Structures of Matter, Properties and Interactions Assignment
VerifiedAdded on 2021/09/28
|8
|1563
|226
Homework Assignment
AI Summary
This homework assignment delves into the fundamental concepts of chemistry, focusing on the structures of matter, their properties, and interactions. The assignment begins by examining ionic bonding, explaining how it arises from the transfer of electrons between atoms, using sodium and chlorine as examples, and discussing the octet rule. It then explores covalent bonds, differentiating between pure and polar covalent bonds, and introducing the concept of electronegativity. The electronic configuration of potassium is described, and its classification as an alkali metal is discussed, highlighting its reactivity and tendency to form ionic bonds. Finally, the assignment critiques the Bohr model of the atom, contrasting it with the more accurate quantum mechanical model and the Heisenberg Uncertainty principle, which accounts for the probabilistic nature of electron positions and momentum.

Structures of matter, properties and
interactions1
STRUCTURES OF MATTER, PROPERTIES AND INTERACTIONS
Name:
Department:
School:
Date:
interactions1
STRUCTURES OF MATTER, PROPERTIES AND INTERACTIONS
Name:
Department:
School:
Date:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Structures of matter, properties and interactions 2
Structures of matter, properties and interactions
Question 1
The attraction amongst oppositely charged molecule is referred to as ionic bonding, and it
is one of the various forms of chemical bonding in chemistry. Ionic bonding is as a result of
electrons (e-) moving from one particle to another. The trends that atoms have eight electrons in
their valence shell are referred to as the octet decree. Sodium in its elemental sort, it has one
valence e- and is firm (Svidzinsky, Scully and Herschbach 2014). It is reactive and does not
need a lot of vigor to dislodge an e- to create the Na+ ions. To form Na2+ from Na+ ions, it will
require a lot of energy that is normally available in chemical reactions. Now, considering a Na
atom in existence of a Cl bit, the two atoms have these Lewis e- dot figures and electronic
configuration (Moruzzi, Janak and Williams 2013).
For a Na particle to have an octet, it needs to lose an e-; for the Cl atom to gain an octet,
it must gain an e-
Structures of matter, properties and interactions
Question 1
The attraction amongst oppositely charged molecule is referred to as ionic bonding, and it
is one of the various forms of chemical bonding in chemistry. Ionic bonding is as a result of
electrons (e-) moving from one particle to another. The trends that atoms have eight electrons in
their valence shell are referred to as the octet decree. Sodium in its elemental sort, it has one
valence e- and is firm (Svidzinsky, Scully and Herschbach 2014). It is reactive and does not
need a lot of vigor to dislodge an e- to create the Na+ ions. To form Na2+ from Na+ ions, it will
require a lot of energy that is normally available in chemical reactions. Now, considering a Na
atom in existence of a Cl bit, the two atoms have these Lewis e- dot figures and electronic
configuration (Moruzzi, Janak and Williams 2013).
For a Na particle to have an octet, it needs to lose an e-; for the Cl atom to gain an octet,
it must gain an e-
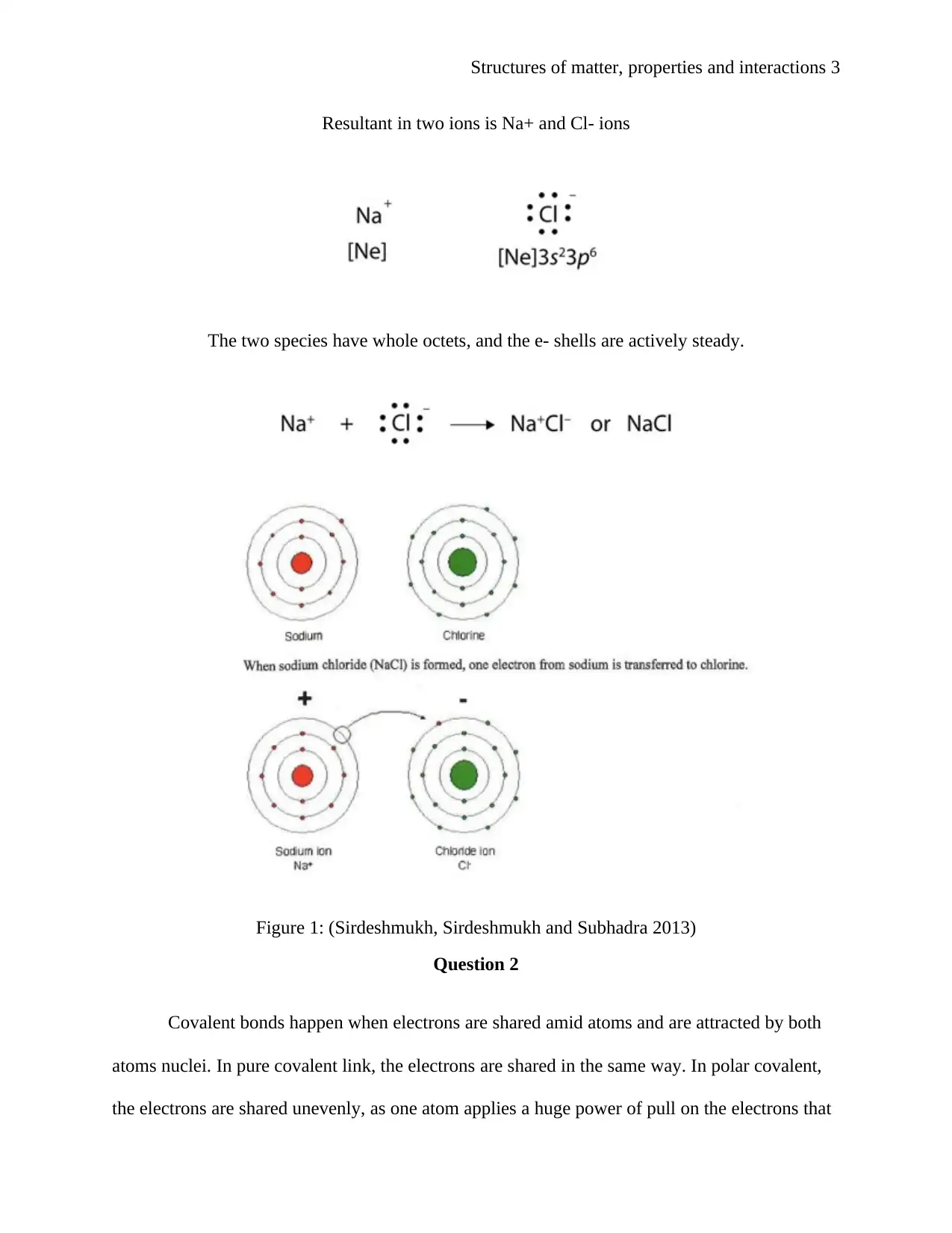
Structures of matter, properties and interactions 3
Resultant in two ions is Na+ and Cl- ions
The two species have whole octets, and the e- shells are actively steady.
Figure 1: (Sirdeshmukh, Sirdeshmukh and Subhadra 2013)
Question 2
Covalent bonds happen when electrons are shared amid atoms and are attracted by both
atoms nuclei. In pure covalent link, the electrons are shared in the same way. In polar covalent,
the electrons are shared unevenly, as one atom applies a huge power of pull on the electrons that
Resultant in two ions is Na+ and Cl- ions
The two species have whole octets, and the e- shells are actively steady.
Figure 1: (Sirdeshmukh, Sirdeshmukh and Subhadra 2013)
Question 2
Covalent bonds happen when electrons are shared amid atoms and are attracted by both
atoms nuclei. In pure covalent link, the electrons are shared in the same way. In polar covalent,
the electrons are shared unevenly, as one atom applies a huge power of pull on the electrons that
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
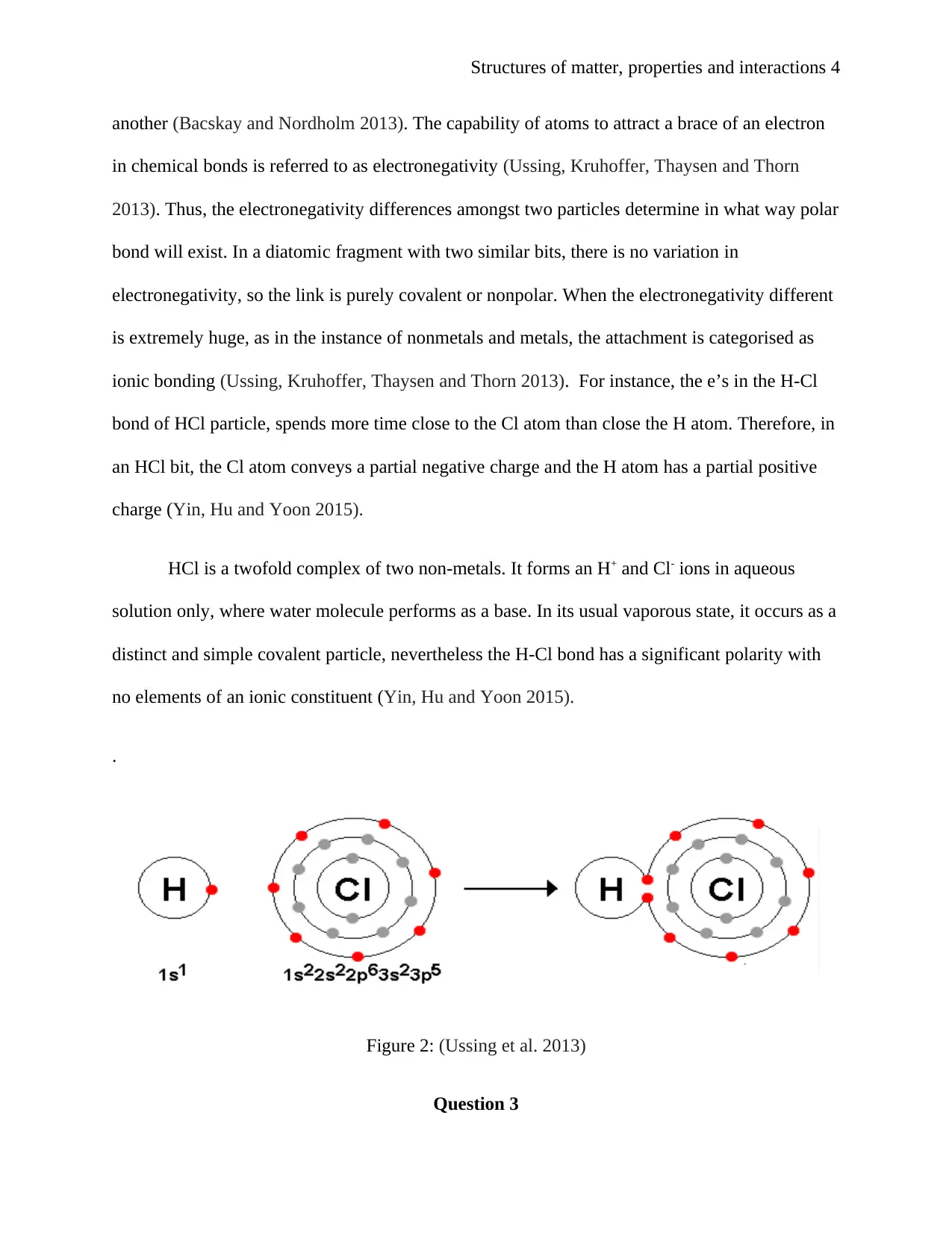
Structures of matter, properties and interactions 4
another (Bacskay and Nordholm 2013). The capability of atoms to attract a brace of an electron
in chemical bonds is referred to as electronegativity (Ussing, Kruhoffer, Thaysen and Thorn
2013). Thus, the electronegativity differences amongst two particles determine in what way polar
bond will exist. In a diatomic fragment with two similar bits, there is no variation in
electronegativity, so the link is purely covalent or nonpolar. When the electronegativity different
is extremely huge, as in the instance of nonmetals and metals, the attachment is categorised as
ionic bonding (Ussing, Kruhoffer, Thaysen and Thorn 2013). For instance, the e’s in the H-Cl
bond of HCl particle, spends more time close to the Cl atom than close the H atom. Therefore, in
an HCl bit, the Cl atom conveys a partial negative charge and the H atom has a partial positive
charge (Yin, Hu and Yoon 2015).
HCl is a twofold complex of two non-metals. It forms an H+ and Cl- ions in aqueous
solution only, where water molecule performs as a base. In its usual vaporous state, it occurs as a
distinct and simple covalent particle, nevertheless the H-Cl bond has a significant polarity with
no elements of an ionic constituent (Yin, Hu and Yoon 2015).
.
Figure 2: (Ussing et al. 2013)
Question 3
another (Bacskay and Nordholm 2013). The capability of atoms to attract a brace of an electron
in chemical bonds is referred to as electronegativity (Ussing, Kruhoffer, Thaysen and Thorn
2013). Thus, the electronegativity differences amongst two particles determine in what way polar
bond will exist. In a diatomic fragment with two similar bits, there is no variation in
electronegativity, so the link is purely covalent or nonpolar. When the electronegativity different
is extremely huge, as in the instance of nonmetals and metals, the attachment is categorised as
ionic bonding (Ussing, Kruhoffer, Thaysen and Thorn 2013). For instance, the e’s in the H-Cl
bond of HCl particle, spends more time close to the Cl atom than close the H atom. Therefore, in
an HCl bit, the Cl atom conveys a partial negative charge and the H atom has a partial positive
charge (Yin, Hu and Yoon 2015).
HCl is a twofold complex of two non-metals. It forms an H+ and Cl- ions in aqueous
solution only, where water molecule performs as a base. In its usual vaporous state, it occurs as a
distinct and simple covalent particle, nevertheless the H-Cl bond has a significant polarity with
no elements of an ionic constituent (Yin, Hu and Yoon 2015).
.
Figure 2: (Ussing et al. 2013)
Question 3
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Structures of matter, properties and interactions 5
In the e conformation for potassium, the first binary electrons will go to 1S orbital. Since
1s can merely grasp two electrons, the subsequent 2 electrons will go in the 2s orbital. The
following six electrons will occupy 2P orbital. The p orbital can only have six electrons. The
succeeding electrons go to the 3s. Since the 3s is filled, 3p orbital will fill the next six electrons.
In the 4s orbital, the remaining electron will fill and hence the electronic configuration of
potassium is 1S22S22P63S23P64S1 or [Ar] 4S1 (Yin, Hu and Yoon 2015).
The general electronic configuration of group 1 elements is ns1. Therefore, the formulae
above shows that potassium is classified as group 1 elements or alkali metals. They have a strong
tendency to donate their valence electrons in the last shell to create strong ionic bonds. They are
also very reactive with water to form metallic hydroxide and hydrogen gas. Additionally,
labelled as the most reactive group of metals in the periodic table, each of the alkali metal is able
to react with different elements to produce different results. The potassium is significant to
atomic bonding due to its ability to donate valence electron to non-metals, forming an ionic bond
(Yin, Hu and Yoon 2015).
Question 4
In the e conformation for potassium, the first binary electrons will go to 1S orbital. Since
1s can merely grasp two electrons, the subsequent 2 electrons will go in the 2s orbital. The
following six electrons will occupy 2P orbital. The p orbital can only have six electrons. The
succeeding electrons go to the 3s. Since the 3s is filled, 3p orbital will fill the next six electrons.
In the 4s orbital, the remaining electron will fill and hence the electronic configuration of
potassium is 1S22S22P63S23P64S1 or [Ar] 4S1 (Yin, Hu and Yoon 2015).
The general electronic configuration of group 1 elements is ns1. Therefore, the formulae
above shows that potassium is classified as group 1 elements or alkali metals. They have a strong
tendency to donate their valence electrons in the last shell to create strong ionic bonds. They are
also very reactive with water to form metallic hydroxide and hydrogen gas. Additionally,
labelled as the most reactive group of metals in the periodic table, each of the alkali metal is able
to react with different elements to produce different results. The potassium is significant to
atomic bonding due to its ability to donate valence electron to non-metals, forming an ionic bond
(Yin, Hu and Yoon 2015).
Question 4

Structures of matter, properties and interactions 6
In the initial step, there is thinking that electrons are very small balls that orbit
around the nucleus in a circular motion. This is known as the Bohr model (Liu, Oulton and
Kivshar 2015). Despite being an excellent model at the introduction level, the entire story is
wrong and oversimplified. There are numerous concerns with an idea that electrons travel in a
circular orbit. The main is one being that they should eventually undergo orbital decay and hence
slow down and crash in the nucleus. Of course, this does not happen. In the 20 century, the
quantum theory came to the rescue and stated electrons could not be treated as a classical particle
and did not have definite momentum and position (Kragh 2012). Therefore, basically, it means
the electron’s position and its momentum at the same time is not known. This relationship is
given by the Heisenberg Uncertainty principle (Svidzinsky, Scully and Herschbach 2014).
Therefore, it makes it hard to plot an orbit for an electron, if one does not know where the
electron is going to be next. There is no way one can project its path. Atomic orbitals draw an
orbit of an electron by plotting electron density probability orbital only. Thus, the methods are
termed as inaccurate and oversimplified.
References
In the initial step, there is thinking that electrons are very small balls that orbit
around the nucleus in a circular motion. This is known as the Bohr model (Liu, Oulton and
Kivshar 2015). Despite being an excellent model at the introduction level, the entire story is
wrong and oversimplified. There are numerous concerns with an idea that electrons travel in a
circular orbit. The main is one being that they should eventually undergo orbital decay and hence
slow down and crash in the nucleus. Of course, this does not happen. In the 20 century, the
quantum theory came to the rescue and stated electrons could not be treated as a classical particle
and did not have definite momentum and position (Kragh 2012). Therefore, basically, it means
the electron’s position and its momentum at the same time is not known. This relationship is
given by the Heisenberg Uncertainty principle (Svidzinsky, Scully and Herschbach 2014).
Therefore, it makes it hard to plot an orbit for an electron, if one does not know where the
electron is going to be next. There is no way one can project its path. Atomic orbitals draw an
orbit of an electron by plotting electron density probability orbital only. Thus, the methods are
termed as inaccurate and oversimplified.
References
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Structures of matter, properties and interactions 7
Bacskay, G.B. and Nordholm, S., 2013. Covalent bonding: The fundamental role of the kinetic
energy. The Journal of Physical Chemistry A, 117(33), pp.7946-7958. [Online]. Available from:
[https://pubs.acs.org/doi/abs/10.1021/jp403284g, [Accessed on 9 November 2018].
Kragh, H., 2012. Niels Bohr and the quantum atom: The Bohr model of atomic structure 1913-
1925. OUP Oxford. [Online]. Available from: https://books.google.com/books?
hl=en&lr=&id=pVyrkndSrkQC&oi=fnd&pg=PP1&dq=bohr+model&ots=dUFom_Anoy&sig=6
WsSjvYX2wHSz7drX6SoxxktHdQ, [Accessed on 9 November 2018].
Liu, W., Oulton, R.F. and Kivshar, Y.S., 2015. Geometric interpretations for resonances of
plasmonic nanoparticles. Scientific reports, 5, p.12148. [Online]. Available from:
https://www.nature.com/articles/srep12148, [Accessed on 9 November 2018].
Moruzzi, V.L., Janak, J.F. and Williams, A.R., 2013. Calculated electronic properties of metals.
Elsevier. [Online]. Available from: https://books.google.com/books?
hl=en&lr=&id=ZzgvBQAAQBAJ&oi=fnd&pg=PP1&dq=alkali+metals+properties&ots=KUVC
hmKiRz&sig=ds_DrTCXV2dJKyRXzCYrMVw7ryY, [Accessed on 9 November 2018].
Sirdeshmukh, D.B., Sirdeshmukh, L. and Subhadra, K.G., 2013. Alkali Halides: A Handbook of
physical properties (Vol. 49). Springer Science & Business Media. [Online]. Available from:
https://books.google.com/books?
hl=en&lr=&id=wCvrCAAAQBAJ&oi=fnd&pg=PR5&dq=alkali+metals+properties&ots=Jq8pN
ljKIO&sig=OYtdZ503wNT61qYRPlZDxI4XVNI, [Accessed on 9 November 2018].
Svidzinsky, A., Scully, M. and Herschbach, D., 2014. BOHR’S. Physics Today, 67(1), p.33.
[Online]. Available from:
Bacskay, G.B. and Nordholm, S., 2013. Covalent bonding: The fundamental role of the kinetic
energy. The Journal of Physical Chemistry A, 117(33), pp.7946-7958. [Online]. Available from:
[https://pubs.acs.org/doi/abs/10.1021/jp403284g, [Accessed on 9 November 2018].
Kragh, H., 2012. Niels Bohr and the quantum atom: The Bohr model of atomic structure 1913-
1925. OUP Oxford. [Online]. Available from: https://books.google.com/books?
hl=en&lr=&id=pVyrkndSrkQC&oi=fnd&pg=PP1&dq=bohr+model&ots=dUFom_Anoy&sig=6
WsSjvYX2wHSz7drX6SoxxktHdQ, [Accessed on 9 November 2018].
Liu, W., Oulton, R.F. and Kivshar, Y.S., 2015. Geometric interpretations for resonances of
plasmonic nanoparticles. Scientific reports, 5, p.12148. [Online]. Available from:
https://www.nature.com/articles/srep12148, [Accessed on 9 November 2018].
Moruzzi, V.L., Janak, J.F. and Williams, A.R., 2013. Calculated electronic properties of metals.
Elsevier. [Online]. Available from: https://books.google.com/books?
hl=en&lr=&id=ZzgvBQAAQBAJ&oi=fnd&pg=PP1&dq=alkali+metals+properties&ots=KUVC
hmKiRz&sig=ds_DrTCXV2dJKyRXzCYrMVw7ryY, [Accessed on 9 November 2018].
Sirdeshmukh, D.B., Sirdeshmukh, L. and Subhadra, K.G., 2013. Alkali Halides: A Handbook of
physical properties (Vol. 49). Springer Science & Business Media. [Online]. Available from:
https://books.google.com/books?
hl=en&lr=&id=wCvrCAAAQBAJ&oi=fnd&pg=PR5&dq=alkali+metals+properties&ots=Jq8pN
ljKIO&sig=OYtdZ503wNT61qYRPlZDxI4XVNI, [Accessed on 9 November 2018].
Svidzinsky, A., Scully, M. and Herschbach, D., 2014. BOHR’S. Physics Today, 67(1), p.33.
[Online]. Available from:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Structures of matter, properties and interactions 8
http://www.fizika.unios.hr/kvm2/wp-content/uploads/sites/76/2015/11/Bohr%E2%80%99s-
molecular-model-a-century-later.pdf, [Accessed on 9 November 2018].
Ussing, H.H., Kruhoffer, P., Thaysen, H.J. and Thorn, N.H., 2013. The Alkali Metal Ions in
Biology: I. The Alkali Metal Ions in Isolated Systems and Tissues. II. The Alkali Metal Ions in the
Organism (Vol. 13). Springer Science & Business Media. [Online]. Available from:
https://books.google.com/books?
hl=en&lr=&id=V17vCAAAQBAJ&oi=fnd&pg=PR13&dq=alkali+metals+properties&ots=OKI
UExpeRj&sig=LhbaVHt04u5fCQI5xAQKlxehMc4, [Accessed on 9 November 2018].
Yin, J., Hu, Y. and Yoon, J., 2015. Fluorescent probes and bioimaging: alkali metals, alkaline
earth metals and pH. Chemical Society Reviews, 44(14), pp.4619-4644. [Online]. Available
from: http://pubs.rsc.org/ru/content/articlehtml/2015/cs/c4cs00275j, [Accessed on 9 November
2018].
http://www.fizika.unios.hr/kvm2/wp-content/uploads/sites/76/2015/11/Bohr%E2%80%99s-
molecular-model-a-century-later.pdf, [Accessed on 9 November 2018].
Ussing, H.H., Kruhoffer, P., Thaysen, H.J. and Thorn, N.H., 2013. The Alkali Metal Ions in
Biology: I. The Alkali Metal Ions in Isolated Systems and Tissues. II. The Alkali Metal Ions in the
Organism (Vol. 13). Springer Science & Business Media. [Online]. Available from:
https://books.google.com/books?
hl=en&lr=&id=V17vCAAAQBAJ&oi=fnd&pg=PR13&dq=alkali+metals+properties&ots=OKI
UExpeRj&sig=LhbaVHt04u5fCQI5xAQKlxehMc4, [Accessed on 9 November 2018].
Yin, J., Hu, Y. and Yoon, J., 2015. Fluorescent probes and bioimaging: alkali metals, alkaline
earth metals and pH. Chemical Society Reviews, 44(14), pp.4619-4644. [Online]. Available
from: http://pubs.rsc.org/ru/content/articlehtml/2015/cs/c4cs00275j, [Accessed on 9 November
2018].
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
