SHC4006 Organic Chemistry Practical: Ketone Synthesis and Reactions
VerifiedAdded on 2022/08/12
|21
|1501
|24
Practical Assignment
AI Summary
This document presents a comprehensive practical assignment on the synthesis of two target molecules: 2,2,6-trimethyl-5-phenylheptan-3-one and 4,4-dimethyl-1-phenylpent-1-en-3-one. The experiment involves a Claisen-Schmidt reaction followed by a Grignard reaction. The assignment details the experimental procedures, including the preparation of reactants, reaction conditions, and purification techniques such as vacuum distillation. It also includes spectral analysis (IR and NMR) of the products to confirm their structures and assess the success of the reactions. The discussion section provides insights into the reaction mechanisms, spectroscopic analysis, and relevant references. The document aims to provide a detailed understanding of organic synthesis techniques and analysis methods.

Chemistry
ORGANIC EXPERIMENTS
The synthesis of a ketone using a Claisen - Schimdt reaction and a Grignard
Reaction
Aim :
The aim is the synthesis of two target molecules.
Experiment No. 1.
Preparation of 2,2,6-trimethyl-5-phenylheptan-3-one
Here, 2,2,6-trimethyl-5-phenylheptan-3-one is prepared by a reaction sequence involving a
Claisen-Schmidt reaction followed by the 1,4-addition of a Grignard reagent to a conjugated
ketone:
Stage 1
C6H5-CH=O + CH3-CO-C(CH3)3 C6H5-CH=CH-CO-C(CH3)3 + H2O
Stage 2
(CH3)2CH-Br + Mg (CH3)2CH-Mg-Br
C6H5-CH=CH-CO-C(CH3)3 C6H5-CH-CH2-CO-C(CH3)3
OH-
ether
(i) (CH3)2CH-Mg-Br
(ii) H3O+
CH3-CH-CH3
ORGANIC EXPERIMENTS
The synthesis of a ketone using a Claisen - Schimdt reaction and a Grignard
Reaction
Aim :
The aim is the synthesis of two target molecules.
Experiment No. 1.
Preparation of 2,2,6-trimethyl-5-phenylheptan-3-one
Here, 2,2,6-trimethyl-5-phenylheptan-3-one is prepared by a reaction sequence involving a
Claisen-Schmidt reaction followed by the 1,4-addition of a Grignard reagent to a conjugated
ketone:
Stage 1
C6H5-CH=O + CH3-CO-C(CH3)3 C6H5-CH=CH-CO-C(CH3)3 + H2O
Stage 2
(CH3)2CH-Br + Mg (CH3)2CH-Mg-Br
C6H5-CH=CH-CO-C(CH3)3 C6H5-CH-CH2-CO-C(CH3)3
OH-
ether
(i) (CH3)2CH-Mg-Br
(ii) H3O+
CH3-CH-CH3
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Mechanisms
In these reactions, extended reaction times are used, vacuum distillation is done under
reduced pressure, a water-free system is used, the differences in the IR absorption frequencies
of conjugated and non-conjugated ketones are used and NMR is used in the determination of
stereochemistry and structure.
1) Synthesis of an enone
A CRUDE sample of 4,4–dimethyl-1-phenylpent-1-en-3-one is synthesized. The individual
spectra for pinacolone and benzaldehyde is recorded. The carbonyl stretching
frequencies are noted for these 2 compounds.
A mixture of 3,3-dimethylbutan-2-one (pinacolone; 20.0 g, 0.200 moles), benzaldehyde (24.0
g, 0.226 moles; see Note 1), 95% ethanol ( ‘rectified spirit’; 75 mL), water (26 mL) and 10%
aqueous sodium hydroxide solution (20 mL) in a 500 mL round-bottomed flask overhead
mechanical stirrer is prepared.
Then, it is stirred for 6 hours at ambient temperature. The IR spectra of the two starting
materials is recorded and the boiling point for pinacolone and benzaldehyde at 760 mmHg
and at 10 mmHg is found.
It is diluted using the same amount of water. The organic product is extracted with three
separate portions of diethyl ether (60 mL) using an appropriately sized separating funnel. The
mixed ethereal extracts are washed using water (4 x 60 mL), then dried using anhydrous
magnesium sulphate.
The desiccant is removed by filtration (fluted filter paper, gravity) and the solvent by rotary
evaporation over a water-bath using a tared RB flask. The mass of the crude product is
determined.
In these reactions, extended reaction times are used, vacuum distillation is done under
reduced pressure, a water-free system is used, the differences in the IR absorption frequencies
of conjugated and non-conjugated ketones are used and NMR is used in the determination of
stereochemistry and structure.
1) Synthesis of an enone
A CRUDE sample of 4,4–dimethyl-1-phenylpent-1-en-3-one is synthesized. The individual
spectra for pinacolone and benzaldehyde is recorded. The carbonyl stretching
frequencies are noted for these 2 compounds.
A mixture of 3,3-dimethylbutan-2-one (pinacolone; 20.0 g, 0.200 moles), benzaldehyde (24.0
g, 0.226 moles; see Note 1), 95% ethanol ( ‘rectified spirit’; 75 mL), water (26 mL) and 10%
aqueous sodium hydroxide solution (20 mL) in a 500 mL round-bottomed flask overhead
mechanical stirrer is prepared.
Then, it is stirred for 6 hours at ambient temperature. The IR spectra of the two starting
materials is recorded and the boiling point for pinacolone and benzaldehyde at 760 mmHg
and at 10 mmHg is found.
It is diluted using the same amount of water. The organic product is extracted with three
separate portions of diethyl ether (60 mL) using an appropriately sized separating funnel. The
mixed ethereal extracts are washed using water (4 x 60 mL), then dried using anhydrous
magnesium sulphate.
The desiccant is removed by filtration (fluted filter paper, gravity) and the solvent by rotary
evaporation over a water-bath using a tared RB flask. The mass of the crude product is
determined.

2) Purification of enome by vacuum distillation
A vacuum distillation of the crude product is performed. The temperature and
pressure at which fractions are recovered are noted. A record of the weight of each fraction is
made. Individual IR spectra are recorded for each unique fraction. A yield of pure enone is
determined.
The product is purified by distillation under reduced pressure, using an efficient vacuum-
pump. The lowish-boiling fore-run is collected in receiver flask 1 and 2, and the constant-
boiling pure product in receiver flask 3, over a temperature range of no more than 20.The
bp/pressure is recorded at which the product distils, its mass is recorded and the percentage
yield is calculated. An IR spectrum of different fractions is run and the purity of the product
is checked. A 1H spectrum of the sample is checked.
3) Stage 2 : Preparation of 2,2,6-trimethyl-5-phenylheptan-3-one
(a) Use of the Grignard solution to prepare 2,2,6-trimethyl-5-phenylheptan-3-one
A Grignard Reagent was prepared and reacted with a pure sample of their enone. A solution
of the enone (5.64 g; 0.030 moles) was prepared in dry ether (30 mL) and added to the
dropping funnel. Also, 2-propyl magnesium bromide is added. The stirring was done for 30
minutes.
The weight of the crude product obtained was determined. An IR and a1H spectrum of the
sample was obtained and the percentage yield was calculated.
(b) Preparation of 2-propyl magnesium bromide
A 3-neck 250 mL RB flask was equipped with a magnetic flea, a stopper, a Liebig condenser
surmounted by a calcium chloride drying tube, and a stoppered dropping funnel. The
apparatus was mounted on a retort stand, on the base-plate of which stood a partially jacked
up lab-jack, supporting a heater/magnetic stirrer unit.
A vacuum distillation of the crude product is performed. The temperature and
pressure at which fractions are recovered are noted. A record of the weight of each fraction is
made. Individual IR spectra are recorded for each unique fraction. A yield of pure enone is
determined.
The product is purified by distillation under reduced pressure, using an efficient vacuum-
pump. The lowish-boiling fore-run is collected in receiver flask 1 and 2, and the constant-
boiling pure product in receiver flask 3, over a temperature range of no more than 20.The
bp/pressure is recorded at which the product distils, its mass is recorded and the percentage
yield is calculated. An IR spectrum of different fractions is run and the purity of the product
is checked. A 1H spectrum of the sample is checked.
3) Stage 2 : Preparation of 2,2,6-trimethyl-5-phenylheptan-3-one
(a) Use of the Grignard solution to prepare 2,2,6-trimethyl-5-phenylheptan-3-one
A Grignard Reagent was prepared and reacted with a pure sample of their enone. A solution
of the enone (5.64 g; 0.030 moles) was prepared in dry ether (30 mL) and added to the
dropping funnel. Also, 2-propyl magnesium bromide is added. The stirring was done for 30
minutes.
The weight of the crude product obtained was determined. An IR and a1H spectrum of the
sample was obtained and the percentage yield was calculated.
(b) Preparation of 2-propyl magnesium bromide
A 3-neck 250 mL RB flask was equipped with a magnetic flea, a stopper, a Liebig condenser
surmounted by a calcium chloride drying tube, and a stoppered dropping funnel. The
apparatus was mounted on a retort stand, on the base-plate of which stood a partially jacked
up lab-jack, supporting a heater/magnetic stirrer unit.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

To the flask, the dry magnesium (1.08 g, 0.045 moles), and enough dry ether was added to
cover it (ca. 10 mL). A solution of 2-bromopropane (isopropyl bromide; 5.54 g, 0.045 moles)
was placed in dry ether (35 mL) in the dropping funnel. A few mLs of this solution were
added rapidly to the magnesium, at ambient temperature, and reaction was allowed to
proceed ( Ghaffar, 2013 ).
If nothing happened during the next 5 min, a clean, dry glass-rod (through the stoppered
opening of the flask) was used to scrape firmly some of the magnesium against the sides of
the flask, beneath the surface of the ether. The onset of reaction was indicated by the
occasional bubble rising from the magnesium to the surface. Soon, a stream of bubbles was
observed. The temperature of the solution increased, and the solution began to reflux
spontaneously. At this point, the stirrer was started, and slowly the remainder of the halide
solution was added to maintain self-sustaining, but controlled, reflux.
When addition of the halide was completed, and the reflux slackened, it was stirred and
heated using reflux for 15 minutes, until almost all of the magnesium had been used up. A
solution of 2-propyl magnesium bromide was obtained containing a few darkish particles of
unreacted magnesium.
Experimental/Results
Stage 1 Enone Method
Spectral Int.
cover it (ca. 10 mL). A solution of 2-bromopropane (isopropyl bromide; 5.54 g, 0.045 moles)
was placed in dry ether (35 mL) in the dropping funnel. A few mLs of this solution were
added rapidly to the magnesium, at ambient temperature, and reaction was allowed to
proceed ( Ghaffar, 2013 ).
If nothing happened during the next 5 min, a clean, dry glass-rod (through the stoppered
opening of the flask) was used to scrape firmly some of the magnesium against the sides of
the flask, beneath the surface of the ether. The onset of reaction was indicated by the
occasional bubble rising from the magnesium to the surface. Soon, a stream of bubbles was
observed. The temperature of the solution increased, and the solution began to reflux
spontaneously. At this point, the stirrer was started, and slowly the remainder of the halide
solution was added to maintain self-sustaining, but controlled, reflux.
When addition of the halide was completed, and the reflux slackened, it was stirred and
heated using reflux for 15 minutes, until almost all of the magnesium had been used up. A
solution of 2-propyl magnesium bromide was obtained containing a few darkish particles of
unreacted magnesium.
Experimental/Results
Stage 1 Enone Method
Spectral Int.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
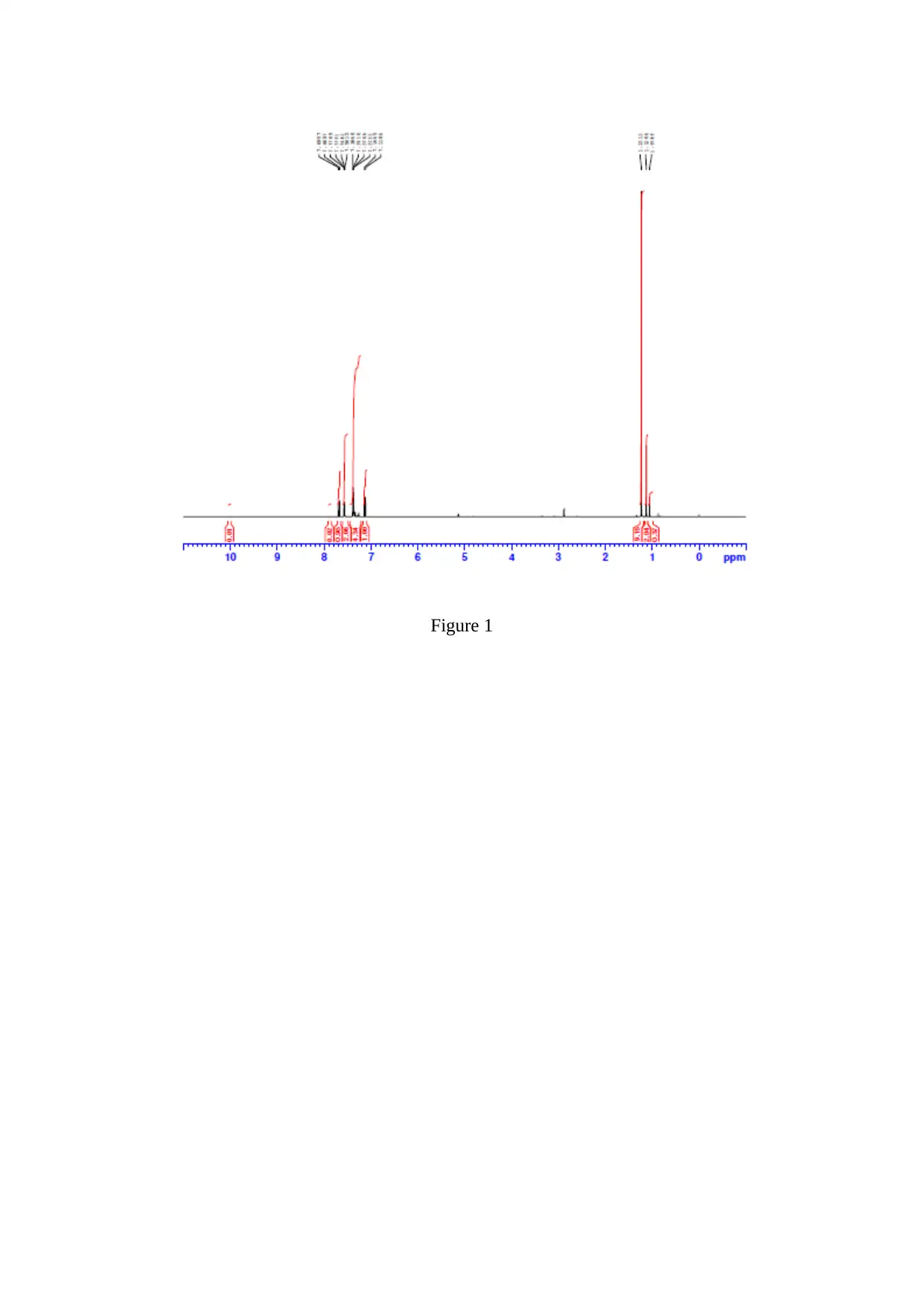
Figure 1
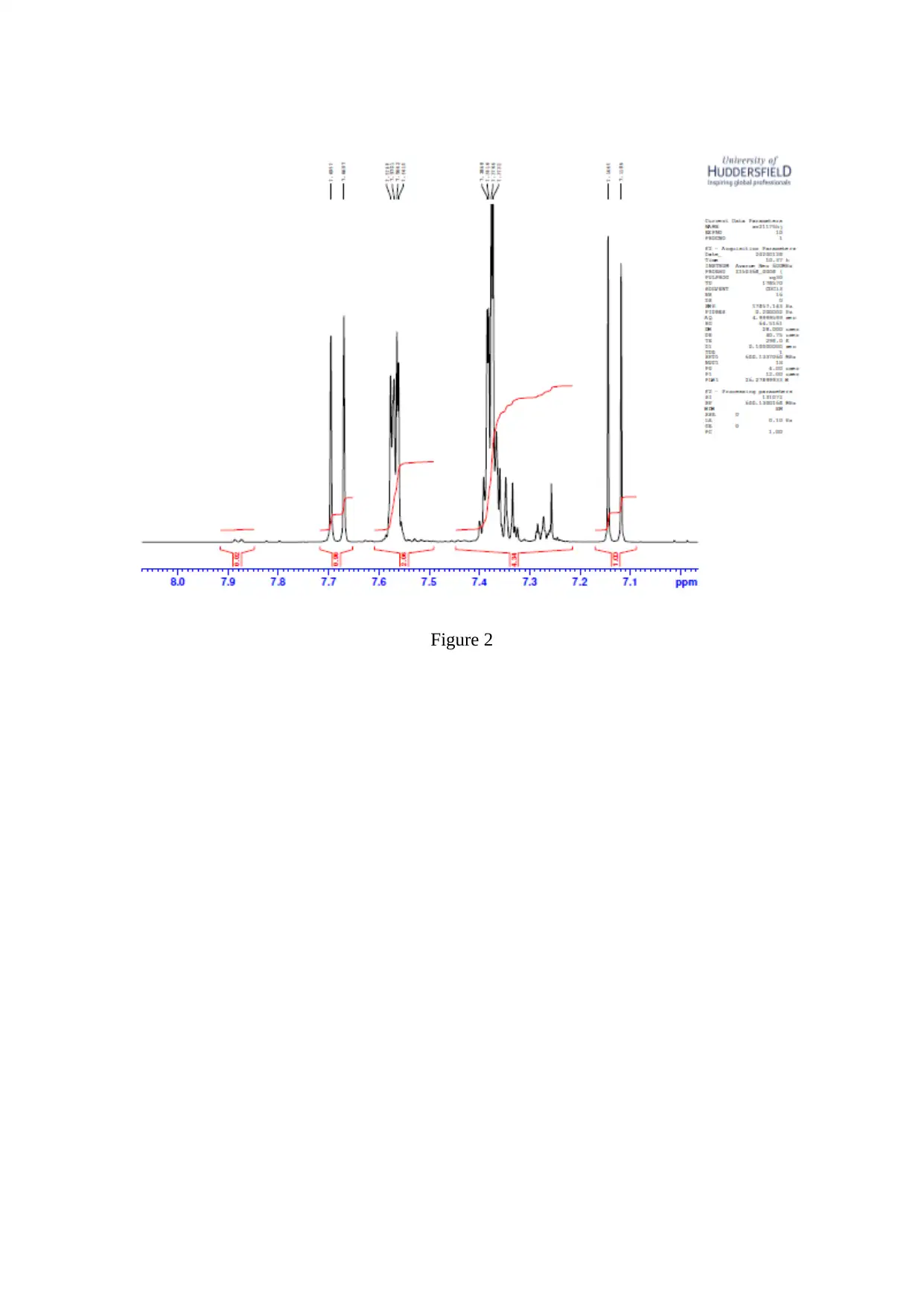
Figure 2
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
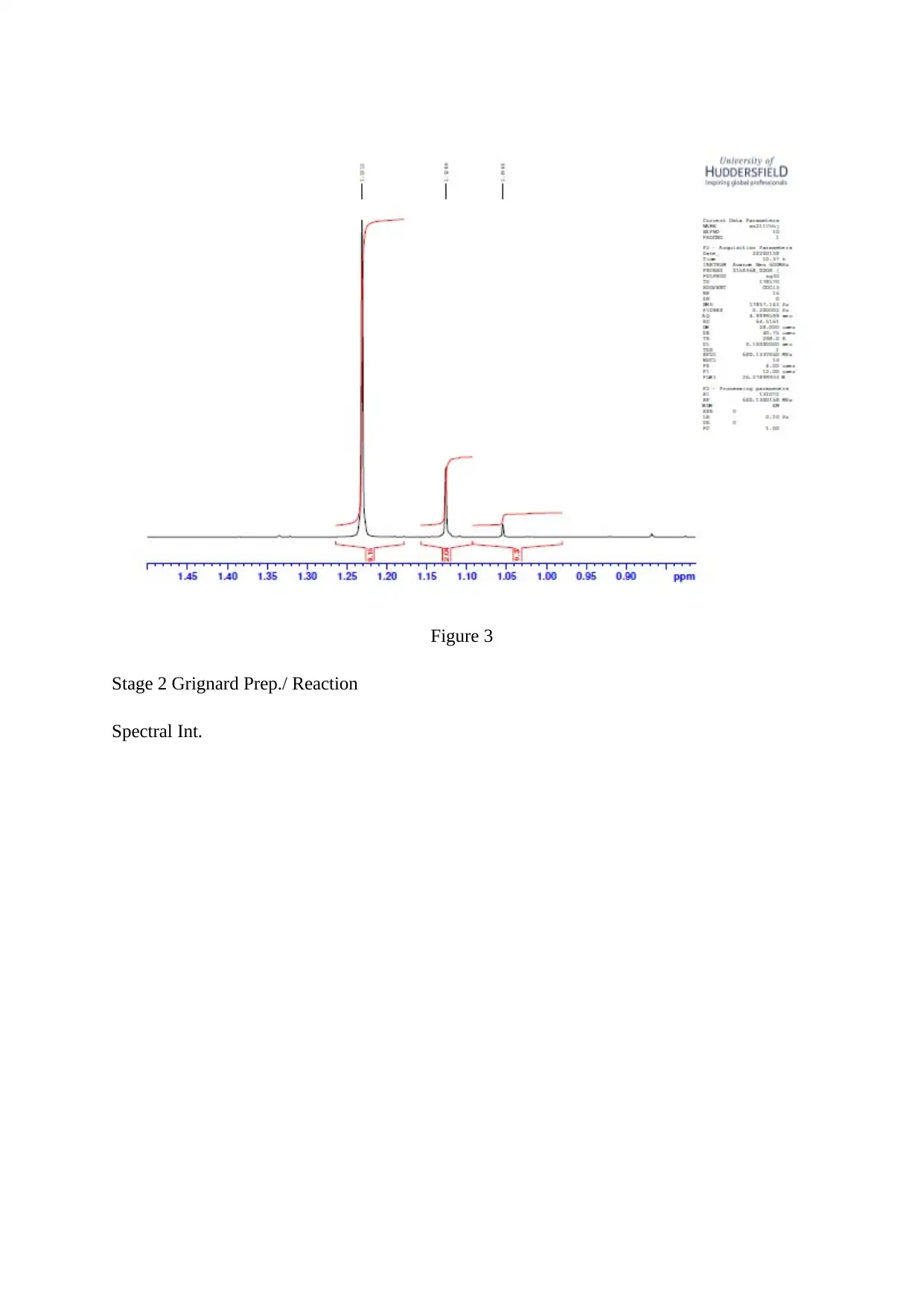
Figure 3
Stage 2 Grignard Prep./ Reaction
Spectral Int.
Stage 2 Grignard Prep./ Reaction
Spectral Int.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
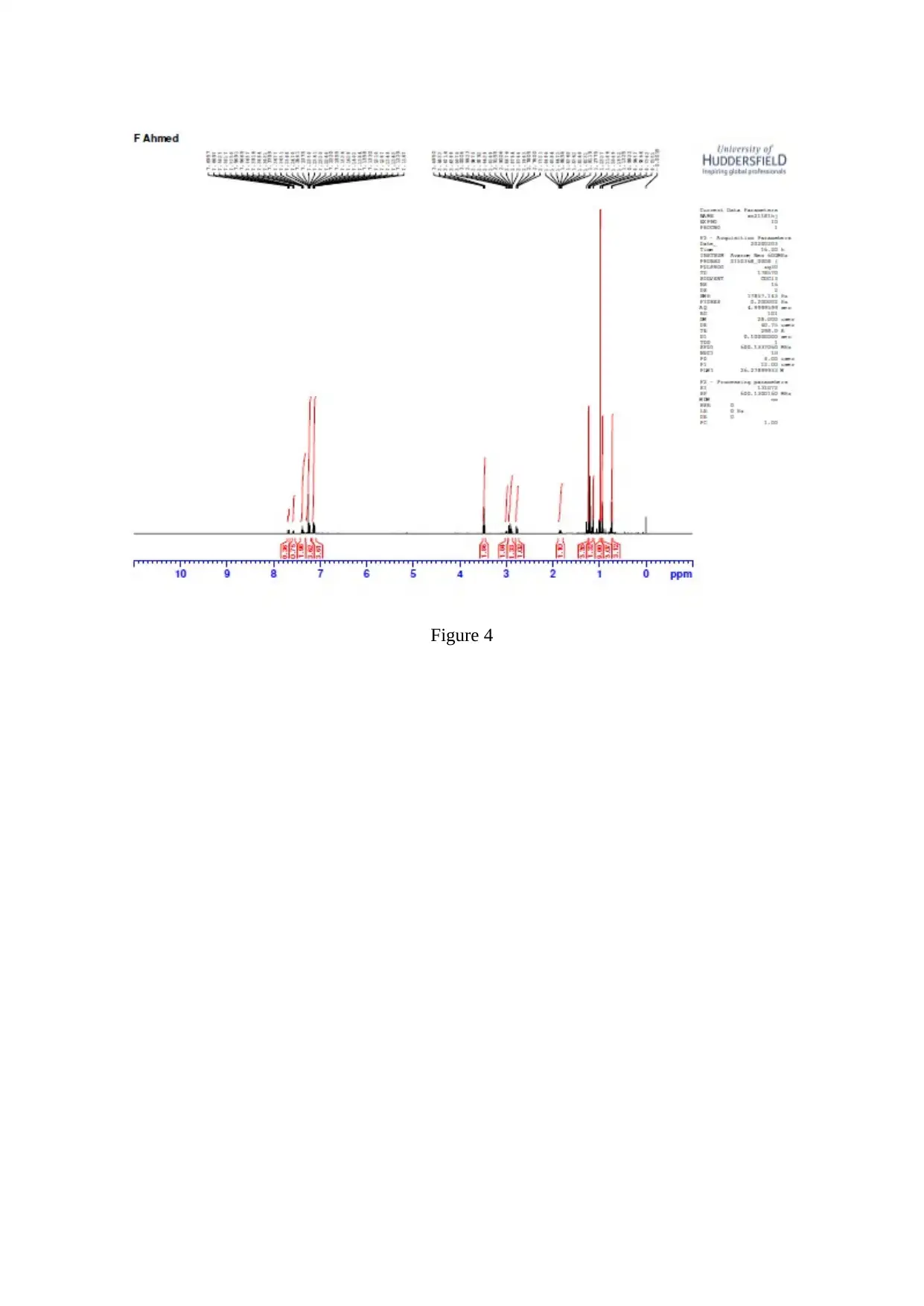
Figure 4
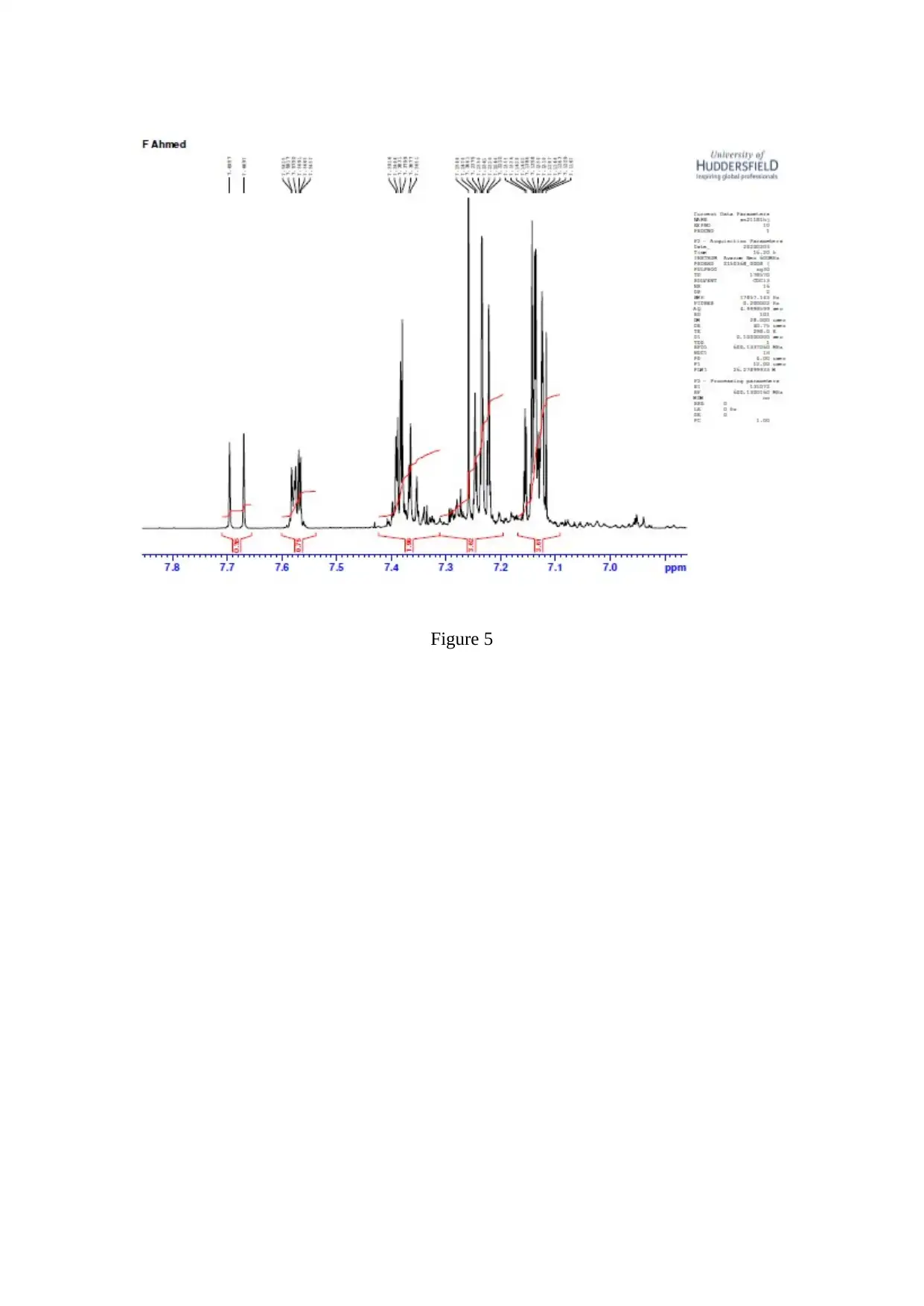
Figure 5
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
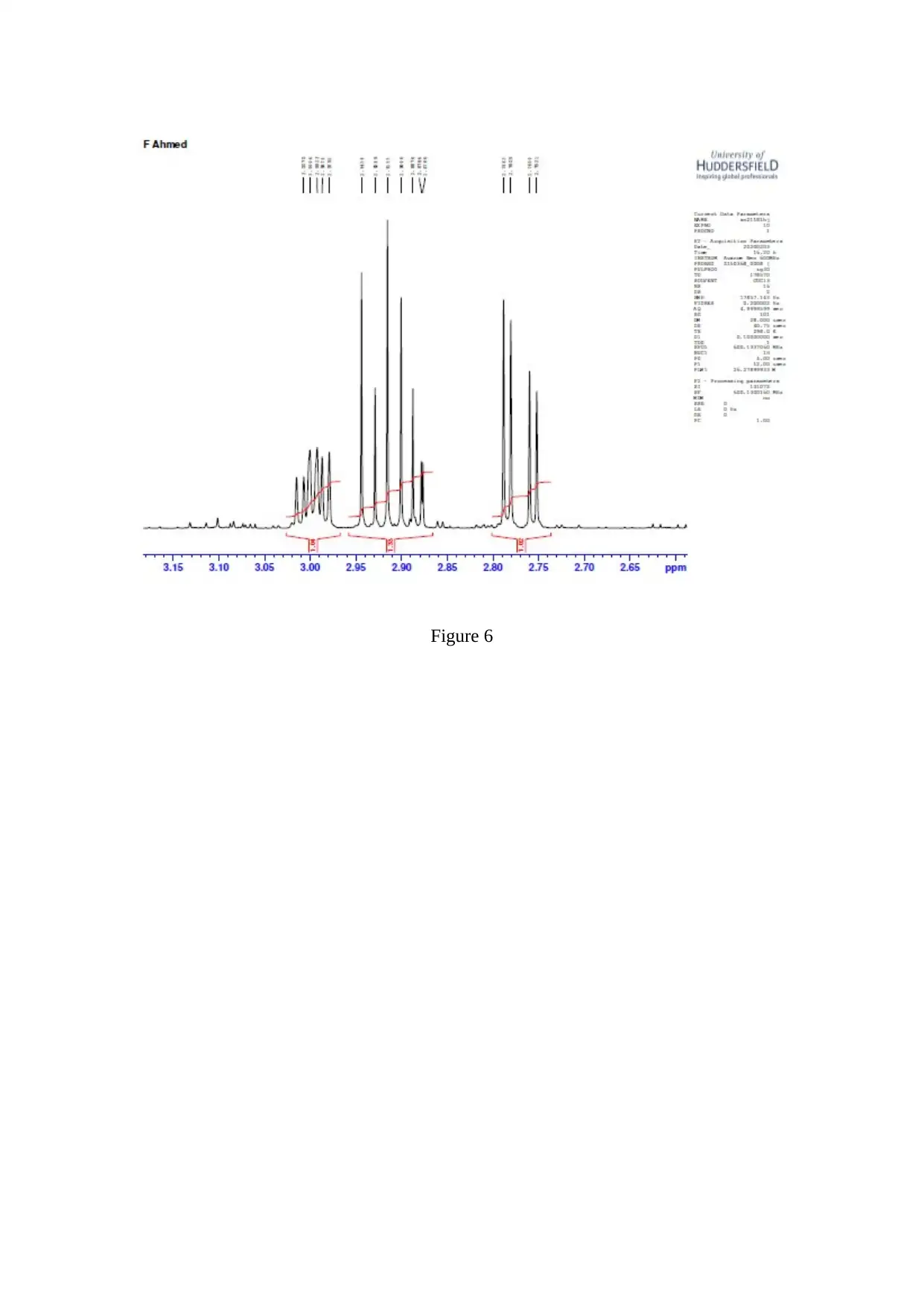
Figure 6
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
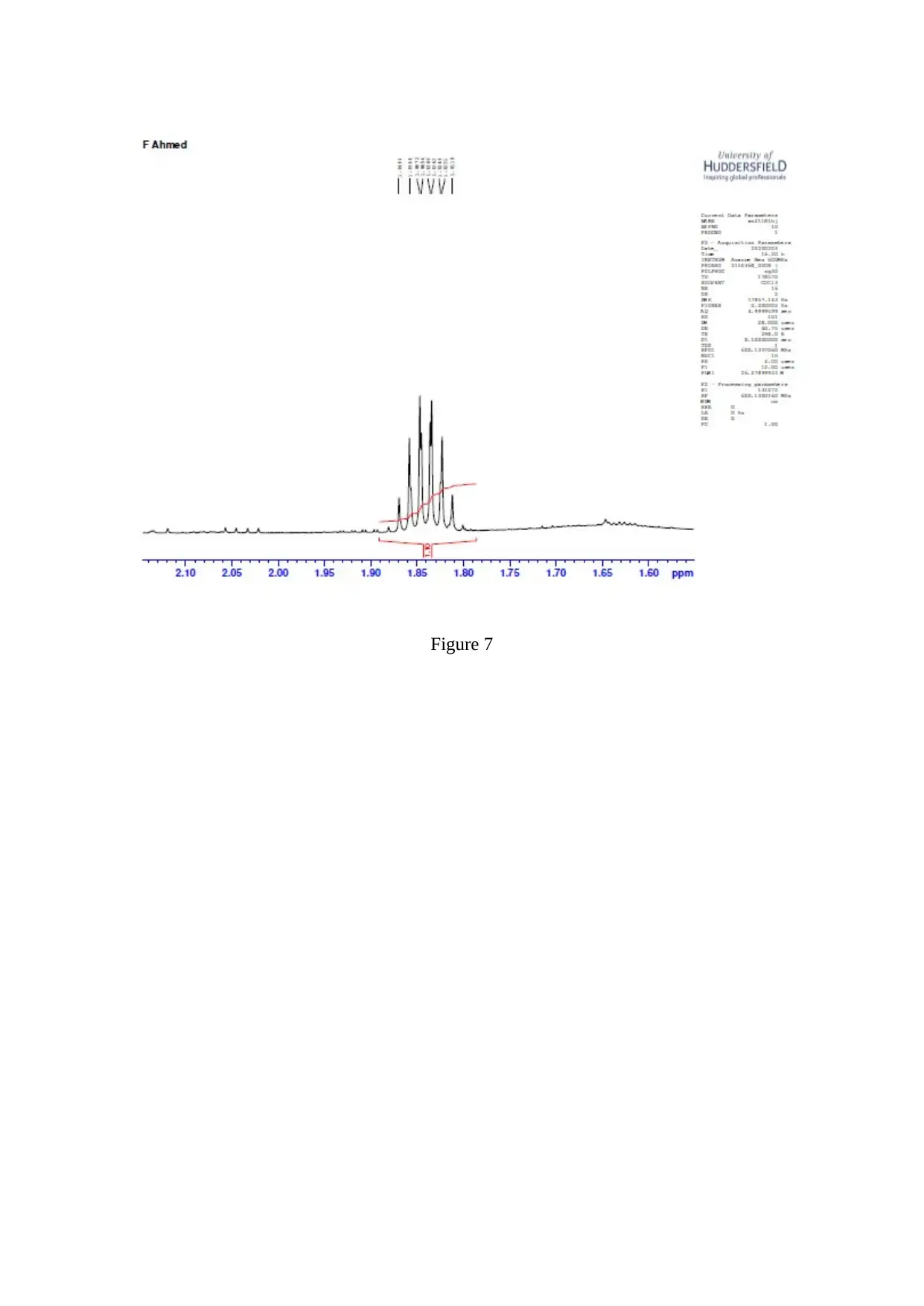
Figure 7
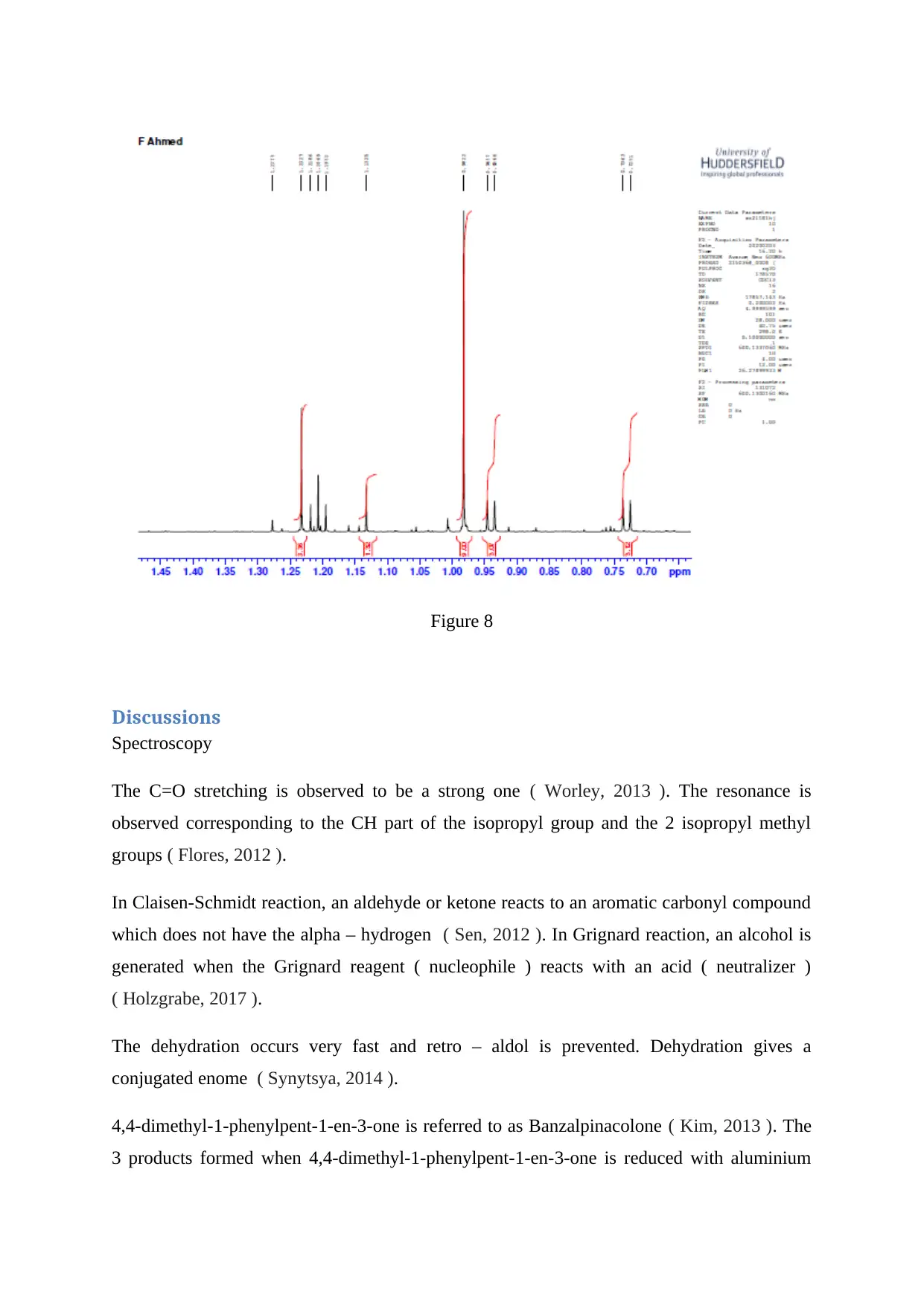
Figure 8
Discussions
Spectroscopy
The C=O stretching is observed to be a strong one ( Worley, 2013 ). The resonance is
observed corresponding to the CH part of the isopropyl group and the 2 isopropyl methyl
groups ( Flores, 2012 ).
In Claisen-Schmidt reaction, an aldehyde or ketone reacts to an aromatic carbonyl compound
which does not have the alpha – hydrogen ( Sen, 2012 ). In Grignard reaction, an alcohol is
generated when the Grignard reagent ( nucleophile ) reacts with an acid ( neutralizer )
( Holzgrabe, 2017 ).
The dehydration occurs very fast and retro – aldol is prevented. Dehydration gives a
conjugated enome ( Synytsya, 2014 ).
4,4-dimethyl-1-phenylpent-1-en-3-one is referred to as Banzalpinacolone ( Kim, 2013 ). The
3 products formed when 4,4-dimethyl-1-phenylpent-1-en-3-one is reduced with aluminium
Discussions
Spectroscopy
The C=O stretching is observed to be a strong one ( Worley, 2013 ). The resonance is
observed corresponding to the CH part of the isopropyl group and the 2 isopropyl methyl
groups ( Flores, 2012 ).
In Claisen-Schmidt reaction, an aldehyde or ketone reacts to an aromatic carbonyl compound
which does not have the alpha – hydrogen ( Sen, 2012 ). In Grignard reaction, an alcohol is
generated when the Grignard reagent ( nucleophile ) reacts with an acid ( neutralizer )
( Holzgrabe, 2017 ).
The dehydration occurs very fast and retro – aldol is prevented. Dehydration gives a
conjugated enome ( Synytsya, 2014 ).
4,4-dimethyl-1-phenylpent-1-en-3-one is referred to as Banzalpinacolone ( Kim, 2013 ). The
3 products formed when 4,4-dimethyl-1-phenylpent-1-en-3-one is reduced with aluminium
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 21
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.