Spectrophotometric Determination of Acid Dissociation Constant (Ka)
VerifiedAdded on 2023/02/01
|7
|1218
|23
Report
AI Summary
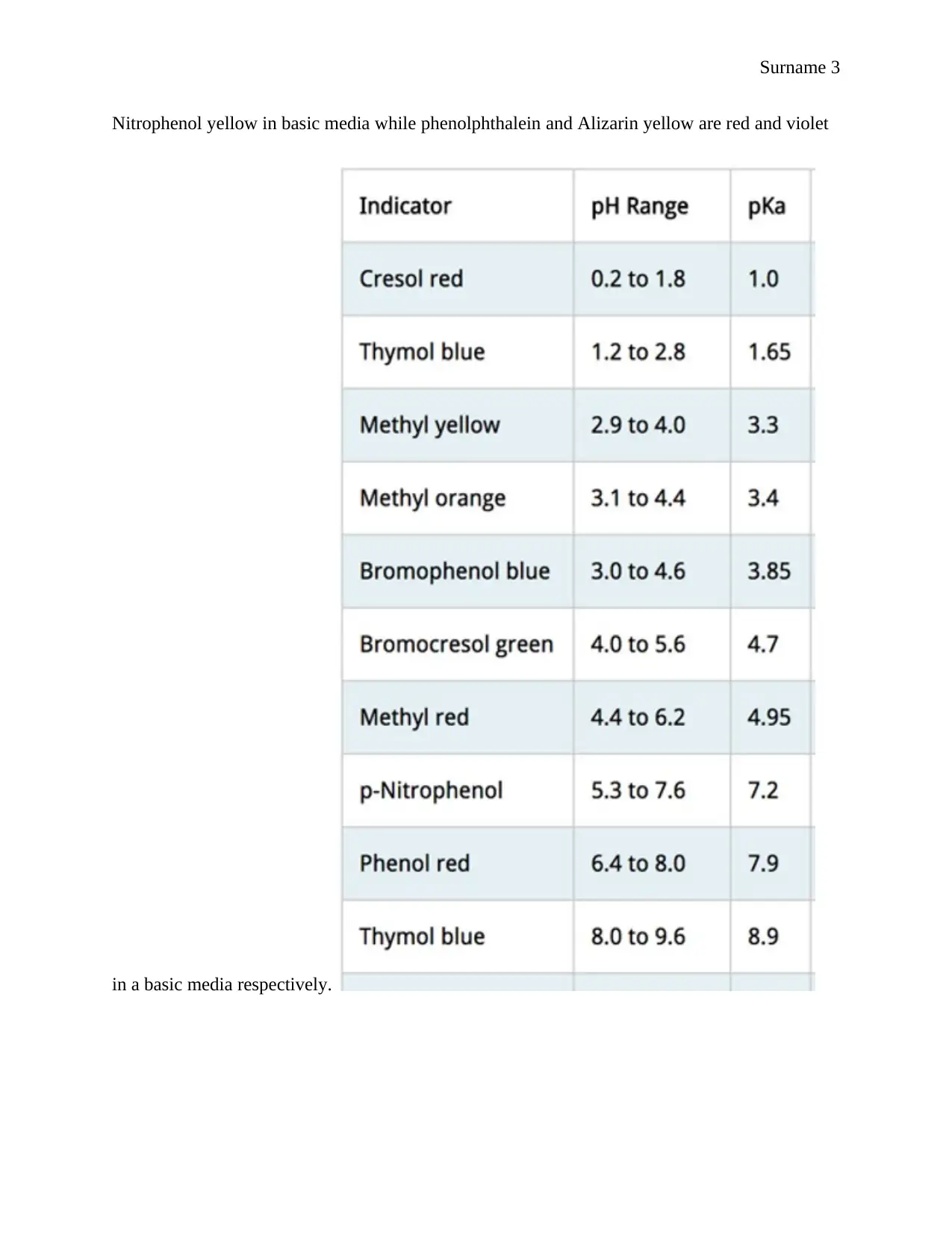
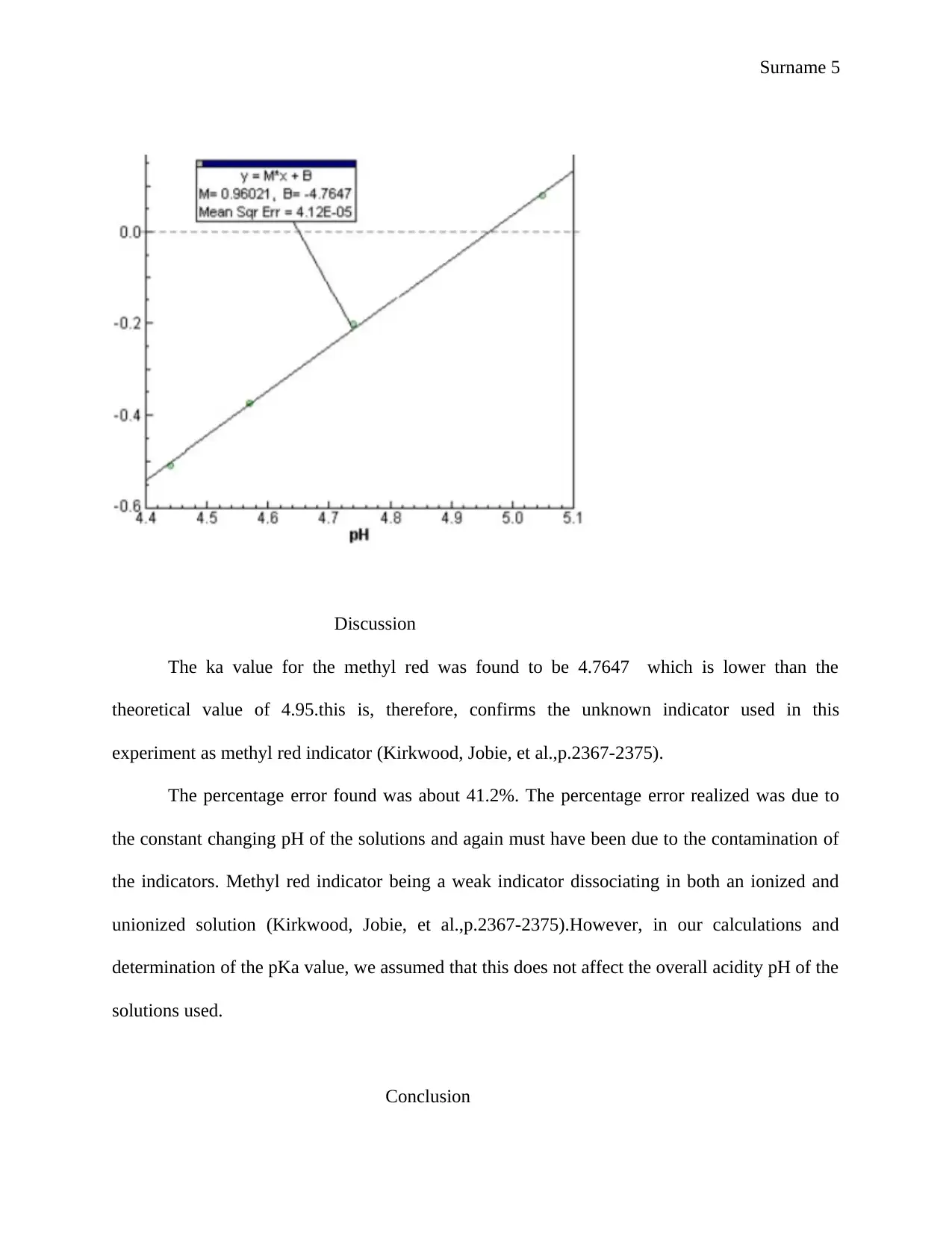
This report details an experiment focused on determining the acid dissociation constant (Ka) of an unknown indicator using spectrophotometry. The introduction explains the purpose of the experiment, the concepts of Ka, acid-base indicators, and the application of spectrophotometry. The experimental section outlines the procedure, including the preparation of solutions and absorbance measurements. The results section presents the experimental pKa values, calculations, and a comparison to the theoretical values, with a discussion on the sources of error, such as pH changes and contamination. The report utilizes the Henderson-Hasselbalch equation to calculate the pKa values and identify the unknown indicator as methyl red. The discussion compares the experimental and theoretical Ka values and includes a percentage error analysis. The conclusion summarizes the successful determination of the pKa value and identifies the indicator. The bibliography provides a list of cited references. The report aims to provide a comprehensive understanding of the spectrophotometric method for determining Ka values, incorporating all the necessary components of a formal scientific report.
1 out of 7







![[object Object]](/_next/static/media/star-bottom.7253800d.svg)