Analyzing Intensive Statin Regimens on Coronary Disease Progression
VerifiedAdded on 2023/01/19
|13
|2068
|23
Report
AI Summary
This report analyzes a randomized, double-blind, multicenter trial investigating the effects of two intensive statin regimens—atorvastatin and rosuvastatin—on the progression of coronary atherosclerosis. The study included patients aged 18-75 with coronary artery disease, comparing the efficacy of the two statins in reducing LDL cholesterol levels and impacting the progression of the disease. The research involved 1578 patients across 208 centers, with participants randomly assigned to receive either atorvastatin or rosuvastatin. Key findings indicate that both statins led to significant regression of coronary atherosclerosis, with rosuvastatin showing slightly better results in terms of LDL and HDL cholesterol levels, though the differences in the primary efficacy endpoint (percent atheroma volume) were not statistically significant. The study concludes that maximal doses of both statins effectively regress coronary atherosclerosis, highlighting the importance of achieving optimal LDL and HDL cholesterol levels, while also acknowledging the residual risk even with intensive statin therapy. The report discusses the study's strengths, such as its low bias and concealed allocation, as well as its limitations, including the absence of a placebo group and the inability to apply the findings to asymptomatic patients. The results suggest the potential of statin therapies in preventing the progression of atherosclerotic cardiovascular disease.

EFFECT OF TWO INTENSIVE
STATIN REGIMENS
ON PROGRESSION OF CORONARY
DISEASE
Presented by
STATIN REGIMENS
ON PROGRESSION OF CORONARY
DISEASE
Presented by
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Background information
• Stains results in the reduction of adverse cardiovascular outcomes and also results in slowing down the progression of coronary
atherosclerosis in proportion with the ability of reducing the low density lipoprotein (LDL) ( Stephen J. N, Christie M. B, Philip J. B,
John C, Raimund M. E, Peter L and et.al., 2011)1
• Studies have assesses an ability of intensive statin treatments in order to achieve approaches to administer maximal statin. Satin are the
class of drugs which are normally prescribed by doctors to the patients who are suffering from high level of cholesterol in their blood.
The aim of the study/ The primary hypothesis
To analyse the effect of two intensive statin regimes on progression of coronary disease could decrease the risks of LDL cholesterol leve
in humans.
Þ Clinical question of the study : Can Statin treatment help patients in decreasing the cholesterol level in their blood to reduce the hear
attack or stroke and other cardiovascular disease in patients?
The study design
• Randomised, multicentre
• Double-blind trial,
• From January 22, 2008 to June 12, 2009.
• Stains results in the reduction of adverse cardiovascular outcomes and also results in slowing down the progression of coronary
atherosclerosis in proportion with the ability of reducing the low density lipoprotein (LDL) ( Stephen J. N, Christie M. B, Philip J. B,
John C, Raimund M. E, Peter L and et.al., 2011)1
• Studies have assesses an ability of intensive statin treatments in order to achieve approaches to administer maximal statin. Satin are the
class of drugs which are normally prescribed by doctors to the patients who are suffering from high level of cholesterol in their blood.
The aim of the study/ The primary hypothesis
To analyse the effect of two intensive statin regimes on progression of coronary disease could decrease the risks of LDL cholesterol leve
in humans.
Þ Clinical question of the study : Can Statin treatment help patients in decreasing the cholesterol level in their blood to reduce the hear
attack or stroke and other cardiovascular disease in patients?
The study design
• Randomised, multicentre
• Double-blind trial,
• From January 22, 2008 to June 12, 2009.

Exclusion/ Inclusion Criteria
Inclusion criteria
• Participants of this research were the patients of 18 to 75 years of age.
• The participants chosen had minimum one vessel with 20 percent of stenosis on clinically indicated coronary angiography. Also, the patients which
were chosen had a target vessel for imaging which was less than 50 percent obstruction.
• Patients which used for the study and who matched to the inclusion criteria have to go under preliminary randomisation through the means of
interactive voice-response system.
• Also, they were randomly assigned within the ratio of 1:1 in order to receive either rosuvastatin at a dose of 20 mg or 40 mg doses of atorvastatin
daily for two weeks in order to ascertain the side effects as well as complaints.
Exclusion criteria
• Some patients which were excluded from the study were the patients which had received very high or intensive lipid lowering therapy and who have
received this therapy for more than three months in a previous year.
• Also, such patients where excluded form the study who had uncontrolled hypertension or had uncontrolled heart failure, or were suffering from renal
infection dysfunction or they were suffering from the liver disease.
• Such were the patients in the study that had been excluded and did not meet the inclusion criteria.
Inclusion criteria
• Participants of this research were the patients of 18 to 75 years of age.
• The participants chosen had minimum one vessel with 20 percent of stenosis on clinically indicated coronary angiography. Also, the patients which
were chosen had a target vessel for imaging which was less than 50 percent obstruction.
• Patients which used for the study and who matched to the inclusion criteria have to go under preliminary randomisation through the means of
interactive voice-response system.
• Also, they were randomly assigned within the ratio of 1:1 in order to receive either rosuvastatin at a dose of 20 mg or 40 mg doses of atorvastatin
daily for two weeks in order to ascertain the side effects as well as complaints.
Exclusion criteria
• Some patients which were excluded from the study were the patients which had received very high or intensive lipid lowering therapy and who have
received this therapy for more than three months in a previous year.
• Also, such patients where excluded form the study who had uncontrolled hypertension or had uncontrolled heart failure, or were suffering from renal
infection dysfunction or they were suffering from the liver disease.
• Such were the patients in the study that had been excluded and did not meet the inclusion criteria.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

The additional information for the treatment process
• During the study, patients which had LDL cholesterol level less than 116 mg per decilitre which is almost 3.0 mmol per litre and a triglycerde level of less than 500 mg per decilitre
which is almost 5.6 mmol per litre.
• Such patients again had to undergo through the randomisation with a ratio of 1:1. At this time full time dose treatment of atrovastatin i.e. 80 gm/day or rosuvastatin i.e. 40 gm/per day
was given for 104 weeks.
• The data which was gathered by the academic authors was held and then analysed and after data was analysed effectively decision was made and submitted to manuscript for the
publication.
• All the protocols were followed during the study no confidentiality were breached during the study as coordinating committee and steering committee had confidentiality agreements
with the sponsors.
• A copy of study data base was provided to the coordinating centre and independent analysis was granted also academic authors were given unrestricted for publishing the results that
were gathered from the study.
The sampling method
In order to conduct a research total sample size where n = 1578 patients were selected from 208 centres. Sample size which was selected was randomly assigned for two weeks of
treatment with a half maximal doses of either rosuvastatin or atorvastatin.
Concealed allocation
This procedure was implemented in a randomised control trial in which the individual separating and screening was done is two groups such as atorvastatin and rosuvastatin groups.
This is referred as a kind of consideration which is beyond the blinding the practitioner in order to deliver the care or the patients who are receiving the care during the treatment process.
• During the study, patients which had LDL cholesterol level less than 116 mg per decilitre which is almost 3.0 mmol per litre and a triglycerde level of less than 500 mg per decilitre
which is almost 5.6 mmol per litre.
• Such patients again had to undergo through the randomisation with a ratio of 1:1. At this time full time dose treatment of atrovastatin i.e. 80 gm/day or rosuvastatin i.e. 40 gm/per day
was given for 104 weeks.
• The data which was gathered by the academic authors was held and then analysed and after data was analysed effectively decision was made and submitted to manuscript for the
publication.
• All the protocols were followed during the study no confidentiality were breached during the study as coordinating committee and steering committee had confidentiality agreements
with the sponsors.
• A copy of study data base was provided to the coordinating centre and independent analysis was granted also academic authors were given unrestricted for publishing the results that
were gathered from the study.
The sampling method
In order to conduct a research total sample size where n = 1578 patients were selected from 208 centres. Sample size which was selected was randomly assigned for two weeks of
treatment with a half maximal doses of either rosuvastatin or atorvastatin.
Concealed allocation
This procedure was implemented in a randomised control trial in which the individual separating and screening was done is two groups such as atorvastatin and rosuvastatin groups.
This is referred as a kind of consideration which is beyond the blinding the practitioner in order to deliver the care or the patients who are receiving the care during the treatment process.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
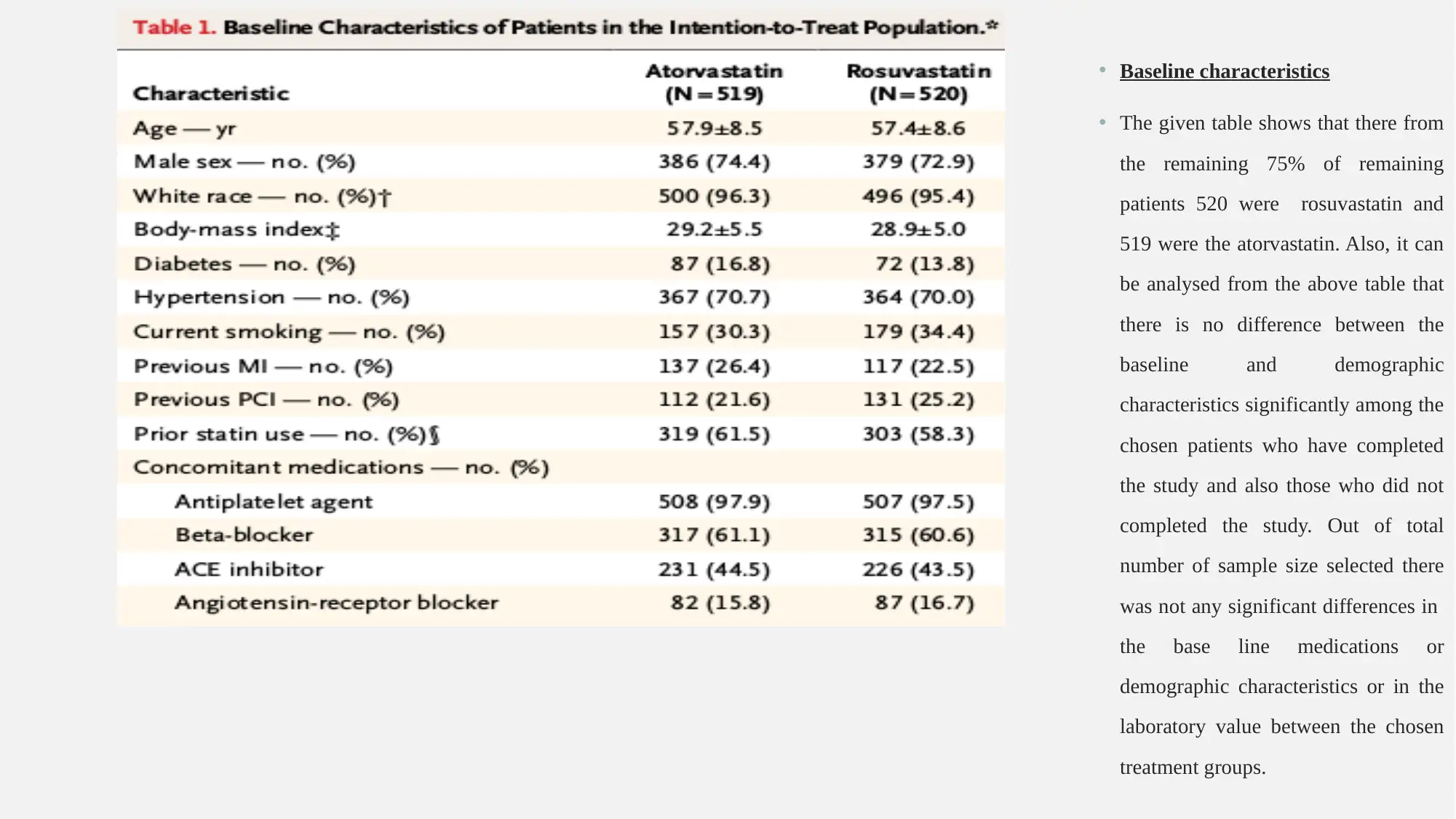
• Baseline characteristics
• The given table shows that there from
the remaining 75% of remaining
patients 520 were rosuvastatin and
519 were the atorvastatin. Also, it can
be analysed from the above table that
there is no difference between the
baseline and demographic
characteristics significantly among the
chosen patients who have completed
the study and also those who did not
completed the study. Out of total
number of sample size selected there
was not any significant differences in
the base line medications or
demographic characteristics or in the
laboratory value between the chosen
treatment groups.
• The given table shows that there from
the remaining 75% of remaining
patients 520 were rosuvastatin and
519 were the atorvastatin. Also, it can
be analysed from the above table that
there is no difference between the
baseline and demographic
characteristics significantly among the
chosen patients who have completed
the study and also those who did not
completed the study. Out of total
number of sample size selected there
was not any significant differences in
the base line medications or
demographic characteristics or in the
laboratory value between the chosen
treatment groups.
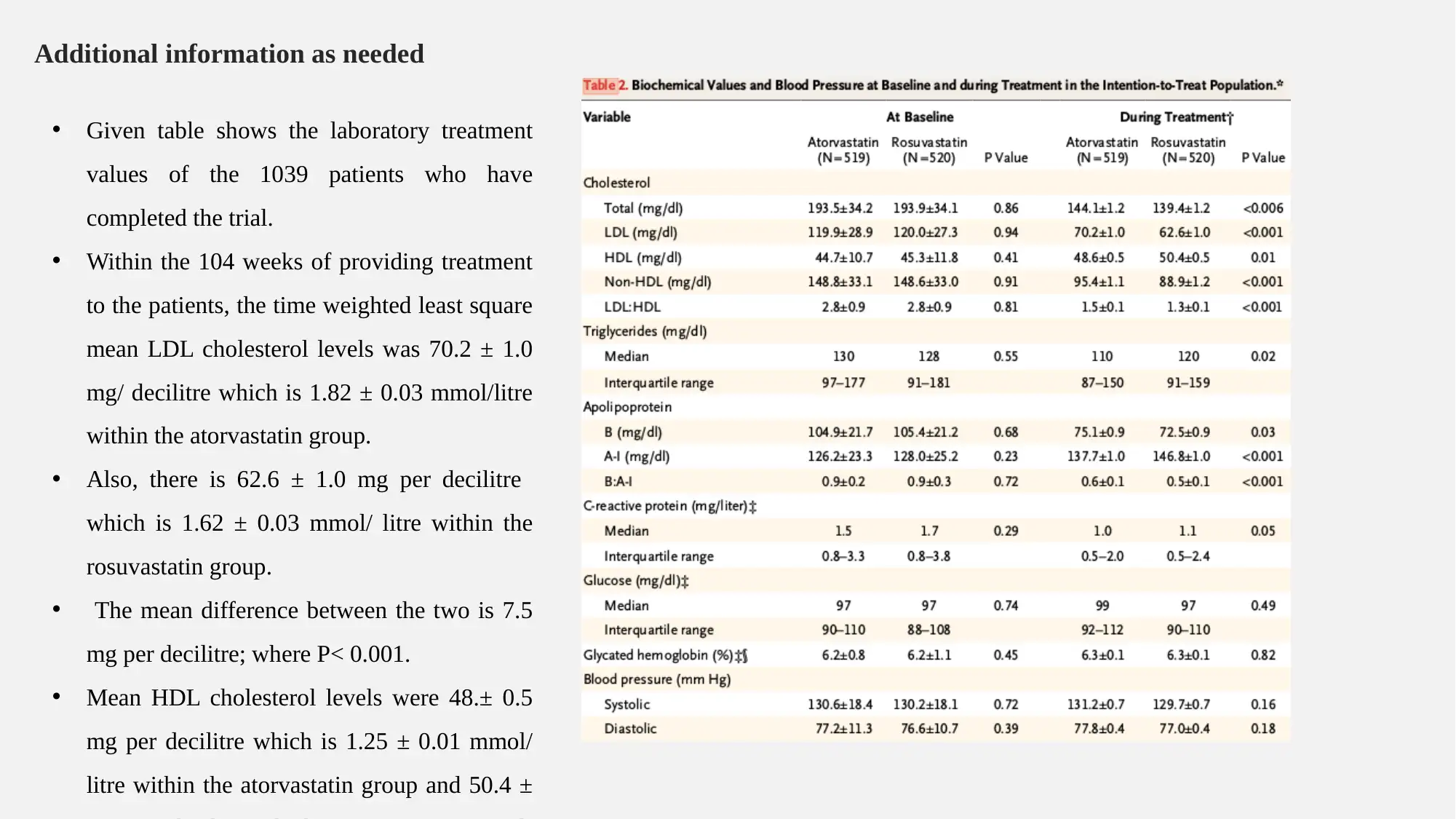
Additional information as needed
• Given table shows the laboratory treatment
values of the 1039 patients who have
completed the trial.
• Within the 104 weeks of providing treatment
to the patients, the time weighted least square
mean LDL cholesterol levels was 70.2 ± 1.0
mg/ decilitre which is 1.82 ± 0.03 mmol/litre
within the atorvastatin group.
• Also, there is 62.6 ± 1.0 mg per decilitre
which is 1.62 ± 0.03 mmol/ litre within the
rosuvastatin group.
• The mean difference between the two is 7.5
mg per decilitre; where P< 0.001.
• Mean HDL cholesterol levels were 48.± 0.5
mg per decilitre which is 1.25 ± 0.01 mmol/
litre within the atorvastatin group and 50.4 ±
• Given table shows the laboratory treatment
values of the 1039 patients who have
completed the trial.
• Within the 104 weeks of providing treatment
to the patients, the time weighted least square
mean LDL cholesterol levels was 70.2 ± 1.0
mg/ decilitre which is 1.82 ± 0.03 mmol/litre
within the atorvastatin group.
• Also, there is 62.6 ± 1.0 mg per decilitre
which is 1.62 ± 0.03 mmol/ litre within the
rosuvastatin group.
• The mean difference between the two is 7.5
mg per decilitre; where P< 0.001.
• Mean HDL cholesterol levels were 48.± 0.5
mg per decilitre which is 1.25 ± 0.01 mmol/
litre within the atorvastatin group and 50.4 ±
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
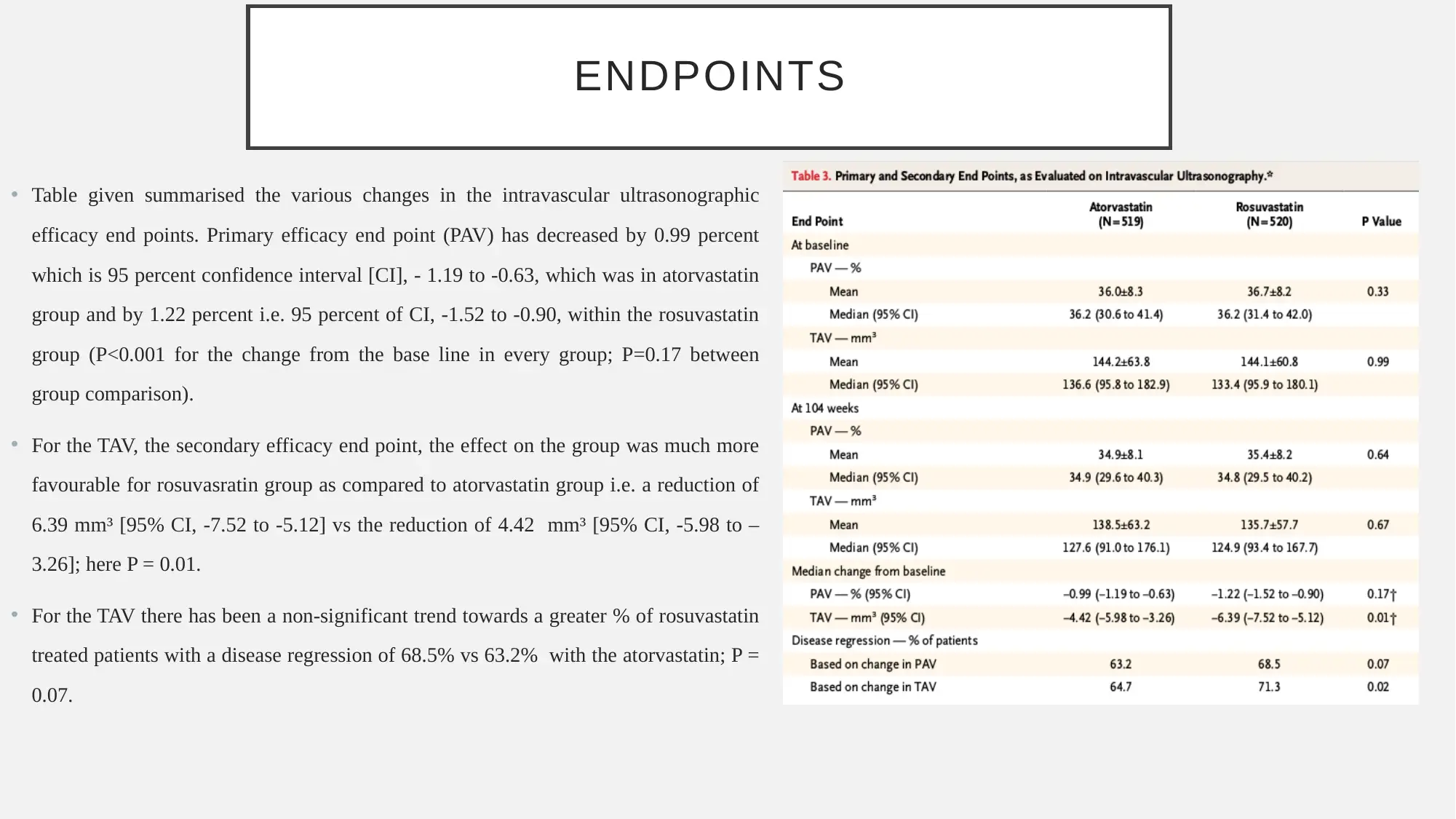
ENDPOINTS
• Table given summarised the various changes in the intravascular ultrasonographic
efficacy end points. Primary efficacy end point (PAV) has decreased by 0.99 percent
which is 95 percent confidence interval [CI], - 1.19 to -0.63, which was in atorvastatin
group and by 1.22 percent i.e. 95 percent of CI, -1.52 to -0.90, within the rosuvastatin
group (P<0.001 for the change from the base line in every group; P=0.17 between
group comparison).
• For the TAV, the secondary efficacy end point, the effect on the group was much more
favourable for rosuvasratin group as compared to atorvastatin group i.e. a reduction of
6.39 mm³ [95% CI, -7.52 to -5.12] vs the reduction of 4.42 mm³ [95% CI, -5.98 to –
3.26]; here P = 0.01.
• For the TAV there has been a non-significant trend towards a greater % of rosuvastatin
treated patients with a disease regression of 68.5% vs 63.2% with the atorvastatin; P =
0.07.
• Table given summarised the various changes in the intravascular ultrasonographic
efficacy end points. Primary efficacy end point (PAV) has decreased by 0.99 percent
which is 95 percent confidence interval [CI], - 1.19 to -0.63, which was in atorvastatin
group and by 1.22 percent i.e. 95 percent of CI, -1.52 to -0.90, within the rosuvastatin
group (P<0.001 for the change from the base line in every group; P=0.17 between
group comparison).
• For the TAV, the secondary efficacy end point, the effect on the group was much more
favourable for rosuvasratin group as compared to atorvastatin group i.e. a reduction of
6.39 mm³ [95% CI, -7.52 to -5.12] vs the reduction of 4.42 mm³ [95% CI, -5.98 to –
3.26]; here P = 0.01.
• For the TAV there has been a non-significant trend towards a greater % of rosuvastatin
treated patients with a disease regression of 68.5% vs 63.2% with the atorvastatin; P =
0.07.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
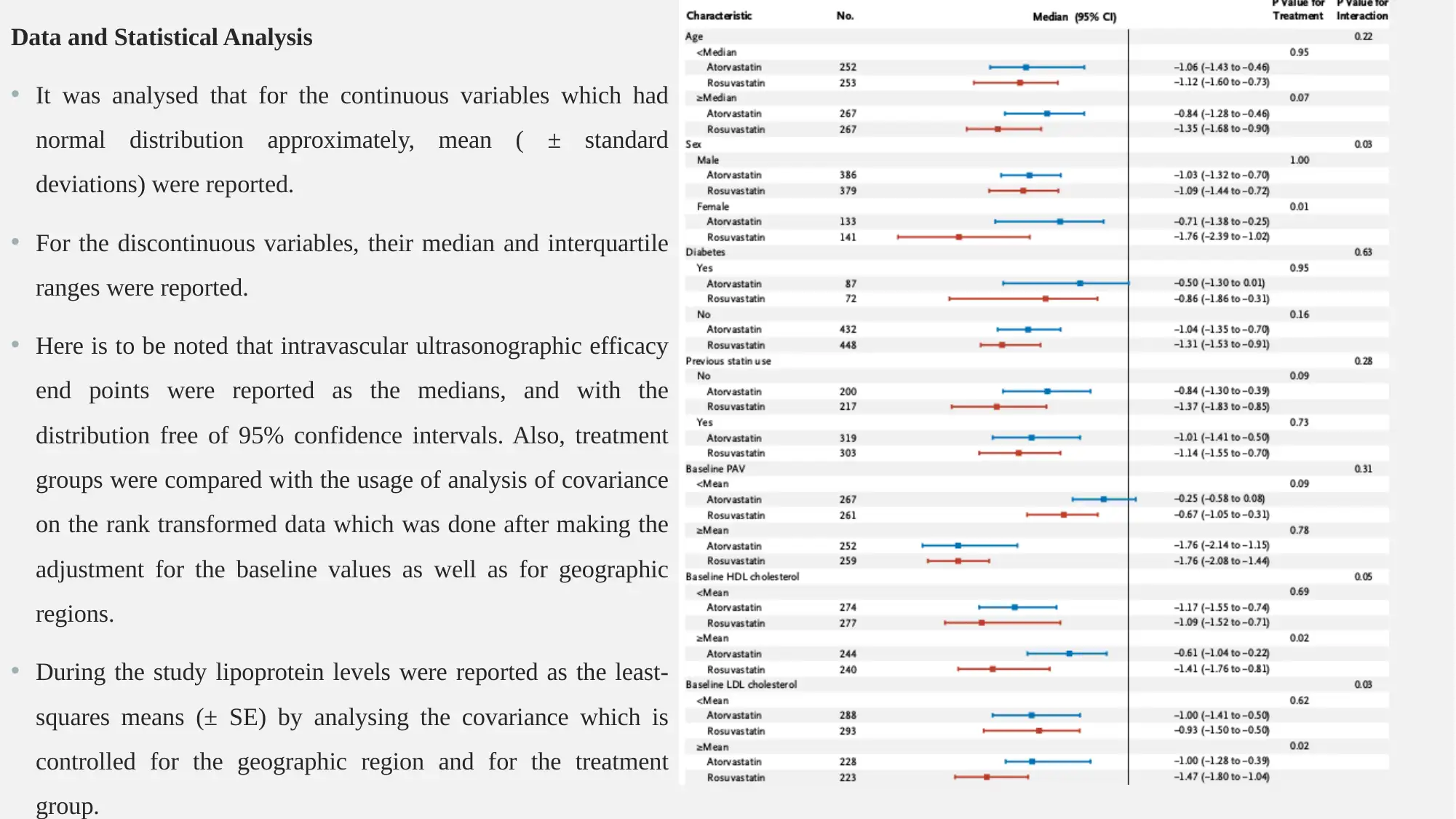
Data and Statistical Analysis
• It was analysed that for the continuous variables which had
normal distribution approximately, mean ( ± standard
deviations) were reported.
• For the discontinuous variables, their median and interquartile
ranges were reported.
• Here is to be noted that intravascular ultrasonographic efficacy
end points were reported as the medians, and with the
distribution free of 95% confidence intervals. Also, treatment
groups were compared with the usage of analysis of covariance
on the rank transformed data which was done after making the
adjustment for the baseline values as well as for geographic
regions.
• During the study lipoprotein levels were reported as the least-
squares means (± SE) by analysing the covariance which is
controlled for the geographic region and for the treatment
group.
• It was analysed that for the continuous variables which had
normal distribution approximately, mean ( ± standard
deviations) were reported.
• For the discontinuous variables, their median and interquartile
ranges were reported.
• Here is to be noted that intravascular ultrasonographic efficacy
end points were reported as the medians, and with the
distribution free of 95% confidence intervals. Also, treatment
groups were compared with the usage of analysis of covariance
on the rank transformed data which was done after making the
adjustment for the baseline values as well as for geographic
regions.
• During the study lipoprotein levels were reported as the least-
squares means (± SE) by analysing the covariance which is
controlled for the geographic region and for the treatment
group.

• Results
• The result shows various data which was found in the 104 weeks of therapy.
• The rosuvastatin group has lower cholesterol than the other atorvastatin group(62.6 vs 70.2 milligram /
decilitre[1.62 vs 1.82 mmol / litre] , p<0.001), high level of high-dense lipoprotein cholesterol(50.4 vs 48.6
milligram/decilitre[1.30 vs 1.26 mmol / litre], p=0.01 ).
• The main efficacy end point or percent atheroma volume, is decreased by 0.99%(95% confidence interval[CI], -1.19
to -0.63) and atorvastatin and by 1.22%(95% CI, -1.52 to -0.90) with rosuvastatin [P=0.17].
• There is effect on secondary efficacy end point, and normalised total atheroma volume and they both were more
favourable with rosuvastatin than with the atorvastatin which is: -6.39mm³(95% CI, -7.52 to -5.12), compared with -
4.42mm³ (95% CI, -5.98 to -3.26) (let P=0.01).
• Both agents persuaded regression in many of the patients: 63.2% were with atorvastatin and 68.5% were with
rosuvastatin for PAV in which P=0.07 and 64.7% and 71.3% respectively and for TAV P=0.02. Both the agents had
admissible side-effect profiles and with a low incidence of lab abnormalities and less cardiovascular events.
• The result shows various data which was found in the 104 weeks of therapy.
• The rosuvastatin group has lower cholesterol than the other atorvastatin group(62.6 vs 70.2 milligram /
decilitre[1.62 vs 1.82 mmol / litre] , p<0.001), high level of high-dense lipoprotein cholesterol(50.4 vs 48.6
milligram/decilitre[1.30 vs 1.26 mmol / litre], p=0.01 ).
• The main efficacy end point or percent atheroma volume, is decreased by 0.99%(95% confidence interval[CI], -1.19
to -0.63) and atorvastatin and by 1.22%(95% CI, -1.52 to -0.90) with rosuvastatin [P=0.17].
• There is effect on secondary efficacy end point, and normalised total atheroma volume and they both were more
favourable with rosuvastatin than with the atorvastatin which is: -6.39mm³(95% CI, -7.52 to -5.12), compared with -
4.42mm³ (95% CI, -5.98 to -3.26) (let P=0.01).
• Both agents persuaded regression in many of the patients: 63.2% were with atorvastatin and 68.5% were with
rosuvastatin for PAV in which P=0.07 and 64.7% and 71.3% respectively and for TAV P=0.02. Both the agents had
admissible side-effect profiles and with a low incidence of lab abnormalities and less cardiovascular events.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

• Discussion
• The study compared various affects on the progression of coronary atherosclerosis of the highest dose of atorvastatin and also rosuvastatin.
• These are the two most intensive statin regimes which are used in the clinical practise done today. Very low LDL level were achieved in
treating both groups with value less than 70 milligram per decilitre.
• During treatment HDL concentrations in both groups reached 50 milligrams per decilitre which is acceptable for the prevention of
cardiovascular problems.
• The data says that coronary artery disease can regress if favourable level of both the HDL and LDL are attained with the statin therapy.
• As comparing both the regimen, the rosuvastatin showed lower LDL cholesterol and slightly higher HDL cholesterol.
• These differences did not showed significant incremental effect on disease as assessed according to intravascular ultrasonographic end point.
• Rosuvastatin showed a benefit with respect to disease regression assessed according to TAV, but the difference between two regimes are
modest.
• The data shows that the two regimes are similar in their abilities to limit progression although a small difference in efficacy cannot be ruled
out on the basis of differences observed in TAV.
• The study compared various affects on the progression of coronary atherosclerosis of the highest dose of atorvastatin and also rosuvastatin.
• These are the two most intensive statin regimes which are used in the clinical practise done today. Very low LDL level were achieved in
treating both groups with value less than 70 milligram per decilitre.
• During treatment HDL concentrations in both groups reached 50 milligrams per decilitre which is acceptable for the prevention of
cardiovascular problems.
• The data says that coronary artery disease can regress if favourable level of both the HDL and LDL are attained with the statin therapy.
• As comparing both the regimen, the rosuvastatin showed lower LDL cholesterol and slightly higher HDL cholesterol.
• These differences did not showed significant incremental effect on disease as assessed according to intravascular ultrasonographic end point.
• Rosuvastatin showed a benefit with respect to disease regression assessed according to TAV, but the difference between two regimes are
modest.
• The data shows that the two regimes are similar in their abilities to limit progression although a small difference in efficacy cannot be ruled
out on the basis of differences observed in TAV.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Conclusion
• It has been concluded from the study that the maximal doses of atoravastatin and rosuvastatin have resulted in the significant
regression of coronary atherosclerosis.
• It can also be concluded that despite that higher the levels of HDL cholesterol and lower the level of LDL cholesterol achieved with
the rosuvastatin, the similar degree of regression of PAV has been observed within the two groups to whom treatment was provided.
• It can also be concluded that the levels of LDL as well as HDL cholesterol observed within the study can not be achieved with help of
stains alone.
• Also, this study suggests that one third of patients which are chosen for the study were having disease progression despite of maximal
intensive statin therapy.
• It can also be concluded from the study that there remains the substantial residual risk sill remains in the most secondary prevention
population whether of their using the most effective present medical therapies available to them.
• Also, this study suggests that the findings presents the useful steps towards the efforts of preventing devastating clinical sequelae of
the atherosclerotic cardiovascular disease.
•
• It has been concluded from the study that the maximal doses of atoravastatin and rosuvastatin have resulted in the significant
regression of coronary atherosclerosis.
• It can also be concluded that despite that higher the levels of HDL cholesterol and lower the level of LDL cholesterol achieved with
the rosuvastatin, the similar degree of regression of PAV has been observed within the two groups to whom treatment was provided.
• It can also be concluded that the levels of LDL as well as HDL cholesterol observed within the study can not be achieved with help of
stains alone.
• Also, this study suggests that one third of patients which are chosen for the study were having disease progression despite of maximal
intensive statin therapy.
• It can also be concluded from the study that there remains the substantial residual risk sill remains in the most secondary prevention
population whether of their using the most effective present medical therapies available to them.
• Also, this study suggests that the findings presents the useful steps towards the efforts of preventing devastating clinical sequelae of
the atherosclerotic cardiovascular disease.
•

• The strength of the study
One of the major strength of this study was the bias within the research was very low and conceal allocation has been achieved
effectively
• The weakness of the study
• One of the major weakness of the study was that it could not be ethically possible to measure the disease progression in a
placebo-treated patients.
• Also, this remain quite uncertain that findings of the study can apply to primary prevention within the asymptomatic patients.
• Another major limitation was that some of the patients did not agree to undergo follow-up cardiac catheterisation so, calculation
of intravascular ultrasonographic end points could not be considered.
One of the major strength of this study was the bias within the research was very low and conceal allocation has been achieved
effectively
• The weakness of the study
• One of the major weakness of the study was that it could not be ethically possible to measure the disease progression in a
placebo-treated patients.
• Also, this remain quite uncertain that findings of the study can apply to primary prevention within the asymptomatic patients.
• Another major limitation was that some of the patients did not agree to undergo follow-up cardiac catheterisation so, calculation
of intravascular ultrasonographic end points could not be considered.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 13
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.