that use of silicone foam dressings in combination with thorough risk assessment and
evidence-based pressure ulcer prevention strategies had a substantial impact on the reducti
of pressure ulcer incidence.
Upon review of the articles, there is efficacy and utility of silicone foam dressings among oth
forms of barriers in the prevention of pressure ulcers. This literature review shows that
standard traditional methods were helpful in preventing pressure ulcers, but silicone foam
dressings were even more effective in reducing the incidence of pressure ulcer formation (3.
versus 42.9% in the study conducted by Forni, et al.) [9]. Not only should silicone foam
dressings be considered due to their effectiveness, Santamaria, et al. concluded in their stud
that they should be considered due to their economic savings to health care institutions
(average net cost of intervention compared to control, $52.87 versus $107.91) [12]. There w
still limitations, which were evident when reviewing the literature, even though each article
provided evidence regarding the effectiveness of silicone foam dressings. Research conduct
by Huang, et al. provided information regarding protection of pressure ulcer formation, but t
paper did not include the methods of data collection or methods of analyses [6]. The researc
Forni, et al. and Chaiken was limited by a lack of a parallel control within these respective
studies and used past patient data as a control instead [9, 11]. Brindle and Wegelin mention
that a major limitation of their study was indeed the duration of the experiment and sample
size [4].
Conclusions
We would like to recommend that future research would not only benefit from increasing the
sample size and diversifying the population but should strive to conduct trials over longer
periods of time in order to obtain adequate data and use the maximal amount of data it can
analysis. Additionally, the research may be expanded to other clinical areas where the incide
of pressure ulcer formation is high, such as patients with incontinence, recurrent pressure
ulcers, and the elderly hospitalized with altered mental status.
Proper skin care is very important in the prevention of these ulcers. Even though guidelines
in place for the prevention of pressure ulcers, the incidence is still very high in patients who
in the ICU or who have had major surgeries. Prevention is extremely important because not
only does the patient suffer from the pressure ulcer, but there is an economic impact related
them as the hospital may incur additional costs related to pressure ulcer management. In
addition to standard protocols, the use of silicone foam dressings as a barrier against irritatio
and constant pressure to the skin should be effectively utilized in pressure ulcer prevention.
2016 Truong et al. Cureus 8(8): e730. DOI 10.7759/cureus.730 5 of 6

Appendices
Additional Information
Disclosures
This study did not involve human participants or tissue. This study did not involve animal
subjects or tissue. Conflicts of interest: The authors have declared that no conflicts of inte
exist except for the following: Other relationships: A research grant and supply of dressin
was provided by a Mepilex® supplier in one of the studies cited within this article.
References
1. Moore Z: US Medicare data show incidence of hospital-acquired pressure ulcers is 4.5%, and
they are associated with longer hospital stay and higher risk of death. Evid Based Nurs. 2013
16:118–19. 10.1136/eb-2012-101112
2. Moore Z, Cowman S, Posnett J: An economic analysis of repositioning for the prevention of
pressure ulcers. J Clin Nurs. 2013, 22:2354–60. 10.1111/j.1365-2702.2012.04310.x
3. Wake WT: Pressure ulcers: what clinicians need to know . Perm J. 2010, 14:56–60.
4. Brindle CT, Wegelin JA: Prophylactic dressing application to reduce pressure ulcer formation
in cardiac surgery patients. J Wound Ostomy Continence Nurs. 2012, 39:133–42.
10.1097/WON.0b013e318247cb82
5. Bliss DZ, Zehrer C, Savik K, Smith G, Hedblom E:An economic evaluation of four skin damage
prevention regimens in nursing home residents with incontinence: economics of skin damage
prevention. J Wound Ostomy Continence Nurs. 2007, 34:143-52.
10.1097/01.WON.0000264825.03485.40
6. Huang TT, Tseng CE, Lee TM, Yeh JY, Lai YY: Preventing pressure sores of the nasal ala after
nasotracheal tube intubation: from animal model to clinical application. J Oral Maxillofac
Surg. 2009, 67:543–51. 10.1016/j.joms.2008.06.100
7. Barrett S: Mepilex® Ag: an antimicrobial, absorbent foam dressing with Safetac® technology
Br J Nurs. 2009, 18:S28, S30-6. 10.12968/bjon.2009.18.Sup7.45133
8. Chuangsuwanich A, Chortrakarnkij P, Kangwanpoom J: Cost-effectiveness analysis in
comparing alginate silver dressing with silver zinc sulfadiazine cream in the treatment of
pressure ulcers. Arch Plast Surg. 2013, 40:589–96. 10.5999/aps.2013.40.5.589
9. Forni C, Loro L, Tremosini M, Mini S, Pignotti E, Bigoni O, Guzzo G, Bellini L, Trofa C, Di
Cataldo AM, Guzzi M: Use of polyurethane foam inside plaster casts to prevent the onset of
heel sores in the population at risk. A controlled clinical study. J Clin Nurs. 2011, 20:675–80.
10.1111/j.1365-2702.2010.03458.x
10. Chen HL, Chen XY, Wu J: The incidence of pressure ulcers in surgical patients of the last 5
years: a systematic review. Wounds. 2012, 24:234-41.
11. Chaiken N: Reduction of sacral pressure ulcers in the intensive care unit using a silicone
border foam dressing. J Wound Ostomy Continence Nurs. 2012, 39:143–45.
12. Santamaria N, Gerdtz M, Sage S, McCann J, Freeman A, Vassiliou T, De Vincentis S, Ng AW,
Manias E, Liu W, Knott J: A randomised controlled trial of the effectiveness of soft silicone
multi-layered foam dressings in the prevention of sacral and heel pressure ulcers in trauma
and critically ill patients: the border trial. Int Wound J. 2015, 12:302–8. 10.1111/iwj.12101
2016 Truong et al. Cureus 8(8): e730. DOI 10.7759/cureus.730 6 of 6
Additional Information
Disclosures
This study did not involve human participants or tissue. This study did not involve animal
subjects or tissue. Conflicts of interest: The authors have declared that no conflicts of inte
exist except for the following: Other relationships: A research grant and supply of dressin
was provided by a Mepilex® supplier in one of the studies cited within this article.
References
1. Moore Z: US Medicare data show incidence of hospital-acquired pressure ulcers is 4.5%, and
they are associated with longer hospital stay and higher risk of death. Evid Based Nurs. 2013
16:118–19. 10.1136/eb-2012-101112
2. Moore Z, Cowman S, Posnett J: An economic analysis of repositioning for the prevention of
pressure ulcers. J Clin Nurs. 2013, 22:2354–60. 10.1111/j.1365-2702.2012.04310.x
3. Wake WT: Pressure ulcers: what clinicians need to know . Perm J. 2010, 14:56–60.
4. Brindle CT, Wegelin JA: Prophylactic dressing application to reduce pressure ulcer formation
in cardiac surgery patients. J Wound Ostomy Continence Nurs. 2012, 39:133–42.
10.1097/WON.0b013e318247cb82
5. Bliss DZ, Zehrer C, Savik K, Smith G, Hedblom E:An economic evaluation of four skin damage
prevention regimens in nursing home residents with incontinence: economics of skin damage
prevention. J Wound Ostomy Continence Nurs. 2007, 34:143-52.
10.1097/01.WON.0000264825.03485.40
6. Huang TT, Tseng CE, Lee TM, Yeh JY, Lai YY: Preventing pressure sores of the nasal ala after
nasotracheal tube intubation: from animal model to clinical application. J Oral Maxillofac
Surg. 2009, 67:543–51. 10.1016/j.joms.2008.06.100
7. Barrett S: Mepilex® Ag: an antimicrobial, absorbent foam dressing with Safetac® technology
Br J Nurs. 2009, 18:S28, S30-6. 10.12968/bjon.2009.18.Sup7.45133
8. Chuangsuwanich A, Chortrakarnkij P, Kangwanpoom J: Cost-effectiveness analysis in
comparing alginate silver dressing with silver zinc sulfadiazine cream in the treatment of
pressure ulcers. Arch Plast Surg. 2013, 40:589–96. 10.5999/aps.2013.40.5.589
9. Forni C, Loro L, Tremosini M, Mini S, Pignotti E, Bigoni O, Guzzo G, Bellini L, Trofa C, Di
Cataldo AM, Guzzi M: Use of polyurethane foam inside plaster casts to prevent the onset of
heel sores in the population at risk. A controlled clinical study. J Clin Nurs. 2011, 20:675–80.
10.1111/j.1365-2702.2010.03458.x
10. Chen HL, Chen XY, Wu J: The incidence of pressure ulcers in surgical patients of the last 5
years: a systematic review. Wounds. 2012, 24:234-41.
11. Chaiken N: Reduction of sacral pressure ulcers in the intensive care unit using a silicone
border foam dressing. J Wound Ostomy Continence Nurs. 2012, 39:143–45.
12. Santamaria N, Gerdtz M, Sage S, McCann J, Freeman A, Vassiliou T, De Vincentis S, Ng AW,
Manias E, Liu W, Knott J: A randomised controlled trial of the effectiveness of soft silicone
multi-layered foam dressings in the prevention of sacral and heel pressure ulcers in trauma
and critically ill patients: the border trial. Int Wound J. 2015, 12:302–8. 10.1111/iwj.12101
2016 Truong et al. Cureus 8(8): e730. DOI 10.7759/cureus.730 6 of 6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 6
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





