Technological Innovation: Sources, Problems, and Competitive Advantage
VerifiedAdded on 2022/08/25
|15
|11163
|34
Report
AI Summary
This report delves into the sources of technological innovation, examining how firms develop radical and incremental innovations to sustain a competitive advantage. The study focuses on a conceptual framework of problem-driven innovation, using the pharmaceutical industry, specifically groundbreaking drugs for lung cancer treatment, as a case study. The research suggests that the coevolution of consequential problems and their solutions drives the emergence of radical innovations. Firms are incentivized to find innovative solutions to unsolved problems to achieve a temporary profit monopoly. The report positions this analysis within existing frameworks, reviewing approaches like induced innovations, evolutionary theory, and path-dependent development. The core argument centers on how relevant problems/needs induce problem-solving activities, leading to both incremental and radical innovations. The framework highlights the importance of problem-solving in R&D labs for competitive advantage and technological change in a Schumpeterian world. The research concludes with a hypothesis that relevant problems support new technological paradigms, which induce the development of these innovations over time. The report underscores the high mortality rate of lung cancer as a major unsolved problem that has driven the development of innovative anticancer treatments.
Technology Analysis & Strategic Manageme
ISSN: 0953-7325 (Print) 1465-3990 (Online) Journal homepage: http://www.tandf
Sources of technological innovation: Ra
incremental innovation problem-driven
competitive advantage of firms
Mario Coccia
To cite this article: Mario Coccia (2016): Sources of technological innovation:
incremental innovation problem-driven to support competitive advantage of firm
Analysis & Strategic Management, DOI: 10.1080/09537325.2016.1268682
To link to this article: http://dx.doi.org/10.1080/09537325.2016.1268682
Published online: 29 Dec 2016.
Submit your article to this journal
Article views: 2
View related articles
View Crossmark data
ISSN: 0953-7325 (Print) 1465-3990 (Online) Journal homepage: http://www.tandf
Sources of technological innovation: Ra
incremental innovation problem-driven
competitive advantage of firms
Mario Coccia
To cite this article: Mario Coccia (2016): Sources of technological innovation:
incremental innovation problem-driven to support competitive advantage of firm
Analysis & Strategic Management, DOI: 10.1080/09537325.2016.1268682
To link to this article: http://dx.doi.org/10.1080/09537325.2016.1268682
Published online: 29 Dec 2016.
Submit your article to this journal
Article views: 2
View related articles
View Crossmark data
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Sources of technological innovation:Radical and incremental
innovation problem-driven to support competitive advantage of
firms
Mario Coccia
Arizona State University & CNR – NationalResearch Councilof Italy,Tempe,AZ 85287,USA
ABSTRACT
A fundamentalproblem in the field of management of technology is how
firms develop radicaland incrementalinnovationsthat sustainthe
competitive advantage in markets.Currentframeworksprovide some
explanations butthe generalsources ofmajorand minortechnological
breakthroughs are hardly known.The study here confronts this problem
by developing a conceptualframework of problem-driven innovation.The
inductive studyof the pharmaceuticalindustry(focusing on ground-
breaking drugs for lung cancertreatment)seems to show thatthe co-
evolution ofconsequentialproblemsand their solutionsinducethe
emergence and development ofradicalinnovations.In fact,firms have a
strong incentive to find innovative solutions to unsolved problems in order
to achieve the prospect of a (temporary) profit monopoly and competitive
advantage in marketscharacterised bytechnologicaldynamisms.The
theoreticalframework of this study can be generalised to explain one of
the sourcesof innovation thatsupportstechnologicaland industrial
change in a Schumpeterian world of innovation-based competition.
ARTICLE HISTORY
Received 15 January 2016
Revised 6 September 2016
Accepted 28 November 2016
KEYWORDS
Radicalinnovation;problem
solving;sources of
innovation;innovation
management;technological
paradigm;technological
trajectory
JEL CLASSIFICATION
O31;O39;I19
Overview of the problem
This article has two goals.The first is to develop a theoretical framework,which explains one of the
sources of radicaland incrementalinnovations.The second is to stress the importance of problem-
solving activity in R&D labsof firms for developing innovationsand supporting competitive
advantage.
These topics are basic in the field of the economics of innovation and management of techn
to explain the competitive advantage offirms in markets with technologicaldynamisms (Coccia
2009b, 2010c, 2014b; Tushman and Anderson 1986; Nicholson, Rees, and Brooks-Rooney 199
tensen 1997; Garud et al. 2015). In fact, a main question in these research fields is how firms
and sustain radicaland incrementalinnovations for competitive advantage in markets (cf.Coccia
2016a;Teece,Pisano,and Shuen 1997;von Hippel1988).The study here confronts this question
by developing the approach ofproblem-driven innovation,which endeavours to explain one of
the sources of innovation in markets.As a matter of fact,this framework clarifies the understanding
of how firms generate innovative products/processes to supporttheircompetitive advantage in
markets with rapid change.This study can also provide results in both building a better theoretica
framework of the sources of technological innovation and informing best-practices of R&D ma
ment. In order to position this analysis in a manner that displays similarities and differences w
ing approaches,the study here begins by reviewing some frameworks of technologicalanalysis.
© 2016 Informa UK Limited,trading as Taylor & Francis Group
CONTACT Mario Cocciamario.coccia@cnr.it
TECHNOLOGY ANALYSIS & STRATEGIC MANAGEMENT,2016
http://dx.doi.org/10.1080/09537325.2016.1268682
innovation problem-driven to support competitive advantage of
firms
Mario Coccia
Arizona State University & CNR – NationalResearch Councilof Italy,Tempe,AZ 85287,USA
ABSTRACT
A fundamentalproblem in the field of management of technology is how
firms develop radicaland incrementalinnovationsthat sustainthe
competitive advantage in markets.Currentframeworksprovide some
explanations butthe generalsources ofmajorand minortechnological
breakthroughs are hardly known.The study here confronts this problem
by developing a conceptualframework of problem-driven innovation.The
inductive studyof the pharmaceuticalindustry(focusing on ground-
breaking drugs for lung cancertreatment)seems to show thatthe co-
evolution ofconsequentialproblemsand their solutionsinducethe
emergence and development ofradicalinnovations.In fact,firms have a
strong incentive to find innovative solutions to unsolved problems in order
to achieve the prospect of a (temporary) profit monopoly and competitive
advantage in marketscharacterised bytechnologicaldynamisms.The
theoreticalframework of this study can be generalised to explain one of
the sourcesof innovation thatsupportstechnologicaland industrial
change in a Schumpeterian world of innovation-based competition.
ARTICLE HISTORY
Received 15 January 2016
Revised 6 September 2016
Accepted 28 November 2016
KEYWORDS
Radicalinnovation;problem
solving;sources of
innovation;innovation
management;technological
paradigm;technological
trajectory
JEL CLASSIFICATION
O31;O39;I19
Overview of the problem
This article has two goals.The first is to develop a theoretical framework,which explains one of the
sources of radicaland incrementalinnovations.The second is to stress the importance of problem-
solving activity in R&D labsof firms for developing innovationsand supporting competitive
advantage.
These topics are basic in the field of the economics of innovation and management of techn
to explain the competitive advantage offirms in markets with technologicaldynamisms (Coccia
2009b, 2010c, 2014b; Tushman and Anderson 1986; Nicholson, Rees, and Brooks-Rooney 199
tensen 1997; Garud et al. 2015). In fact, a main question in these research fields is how firms
and sustain radicaland incrementalinnovations for competitive advantage in markets (cf.Coccia
2016a;Teece,Pisano,and Shuen 1997;von Hippel1988).The study here confronts this question
by developing the approach ofproblem-driven innovation,which endeavours to explain one of
the sources of innovation in markets.As a matter of fact,this framework clarifies the understanding
of how firms generate innovative products/processes to supporttheircompetitive advantage in
markets with rapid change.This study can also provide results in both building a better theoretica
framework of the sources of technological innovation and informing best-practices of R&D ma
ment. In order to position this analysis in a manner that displays similarities and differences w
ing approaches,the study here begins by reviewing some frameworks of technologicalanalysis.
© 2016 Informa UK Limited,trading as Taylor & Francis Group
CONTACT Mario Cocciamario.coccia@cnr.it
TECHNOLOGY ANALYSIS & STRATEGIC MANAGEMENT,2016
http://dx.doi.org/10.1080/09537325.2016.1268682

In general,innovation is driven by several concomitant determinants,and scholars of economics,
management oftechnology and related disciplines have described three principalapproaches of
technologicalchange:induced innovations,evolutionary theory oftechnologicalchange,and
path-dependent development of innovations (cf.Dixon 1997).The first approach of induced inno-
vationsshowsthat the demand-pullis an importantfactorfor supporting innovations.Hicks
argued that:‘A change in the relative prices of factors of production is itself a spur to innovation
and to inventions ofa particularkind – directed ateconomising the use ofa factorwhich has
become relatively expensive’(Hicks as quoted by Ruttan 1997,1521).
The second approach is the evolutionary theory of technological change,which abandons the dis-
tinction between factor substitutions and shifts in the production function (Nelson and Winter
This theoretical framework is based on (1) local search for technical innovations, organisationa
and learning processes,(2) imitation of the practices of firms,and (3) satisficing behaviour of firms.
Third approach is the path-dependence developmentof innovation thatconsiders a specific
sequence of micro-level historical events for the evolution of innovations: i.e. current choices
niques may influence the future characteristics oftechnology and knowledge over the long run
(Arthur 1989).Ruttan (1997,1524) argues that:
each of the three approaches to understanding the sources of technical change – induced technical change,
utionary theory,and path dependence – is approaching a dead end.Attempts to construct bridges linking the
separate approaches are now necessary to advance our understanding of the sources of technical change.
Severalstudies in management,to explain how firms achieve and sustain innovation,focus on the
concept of ambidexterity:firms simultaneously engage in exploratory and exploitative activities th
supportboth incrementaland radicalinnovations (cf.Coccia 2009b,2012e;Durisin and Todorova
2012;Lin and McDonough III2014;O’Reilly IIIand Tushman 2004,76,2008;Danneels 2006).Other
approaches argue that a stage-gate model can rationalise and structure the technological dev
of new products (Conforto and Amaral 2016;Cooper 1990).Wuest et al.(2014,33) claim that:
The basic idea of the stage gate model is to divide a process in different phases and create a quality gate a
points in order to secure that the targeted goals are reached before proceeding to the next process phase.The
quality gates represent decision points,which determine on the basis of the current status of the process if the
project is continued,adapted/revised or terminated.The development process cannot pass a gate when it does
not meet all set criteria.(cf.Cooper 2008)
Ambartsoumian et al.(2011) argue that the phases of a new product development process are diffi
cult to plan,especially when goals are not clearly defined (cf.Calabrese,Coccia,and Rolfo 2005;
Cavallo etal. 2014a,2014b;Cavallo,Ferrari,and Coccia 2015).Currenttheoreticalframeworks
analysedifferentcharacteristicsof patternsof technologicalinnovation,2 however,current
approaches in economics and management of technology have trouble explaining some deter
nants that foster incrementaland radicalinnovations in markets.In fact,the generaldriving force
of innovations at micro- and macro-levelis hardly known (Dixon 1997).
In this context,the study here addresses the following questions:What is a general driver of inno-
vations in markets? How firms achieve and sustain innovation? The study here confronts these
scribed questionsby developing aconceptualframeworkof problem-driven based on the
coevolution ofconsequentialproblems and problem-solving activity offirms.In particular,this
study endeavours to explain one of the contributing factors that supports the innovation of firm
in markets. Accordingly, this study does not display a new model of product development but
grative approach within current theoreticalframeworks that can clarify one of the sources of inno-
vations with R&D management implications.
Conceptual framework and working hypothesis
Usher (1954),using the theoretical framework of the Gestalt psychology,shows four main concepts
for explaining the evolution of technology:
2 M.COCCIA
management oftechnology and related disciplines have described three principalapproaches of
technologicalchange:induced innovations,evolutionary theory oftechnologicalchange,and
path-dependent development of innovations (cf.Dixon 1997).The first approach of induced inno-
vationsshowsthat the demand-pullis an importantfactorfor supporting innovations.Hicks
argued that:‘A change in the relative prices of factors of production is itself a spur to innovation
and to inventions ofa particularkind – directed ateconomising the use ofa factorwhich has
become relatively expensive’(Hicks as quoted by Ruttan 1997,1521).
The second approach is the evolutionary theory of technological change,which abandons the dis-
tinction between factor substitutions and shifts in the production function (Nelson and Winter
This theoretical framework is based on (1) local search for technical innovations, organisationa
and learning processes,(2) imitation of the practices of firms,and (3) satisficing behaviour of firms.
Third approach is the path-dependence developmentof innovation thatconsiders a specific
sequence of micro-level historical events for the evolution of innovations: i.e. current choices
niques may influence the future characteristics oftechnology and knowledge over the long run
(Arthur 1989).Ruttan (1997,1524) argues that:
each of the three approaches to understanding the sources of technical change – induced technical change,
utionary theory,and path dependence – is approaching a dead end.Attempts to construct bridges linking the
separate approaches are now necessary to advance our understanding of the sources of technical change.
Severalstudies in management,to explain how firms achieve and sustain innovation,focus on the
concept of ambidexterity:firms simultaneously engage in exploratory and exploitative activities th
supportboth incrementaland radicalinnovations (cf.Coccia 2009b,2012e;Durisin and Todorova
2012;Lin and McDonough III2014;O’Reilly IIIand Tushman 2004,76,2008;Danneels 2006).Other
approaches argue that a stage-gate model can rationalise and structure the technological dev
of new products (Conforto and Amaral 2016;Cooper 1990).Wuest et al.(2014,33) claim that:
The basic idea of the stage gate model is to divide a process in different phases and create a quality gate a
points in order to secure that the targeted goals are reached before proceeding to the next process phase.The
quality gates represent decision points,which determine on the basis of the current status of the process if the
project is continued,adapted/revised or terminated.The development process cannot pass a gate when it does
not meet all set criteria.(cf.Cooper 2008)
Ambartsoumian et al.(2011) argue that the phases of a new product development process are diffi
cult to plan,especially when goals are not clearly defined (cf.Calabrese,Coccia,and Rolfo 2005;
Cavallo etal. 2014a,2014b;Cavallo,Ferrari,and Coccia 2015).Currenttheoreticalframeworks
analysedifferentcharacteristicsof patternsof technologicalinnovation,2 however,current
approaches in economics and management of technology have trouble explaining some deter
nants that foster incrementaland radicalinnovations in markets.In fact,the generaldriving force
of innovations at micro- and macro-levelis hardly known (Dixon 1997).
In this context,the study here addresses the following questions:What is a general driver of inno-
vations in markets? How firms achieve and sustain innovation? The study here confronts these
scribed questionsby developing aconceptualframeworkof problem-driven based on the
coevolution ofconsequentialproblems and problem-solving activity offirms.In particular,this
study endeavours to explain one of the contributing factors that supports the innovation of firm
in markets. Accordingly, this study does not display a new model of product development but
grative approach within current theoreticalframeworks that can clarify one of the sources of inno-
vations with R&D management implications.
Conceptual framework and working hypothesis
Usher (1954),using the theoretical framework of the Gestalt psychology,shows four main concepts
for explaining the evolution of technology:
2 M.COCCIA
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
(1) Perception of the problem:an incomplete pattern in need of resolution is recognised.
(2) Setting stage:assimilation of data related to the problem.
(3) Act of insight:a mentalact finds a solution to the problem.
(4) Critical revision:overall exploration and revision of the problem and improvements by means
new acts of insight.
This theoretical framework focuses on acts of insight that solve technological problems. The
cations of Usher’s theory are the evolution of new technology with a vitalcumulative change.As a
matterof fact,specific technologicalinnovations are the outcome ofa specific problem-solving
activity in the developmentof a specifictechnology in a defined research/technologicalfield
(Coccia 2014c, 2014d, 2016a, 2016b). This conceptual background is important to underpin th
eticalframework here.
We suppose the existence of a relevant problem/need (unsolved) in society.Moreover,we con-
sider firms as purposefulsystems that have purposefulelements with a common purpose,such as
maximising the profit,supporting the market leadership,etc.(cf.Ackoff 1971).
The working hypothesis (HPθ) of the study here is:
HPθ :Relevant and consequentialproblems/needs of consumers induce problem solving activities of firms (by
learning processes and acts ofinsight)that generate incrementaland radicalinnovations in markets,ceteris
paribus.
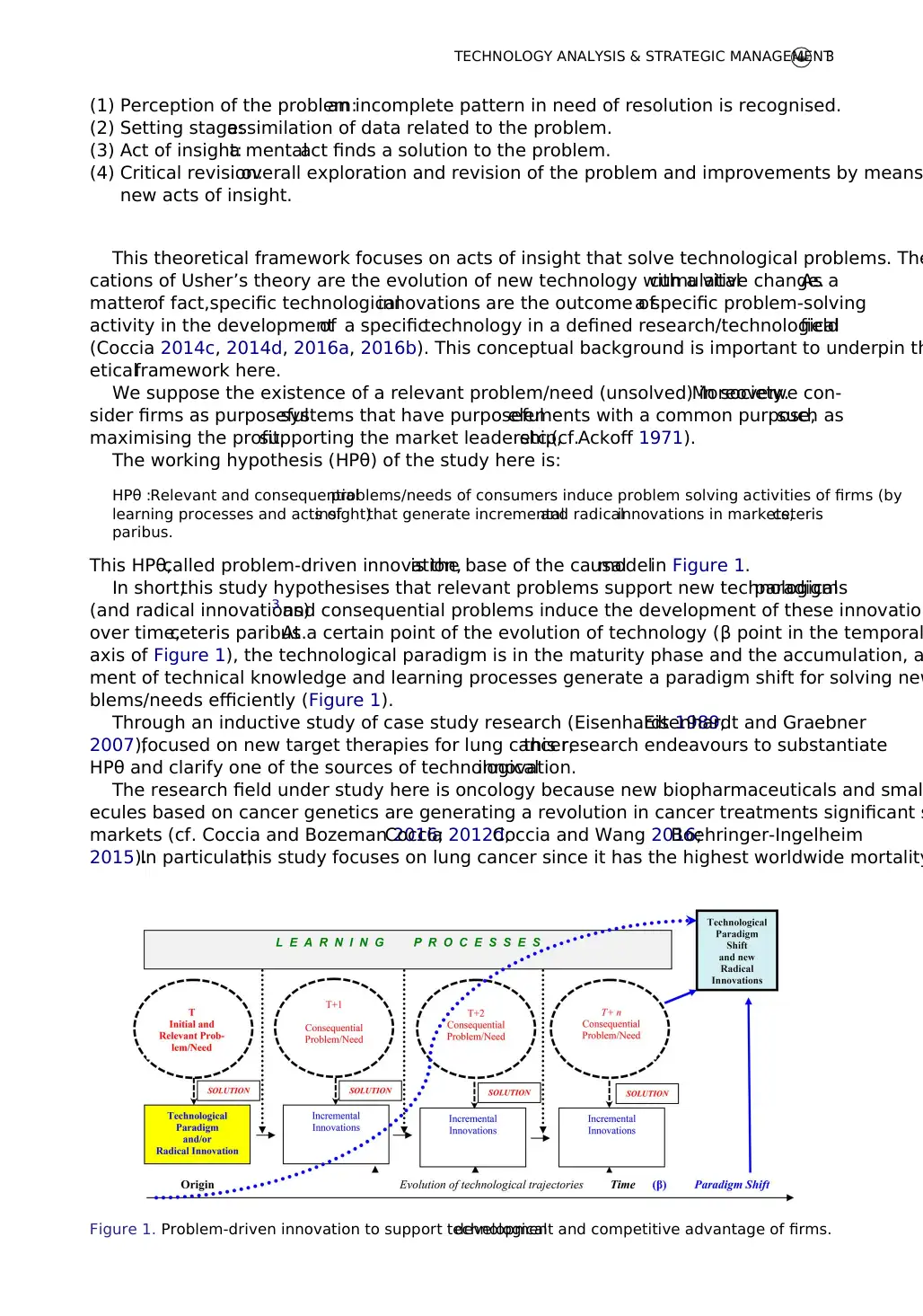
This HPθ,called problem-driven innovation,is the base of the causalmodelin Figure 1.
In short,this study hypothesises that relevant problems support new technologicalparadigms
(and radical innovations)3 and consequential problems induce the development of these innovation
over time,ceteris paribus.At a certain point of the evolution of technology (β point in the temporal
axis of Figure 1), the technological paradigm is in the maturity phase and the accumulation, a
ment of technical knowledge and learning processes generate a paradigm shift for solving new
blems/needs efficiently (Figure 1).
Through an inductive study of case study research (Eisenhardt 1989;Eisenhardt and Graebner
2007),focused on new target therapies for lung cancer,this research endeavours to substantiate
HPθ and clarify one of the sources of technologicalinnovation.
The research field under study here is oncology because new biopharmaceuticals and smal
ecules based on cancer genetics are generating a revolution in cancer treatments significant s
markets (cf. Coccia and Bozeman 2016;Coccia 2012d;Coccia and Wang 2016;Boehringer-Ingelheim
2015).In particular,this study focuses on lung cancer since it has the highest worldwide mortality
Figure 1. Problem-driven innovation to support technologicaldevelopment and competitive advantage of firms.
TECHNOLOGY ANALYSIS & STRATEGIC MANAGEMENT3
(2) Setting stage:assimilation of data related to the problem.
(3) Act of insight:a mentalact finds a solution to the problem.
(4) Critical revision:overall exploration and revision of the problem and improvements by means
new acts of insight.
This theoretical framework focuses on acts of insight that solve technological problems. The
cations of Usher’s theory are the evolution of new technology with a vitalcumulative change.As a
matterof fact,specific technologicalinnovations are the outcome ofa specific problem-solving
activity in the developmentof a specifictechnology in a defined research/technologicalfield
(Coccia 2014c, 2014d, 2016a, 2016b). This conceptual background is important to underpin th
eticalframework here.
We suppose the existence of a relevant problem/need (unsolved) in society.Moreover,we con-
sider firms as purposefulsystems that have purposefulelements with a common purpose,such as
maximising the profit,supporting the market leadership,etc.(cf.Ackoff 1971).
The working hypothesis (HPθ) of the study here is:
HPθ :Relevant and consequentialproblems/needs of consumers induce problem solving activities of firms (by
learning processes and acts ofinsight)that generate incrementaland radicalinnovations in markets,ceteris
paribus.
This HPθ,called problem-driven innovation,is the base of the causalmodelin Figure 1.
In short,this study hypothesises that relevant problems support new technologicalparadigms
(and radical innovations)3 and consequential problems induce the development of these innovation
over time,ceteris paribus.At a certain point of the evolution of technology (β point in the temporal
axis of Figure 1), the technological paradigm is in the maturity phase and the accumulation, a
ment of technical knowledge and learning processes generate a paradigm shift for solving new
blems/needs efficiently (Figure 1).
Through an inductive study of case study research (Eisenhardt 1989;Eisenhardt and Graebner
2007),focused on new target therapies for lung cancer,this research endeavours to substantiate
HPθ and clarify one of the sources of technologicalinnovation.
The research field under study here is oncology because new biopharmaceuticals and smal
ecules based on cancer genetics are generating a revolution in cancer treatments significant s
markets (cf. Coccia and Bozeman 2016;Coccia 2012d;Coccia and Wang 2016;Boehringer-Ingelheim
2015).In particular,this study focuses on lung cancer since it has the highest worldwide mortality
Figure 1. Problem-driven innovation to support technologicaldevelopment and competitive advantage of firms.
TECHNOLOGY ANALYSIS & STRATEGIC MANAGEMENT3
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
rates – for both sexes (cf.Ferlay et al.2013).This study assumes that the lung cancer is a relevant
problem and leading firms in the drug-discovery industry have a primary incentive to find solu
(with innovative and effective anticancer drugs) to this unsolved problem in order to achieve t
spect of a (temporary) profit monopoly in innovation-based markets.New consequentialproblems
and problem-solving activity can generate a main impetus for the development of innovations
markets of anticancer treatments (cf.Coccia 2012a,2012b,2013a,2014a,2014b).Expected evidence
of the inductive study here is to instantiate the HPθ.
Overall,then,the conceptualframework here seeks to explain one of the sources of radicaland
incrementalinnovations forcompetitive advantage offirms.It has also the potentialto explain
one of the generaland basic sources of the technologicalchange in regimes of rapid change.
Evidence to support the hypothesis
The high mortality rate of lung cancer is a major unsolved problem that has had a critical impe
for the development of innovative anticancer treatments.In order to substantiate the HPθ,case
study research here analyses the evolution oftargettherapies (vitalradicalinnovations)for
lung cancer.
. Firstly, as said above, high mortality of the lung cancer is a relevant problem (unsolved) in s
Irigaray et al. (2007) argue that the growing incidence of a variety of cancer in advanced co
is due to several factors such as ageing of the population, progress in health technology, expa
diagnostic and screening programmes,and in particular to the diffusion of environmentalcarcino-
gens (cf. Coccia 2015a; Obe et al. 2011). In fact, cancer incidence (the number of new cases o
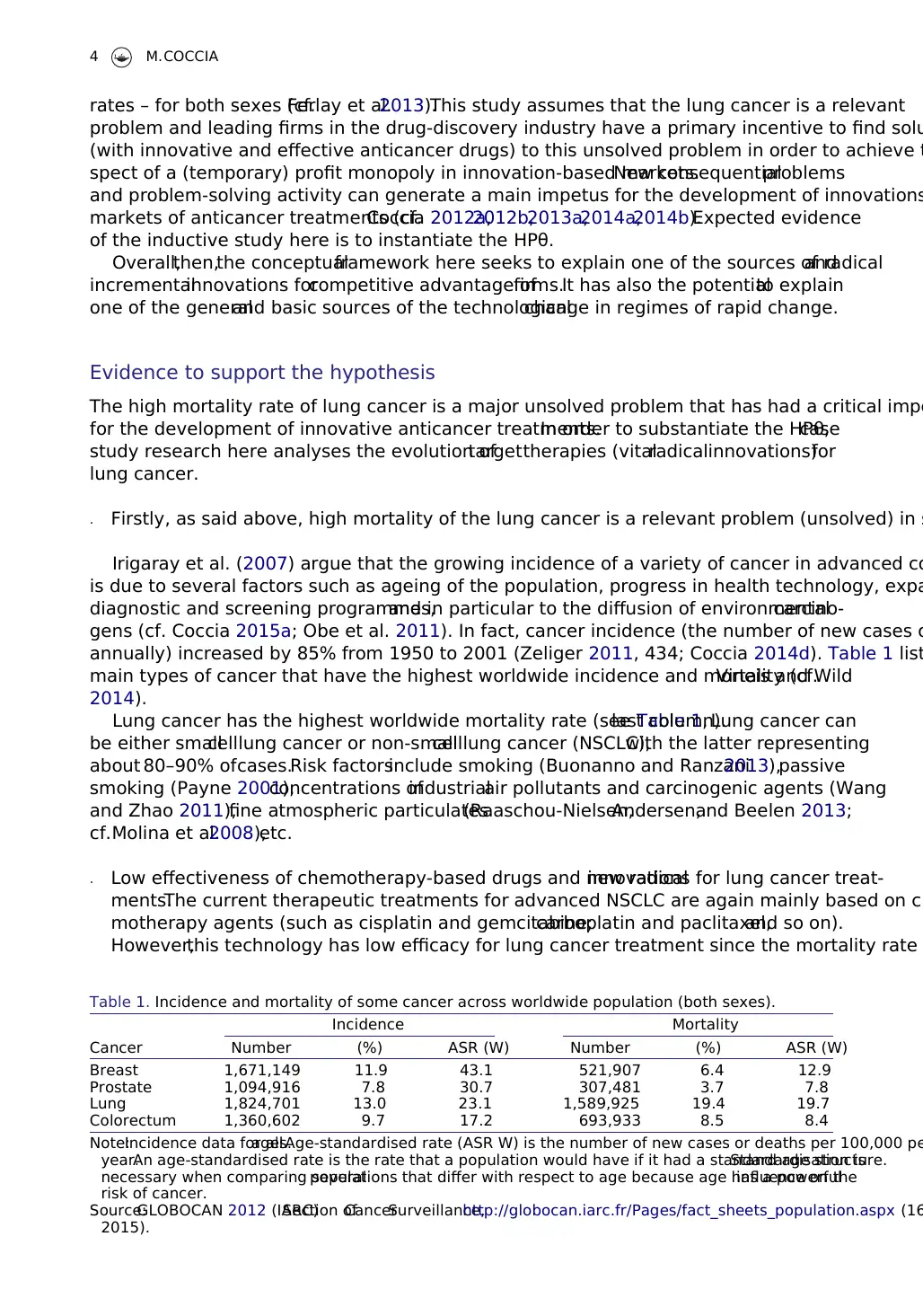
annually) increased by 85% from 1950 to 2001 (Zeliger 2011, 434; Coccia 2014d). Table 1 list
main types of cancer that have the highest worldwide incidence and mortality (cf.Vineis and Wild
2014).
Lung cancer has the highest worldwide mortality rate (see Table 1,last column).Lung cancer can
be either smallcelllung cancer or non-smallcelllung cancer (NSCLC),with the latter representing
about 80–90% ofcases.Risk factorsinclude smoking (Buonanno and Ranzani2013),passive
smoking (Payne 2001),concentrations ofindustrialair pollutants and carcinogenic agents (Wang
and Zhao 2011),fine atmospheric particulates(Raaschou-Nielsen,Andersen,and Beelen 2013;
cf.Molina et al.2008),etc.
. Low effectiveness of chemotherapy-based drugs and new radicalinnovations for lung cancer treat-
ments.The current therapeutic treatments for advanced NSCLC are again mainly based on ch
motherapy agents (such as cisplatin and gemcitabine;carboplatin and paclitaxel,and so on).
However,this technology has low efficacy for lung cancer treatment since the mortality rate
Table 1. Incidence and mortality of some cancer across worldwide population (both sexes).
Cancer
Incidence Mortality
Number (%) ASR (W) Number (%) ASR (W)
Breast 1,671,149 11.9 43.1 521,907 6.4 12.9
Prostate 1,094,916 7.8 30.7 307,481 3.7 7.8
Lung 1,824,701 13.0 23.1 1,589,925 19.4 19.7
Colorectum 1,360,602 9.7 17.2 693,933 8.5 8.4
Note:Incidence data for allages.Age-standardised rate (ASR W) is the number of new cases or deaths per 100,000 pe
year.An age-standardised rate is the rate that a population would have if it had a standard age structure.Standardisation is
necessary when comparing severalpopulations that differ with respect to age because age has a powerfulinfluence on the
risk of cancer.
Source:GLOBOCAN 2012 (IARC)Section ofCancerSurveillance,http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (16
2015).
4 M.COCCIA
problem and leading firms in the drug-discovery industry have a primary incentive to find solu
(with innovative and effective anticancer drugs) to this unsolved problem in order to achieve t
spect of a (temporary) profit monopoly in innovation-based markets.New consequentialproblems
and problem-solving activity can generate a main impetus for the development of innovations
markets of anticancer treatments (cf.Coccia 2012a,2012b,2013a,2014a,2014b).Expected evidence
of the inductive study here is to instantiate the HPθ.
Overall,then,the conceptualframework here seeks to explain one of the sources of radicaland
incrementalinnovations forcompetitive advantage offirms.It has also the potentialto explain
one of the generaland basic sources of the technologicalchange in regimes of rapid change.
Evidence to support the hypothesis
The high mortality rate of lung cancer is a major unsolved problem that has had a critical impe
for the development of innovative anticancer treatments.In order to substantiate the HPθ,case
study research here analyses the evolution oftargettherapies (vitalradicalinnovations)for
lung cancer.
. Firstly, as said above, high mortality of the lung cancer is a relevant problem (unsolved) in s
Irigaray et al. (2007) argue that the growing incidence of a variety of cancer in advanced co
is due to several factors such as ageing of the population, progress in health technology, expa
diagnostic and screening programmes,and in particular to the diffusion of environmentalcarcino-
gens (cf. Coccia 2015a; Obe et al. 2011). In fact, cancer incidence (the number of new cases o
annually) increased by 85% from 1950 to 2001 (Zeliger 2011, 434; Coccia 2014d). Table 1 list
main types of cancer that have the highest worldwide incidence and mortality (cf.Vineis and Wild
2014).
Lung cancer has the highest worldwide mortality rate (see Table 1,last column).Lung cancer can
be either smallcelllung cancer or non-smallcelllung cancer (NSCLC),with the latter representing
about 80–90% ofcases.Risk factorsinclude smoking (Buonanno and Ranzani2013),passive
smoking (Payne 2001),concentrations ofindustrialair pollutants and carcinogenic agents (Wang
and Zhao 2011),fine atmospheric particulates(Raaschou-Nielsen,Andersen,and Beelen 2013;
cf.Molina et al.2008),etc.
. Low effectiveness of chemotherapy-based drugs and new radicalinnovations for lung cancer treat-
ments.The current therapeutic treatments for advanced NSCLC are again mainly based on ch
motherapy agents (such as cisplatin and gemcitabine;carboplatin and paclitaxel,and so on).
However,this technology has low efficacy for lung cancer treatment since the mortality rate
Table 1. Incidence and mortality of some cancer across worldwide population (both sexes).
Cancer
Incidence Mortality
Number (%) ASR (W) Number (%) ASR (W)
Breast 1,671,149 11.9 43.1 521,907 6.4 12.9
Prostate 1,094,916 7.8 30.7 307,481 3.7 7.8
Lung 1,824,701 13.0 23.1 1,589,925 19.4 19.7
Colorectum 1,360,602 9.7 17.2 693,933 8.5 8.4
Note:Incidence data for allages.Age-standardised rate (ASR W) is the number of new cases or deaths per 100,000 pe
year.An age-standardised rate is the rate that a population would have if it had a standard age structure.Standardisation is
necessary when comparing severalpopulations that differ with respect to age because age has a powerfulinfluence on the
risk of cancer.
Source:GLOBOCAN 2012 (IARC)Section ofCancerSurveillance,http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (16
2015).
4 M.COCCIA

still high (19.7% ASR-W)4 in comparison with other cancers (see Table 1). In order to solve this a
other problems concerning the cancer,high R&D investments of advanced countries and leading
corporations have generated vitalscientific advances in genetics,genomics and proteomics,5
which have laid the foundation fornew therapeutic treatments forcancer,such as targeted
cancertherapies,which:‘are drugs orothersubstances thatblock the growth and spread of
cancerby interfering with specific molecules involved in tumorgrowth and progression’(as
defined by NationalCancerInstitute 2015).In particular,molecularbiology has shown that
cancer cells display self-sufficiency of growth signals through the accumulation of genetic a
genetic changes (e.g. Epidermal Growth Factor, EGF). The EGF acts by binding with high affi
the Epidermal Growth Factor Receptor (EGF-R) on the cell surface and by stimulating the in
protein-tyrosine kinase activity of the receptor,which ultimately leads to cancer cell proliferation.
The presence on lung cancer cells of EGF-R (in the exon6 19 and 21),identified by biomarkers,7 is
important to understand patient differences and a precondition for applying target therapie
personalised medicine (cf.,Singerand Marsh 2012).The firstgeneration oftargettherapy for
lung cancer – a technologicalparadigm in lung cancer treatments – is based on the discovery
of the EGF-R blocking agents Gefitinib and Erlotinib to treat patients who have genetic EGF-
Two main radicalinnovations apply these blocking agents to solve the relevantproblem of
high mortality for lung cancer:Iressa® (based on the blocking agent Gefitinib)by AstraZeneca
Company and Tarceva® (based on the blocking agent Erlotinib)commercialised by the Roche
Group.These path-breaking anticancer drugs are generating a revolution in therapeutic trea
ments ofNSCLC with EGF-R because they block-specific enzymes and growth factor receptor
involved in cancer cellproliferation (Laack,Sauter,and Bokemeyer 2010;Mitsudomi2010,101–
102;Mitsudomiet al. 2005;Coccia 2014d,2012d).8 Patients with non-smallcell lung cancer
treated with these targettherapies(Gefitinib and/orErlotinib)have significantly longerpro-
gression-free survivalin comparison with patients who receive a combination ofcarboplatin
plus paclitaxel(chemotherapy agents).In addition,target therapy Erlotinib has the main effect
of reducing the mortality risk by 19% with an increase in median overall survival of patients
ated with lower toxicity (cf.Brugger et al.2009).
. Consequentialproblem:the lung cancer can be resistant to these innovative drugs and grow by
angiogenesis (i.e.cancercells grow by attracting new blood vessels to receive nutrients and
oxygen;cf.Reck and Crinò 2009).This new problem has induced,as solution,the emergence of
the second generation oftarget therapies for lung (and other)cancer (commercialised roughly
from 2014 onwards) that can block the growth of blood vessels feeding tumours9 – angiogenesis
– (Reck and Crinò 2009, 2). These anticancer drugs are multi-inhibitor blocking agents, such
target therapies nintedanib (triple angiokinase inhibitor) and afatinib dimaleate produced b
Boehringer-Ingelheim company (Germany).In July 2013,afatinib dimaleate (commercialname
Gilotrif®)was approved by the U.S.Food and Drug Administration (FDA).Minkovsky and
Berezov (2008) show that afatinib dimaleate can be active against lung cancers resistant to
first generation of anticancer drugs (i.e.Gefitinib and Erlotinib).Instead,Nintedanib,commercial
name Vargate, launched in 2015 for the treatment of NSCLC, can induce endothelial cell ap
Overall, this second generation of ground-breaking anticancer drugs is reducing lung cance
tality,increasing the survivalof patients.
. A new consequentialproblem is that cancer can become drug resistant to previous target thera
and grow with a new mutation.The first and second generation of target therapy (i.e.Gefitinib,
Erlotinib,Gilotrif,etc.)is effective forpatients with NSCLC harbouring activating mutations in
the epidermalgrowth factor receptor (EGFR) kinase domain.However,studies in the biology of
the cancershow thatit can become resistantto these new drugs (cf.Lovly,Horn,and Pao
2015).In particular,approximately 60% of patients typically relapse within 1–3 years of treatme
due to drug resistance to the first and second generation of target therapies. The drug resis
due to a secondary mutation (called T790M)that generates a progression of lung cancer with
severalmetastases.Currently,scientific research to solve the consequentialproblems ofthis
TECHNOLOGY ANALYSIS & STRATEGIC MANAGEMENT5
other problems concerning the cancer,high R&D investments of advanced countries and leading
corporations have generated vitalscientific advances in genetics,genomics and proteomics,5
which have laid the foundation fornew therapeutic treatments forcancer,such as targeted
cancertherapies,which:‘are drugs orothersubstances thatblock the growth and spread of
cancerby interfering with specific molecules involved in tumorgrowth and progression’(as
defined by NationalCancerInstitute 2015).In particular,molecularbiology has shown that
cancer cells display self-sufficiency of growth signals through the accumulation of genetic a
genetic changes (e.g. Epidermal Growth Factor, EGF). The EGF acts by binding with high affi
the Epidermal Growth Factor Receptor (EGF-R) on the cell surface and by stimulating the in
protein-tyrosine kinase activity of the receptor,which ultimately leads to cancer cell proliferation.
The presence on lung cancer cells of EGF-R (in the exon6 19 and 21),identified by biomarkers,7 is
important to understand patient differences and a precondition for applying target therapie
personalised medicine (cf.,Singerand Marsh 2012).The firstgeneration oftargettherapy for
lung cancer – a technologicalparadigm in lung cancer treatments – is based on the discovery
of the EGF-R blocking agents Gefitinib and Erlotinib to treat patients who have genetic EGF-
Two main radicalinnovations apply these blocking agents to solve the relevantproblem of
high mortality for lung cancer:Iressa® (based on the blocking agent Gefitinib)by AstraZeneca
Company and Tarceva® (based on the blocking agent Erlotinib)commercialised by the Roche
Group.These path-breaking anticancer drugs are generating a revolution in therapeutic trea
ments ofNSCLC with EGF-R because they block-specific enzymes and growth factor receptor
involved in cancer cellproliferation (Laack,Sauter,and Bokemeyer 2010;Mitsudomi2010,101–
102;Mitsudomiet al. 2005;Coccia 2014d,2012d).8 Patients with non-smallcell lung cancer
treated with these targettherapies(Gefitinib and/orErlotinib)have significantly longerpro-
gression-free survivalin comparison with patients who receive a combination ofcarboplatin
plus paclitaxel(chemotherapy agents).In addition,target therapy Erlotinib has the main effect
of reducing the mortality risk by 19% with an increase in median overall survival of patients
ated with lower toxicity (cf.Brugger et al.2009).
. Consequentialproblem:the lung cancer can be resistant to these innovative drugs and grow by
angiogenesis (i.e.cancercells grow by attracting new blood vessels to receive nutrients and
oxygen;cf.Reck and Crinò 2009).This new problem has induced,as solution,the emergence of
the second generation oftarget therapies for lung (and other)cancer (commercialised roughly
from 2014 onwards) that can block the growth of blood vessels feeding tumours9 – angiogenesis
– (Reck and Crinò 2009, 2). These anticancer drugs are multi-inhibitor blocking agents, such
target therapies nintedanib (triple angiokinase inhibitor) and afatinib dimaleate produced b
Boehringer-Ingelheim company (Germany).In July 2013,afatinib dimaleate (commercialname
Gilotrif®)was approved by the U.S.Food and Drug Administration (FDA).Minkovsky and
Berezov (2008) show that afatinib dimaleate can be active against lung cancers resistant to
first generation of anticancer drugs (i.e.Gefitinib and Erlotinib).Instead,Nintedanib,commercial
name Vargate, launched in 2015 for the treatment of NSCLC, can induce endothelial cell ap
Overall, this second generation of ground-breaking anticancer drugs is reducing lung cance
tality,increasing the survivalof patients.
. A new consequentialproblem is that cancer can become drug resistant to previous target thera
and grow with a new mutation.The first and second generation of target therapy (i.e.Gefitinib,
Erlotinib,Gilotrif,etc.)is effective forpatients with NSCLC harbouring activating mutations in
the epidermalgrowth factor receptor (EGFR) kinase domain.However,studies in the biology of
the cancershow thatit can become resistantto these new drugs (cf.Lovly,Horn,and Pao
2015).In particular,approximately 60% of patients typically relapse within 1–3 years of treatme
due to drug resistance to the first and second generation of target therapies. The drug resis
due to a secondary mutation (called T790M)that generates a progression of lung cancer with
severalmetastases.Currently,scientific research to solve the consequentialproblems ofthis
TECHNOLOGY ANALYSIS & STRATEGIC MANAGEMENT5
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

secondary mutation is supporting the third generation of inhibitors of mutant lung cancer an
types of cancer (Clovis Oncology 2015).The first and second generation of target therapy is orig-
inally designed to targetwild-type EGFR,whereas new targettherapiesin lung cancerare
designed for EGFR-mutant lung cancer.Clovis Oncology (a smallUS bio-pharmaceuticalfirm) is
developing some of these new drugs such as Rociletinib for the treatment of mutant non-sm
cell lung cancer.In particular,Rociletinib is a novel,oral,targeted covalent (irreversible) inhibitor
to selectively target both the initial activating EGFR mutations and the T790M resistance m
with an improved toxicity profile.Accordingly,it has the potentialto be a first-line treatment in
NSCLC patients with activating EGFR mutations and a second or later-line treatment in NSC
patients who become resistant to previous therapies due to the emergence of the T790M se
ary mutation (Clovis Oncology 2014, 2015). The biopharmaceutical company (AstraZeneca
2015b) has generated a similar selective and irreversible inhibitor for mutant lung cancer: t
anticancer drug is called TAGRISSO™—osimertinib (AZD9291) and wasapproved by US FDA in
2015.
. Future technological trajectories in lung cancer treatments and the potential emergence of
logicalparadigm shift.New generation oftargettherapiestreatsEGFR-mutantlung cancer.
However,Thress et al.(2015,560) analyze the new anticancer therapy AZD9291 by AstraZeneca
for EGFR-mutant lung cancer (a sub type of non-smallcell lung cancer) and show new problems
that will affect the evolution of these innovative target therapies,that is:‘diversity of mechanisms
through which tumors acquire resistance to AZD9291 and highlight the need for therapies t
able to overcome resistance mediated by the EGFRC 797S mutation’.Moreover,cutting-edge
research is opening new scientific frontiers in treatments of oncology by developing therapi
based cancer-fighting viruses and T cells that can generate a technological paradigm shift i
ancer drugs (cf.Ledford 2015).
Overall, the main evidence of this case study above seems in general to support the hypoth
of problem-driven innovation that the origin and development of innovation (with technologica
paradigms,different technological trajectories and paradigm shifts as well) can be explained by
coevolution of consequential problems and problem-solving activity during the evolution of te
nology, ceteris paribus. The innovative anticancer drugs are highly directional and aimed at so
specific problems.In particular,new target therapies embody the development of technological
solutions based on learning processes that generate a vital cumulative change for new techno
cal pathways directed to support the competitive advantage of firms in dynamic markets.In fact,
leading firms,in innovation-based markets – as in the drug-discovery industry – have the chief
incentive to find innovative solutions/products for unsolved problems in order to achieve the p
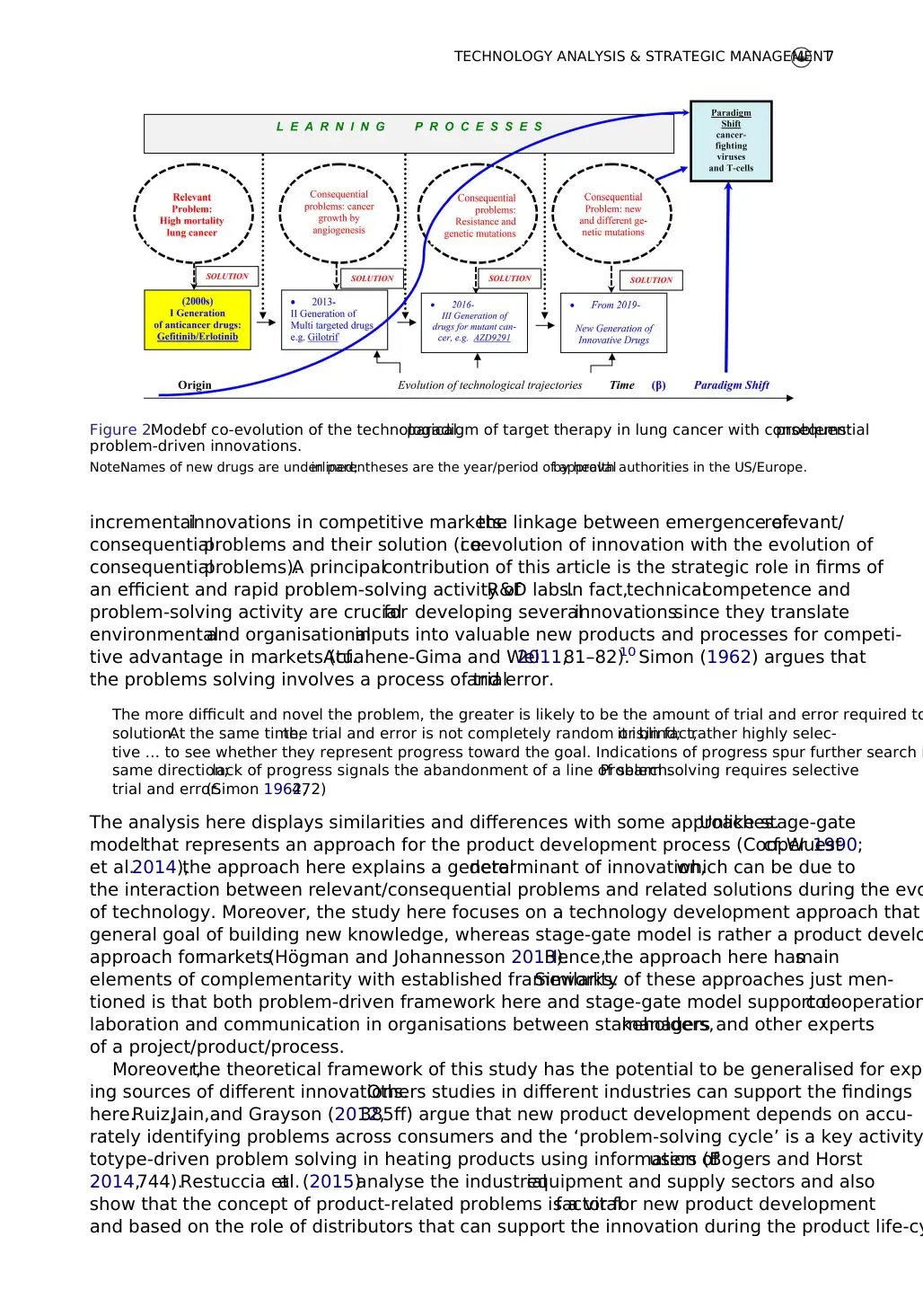
spective goal of a (temporary) monopoly of profits in markets. Figure 2 shows the evolution of
target therapies for lung cancer as hypothesised.This hypothesis of problem-driven innovation,
described here,can explain one ofthe determinants ofradicaland incrementalinnovations in
drug discovery industry.In addition, this conceptualframework hasalso the potentialto
explain a generalsource ofinnovation that supports the industrialand corporate change over
the long run.
Discussion
Sources of innovation in different industries are hardly known. This study endeavors, wheneve
ible, to show thatradical and incremental innovations can be driven by a co-evolution of consequ
tialproblems and problem-solving activities during the evolution of technology.
The analysis of underlying determinants of major and minor technologicalbreakthroughs in
anticancer drugs is a complex task but it is very important to explain some general driving for
of technological change (Coccia 2012c;Gelijns and Rosenberg 1994,30ff;Rosenberg,Gelijns,and
Dawkins 1995).This study suggests a modelthat explains one ofthe sources ofradicaland
6 M.COCCIA
types of cancer (Clovis Oncology 2015).The first and second generation of target therapy is orig-
inally designed to targetwild-type EGFR,whereas new targettherapiesin lung cancerare
designed for EGFR-mutant lung cancer.Clovis Oncology (a smallUS bio-pharmaceuticalfirm) is
developing some of these new drugs such as Rociletinib for the treatment of mutant non-sm
cell lung cancer.In particular,Rociletinib is a novel,oral,targeted covalent (irreversible) inhibitor
to selectively target both the initial activating EGFR mutations and the T790M resistance m
with an improved toxicity profile.Accordingly,it has the potentialto be a first-line treatment in
NSCLC patients with activating EGFR mutations and a second or later-line treatment in NSC
patients who become resistant to previous therapies due to the emergence of the T790M se
ary mutation (Clovis Oncology 2014, 2015). The biopharmaceutical company (AstraZeneca
2015b) has generated a similar selective and irreversible inhibitor for mutant lung cancer: t
anticancer drug is called TAGRISSO™—osimertinib (AZD9291) and wasapproved by US FDA in
2015.
. Future technological trajectories in lung cancer treatments and the potential emergence of
logicalparadigm shift.New generation oftargettherapiestreatsEGFR-mutantlung cancer.
However,Thress et al.(2015,560) analyze the new anticancer therapy AZD9291 by AstraZeneca
for EGFR-mutant lung cancer (a sub type of non-smallcell lung cancer) and show new problems
that will affect the evolution of these innovative target therapies,that is:‘diversity of mechanisms
through which tumors acquire resistance to AZD9291 and highlight the need for therapies t
able to overcome resistance mediated by the EGFRC 797S mutation’.Moreover,cutting-edge
research is opening new scientific frontiers in treatments of oncology by developing therapi
based cancer-fighting viruses and T cells that can generate a technological paradigm shift i
ancer drugs (cf.Ledford 2015).
Overall, the main evidence of this case study above seems in general to support the hypoth
of problem-driven innovation that the origin and development of innovation (with technologica
paradigms,different technological trajectories and paradigm shifts as well) can be explained by
coevolution of consequential problems and problem-solving activity during the evolution of te
nology, ceteris paribus. The innovative anticancer drugs are highly directional and aimed at so
specific problems.In particular,new target therapies embody the development of technological
solutions based on learning processes that generate a vital cumulative change for new techno
cal pathways directed to support the competitive advantage of firms in dynamic markets.In fact,
leading firms,in innovation-based markets – as in the drug-discovery industry – have the chief
incentive to find innovative solutions/products for unsolved problems in order to achieve the p
spective goal of a (temporary) monopoly of profits in markets. Figure 2 shows the evolution of
target therapies for lung cancer as hypothesised.This hypothesis of problem-driven innovation,
described here,can explain one ofthe determinants ofradicaland incrementalinnovations in
drug discovery industry.In addition, this conceptualframework hasalso the potentialto
explain a generalsource ofinnovation that supports the industrialand corporate change over
the long run.
Discussion
Sources of innovation in different industries are hardly known. This study endeavors, wheneve
ible, to show thatradical and incremental innovations can be driven by a co-evolution of consequ
tialproblems and problem-solving activities during the evolution of technology.
The analysis of underlying determinants of major and minor technologicalbreakthroughs in
anticancer drugs is a complex task but it is very important to explain some general driving for
of technological change (Coccia 2012c;Gelijns and Rosenberg 1994,30ff;Rosenberg,Gelijns,and
Dawkins 1995).This study suggests a modelthat explains one ofthe sources ofradicaland
6 M.COCCIA
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
incrementalinnovations in competitive markets:the linkage between emergence ofrelevant/
consequentialproblems and their solution (i.e.coevolution of innovation with the evolution of
consequentialproblems).A principalcontribution of this article is the strategic role in firms of
an efficient and rapid problem-solving activity ofR&D labs.In fact,technicalcompetence and
problem-solving activity are crucialfor developing severalinnovationssince they translate
environmentaland organisationalinputs into valuable new products and processes for competi-
tive advantage in markets (cf.Atuahene-Gima and Wei2011,81–82).10 Simon (1962) argues that
the problems solving involves a process of trialand error.
The more difficult and novel the problem, the greater is likely to be the amount of trial and error required to
solution.At the same time,the trial and error is not completely random or blind;it is,in fact,rather highly selec-
tive … to see whether they represent progress toward the goal. Indications of progress spur further search i
same direction;lack of progress signals the abandonment of a line of search.Problem solving requires selective
trial and error.(Simon 1962,472)
The analysis here displays similarities and differences with some approaches.Unlike stage-gate
modelthat represents an approach for the product development process (Cooper 1990;cf.Wuest
et al.2014),the approach here explains a generaldeterminant of innovation,which can be due to
the interaction between relevant/consequential problems and related solutions during the evo
of technology. Moreover, the study here focuses on a technology development approach that
general goal of building new knowledge, whereas stage-gate model is rather a product develo
approach formarkets(Högman and Johannesson 2013).Hence,the approach here hasmain
elements of complementarity with established frameworks.Similarity of these approaches just men-
tioned is that both problem-driven framework here and stage-gate model support cooperationcol-
laboration and communication in organisations between stakeholders,managers and other experts
of a project/product/process.
Moreover,the theoretical framework of this study has the potential to be generalised for expl
ing sources of different innovations.Others studies in different industries can support the findings
here.Ruiz,Jain,and Grayson (2012,385ff) argue that new product development depends on accu-
rately identifying problems across consumers and the ‘problem-solving cycle’ is a key activity
totype-driven problem solving in heating products using information ofusers (Bogers and Horst
2014,744).Restuccia etal. (2015)analyse the industrialequipment and supply sectors and also
show that the concept of product-related problems is a vitalfactor for new product development
and based on the role of distributors that can support the innovation during the product life-cy
Figure 2.Modelof co-evolution of the technologicalparadigm of target therapy in lung cancer with consequentialproblems:
problem-driven innovations.
Note:Names of new drugs are underlined;in parentheses are the year/period of approvalby health authorities in the US/Europe.
TECHNOLOGY ANALYSIS & STRATEGIC MANAGEMENT7
consequentialproblems and their solution (i.e.coevolution of innovation with the evolution of
consequentialproblems).A principalcontribution of this article is the strategic role in firms of
an efficient and rapid problem-solving activity ofR&D labs.In fact,technicalcompetence and
problem-solving activity are crucialfor developing severalinnovationssince they translate
environmentaland organisationalinputs into valuable new products and processes for competi-
tive advantage in markets (cf.Atuahene-Gima and Wei2011,81–82).10 Simon (1962) argues that
the problems solving involves a process of trialand error.
The more difficult and novel the problem, the greater is likely to be the amount of trial and error required to
solution.At the same time,the trial and error is not completely random or blind;it is,in fact,rather highly selec-
tive … to see whether they represent progress toward the goal. Indications of progress spur further search i
same direction;lack of progress signals the abandonment of a line of search.Problem solving requires selective
trial and error.(Simon 1962,472)
The analysis here displays similarities and differences with some approaches.Unlike stage-gate
modelthat represents an approach for the product development process (Cooper 1990;cf.Wuest
et al.2014),the approach here explains a generaldeterminant of innovation,which can be due to
the interaction between relevant/consequential problems and related solutions during the evo
of technology. Moreover, the study here focuses on a technology development approach that
general goal of building new knowledge, whereas stage-gate model is rather a product develo
approach formarkets(Högman and Johannesson 2013).Hence,the approach here hasmain
elements of complementarity with established frameworks.Similarity of these approaches just men-
tioned is that both problem-driven framework here and stage-gate model support cooperationcol-
laboration and communication in organisations between stakeholders,managers and other experts
of a project/product/process.
Moreover,the theoretical framework of this study has the potential to be generalised for expl
ing sources of different innovations.Others studies in different industries can support the findings
here.Ruiz,Jain,and Grayson (2012,385ff) argue that new product development depends on accu-
rately identifying problems across consumers and the ‘problem-solving cycle’ is a key activity
totype-driven problem solving in heating products using information ofusers (Bogers and Horst
2014,744).Restuccia etal. (2015)analyse the industrialequipment and supply sectors and also
show that the concept of product-related problems is a vitalfactor for new product development
and based on the role of distributors that can support the innovation during the product life-cy
Figure 2.Modelof co-evolution of the technologicalparadigm of target therapy in lung cancer with consequentialproblems:
problem-driven innovations.
Note:Names of new drugs are underlined;in parentheses are the year/period of approvalby health authorities in the US/Europe.
TECHNOLOGY ANALYSIS & STRATEGIC MANAGEMENT7

management.Critical problem-solving activity is also present in the semiconductor industry and
associated with the main variable of speed because in this specific industry,expeditious problem
solving ofR&D lab is an importantperformance goalto supporttechnologicalinnovations with
short market life cycles (Appleyard, Brown, and Sattler 2006). Macher and Mowery (2003), in s
ductormanufacturing,also find thatthe allocation ofengineering resources to problem-solving
activities,associated with information technologyand scheduleproduction,influencesnew
process technologies and manufacturing performance (forpublic research labs cf.Coccia 2001a,
2003;Coccia and Rolfo,2002).
In general,problem-solving competence is an important factor to develop in R&D labs to sust
innovation and competitive advantage of firms.In particular,an efficient R&D management of firms
depends on the ability to speed up the activities of solving complex problems in the presence
environmentalturbulence (cf.Atuahene-Gima and Wei2011).In short,the approach ofproblem-
driven innovation here seems to be a comprehensive framework with the potentialof explaining
one of the generalsources of technicalchange over time and space.
However,innovations are due to manifold factors.For instance,the learning process between
clinicalresearch and clinicalpractice in drug discovery industry is also a vitalfactor that sup-
ports innovative products with the accumulation and advancement oftechnicalknowledge in
specific research fields (cf.Gelijns and Rosenberg 1995b,4ff; Gershon 1998;Kim and Nelson
2000;Morlacchiand Nelson 2011;Gelijns and Rosenberg 1995b,67).Anotherfactorfor the
developmentof innovation isthe dynamic capability:‘the firm’sability to integrate,build,
and reconfigure internaland externalcompetences to address rapidly changing environments’
(Teece,Pisano,and Shuen 1997,516;Helfat et al.2007,4; Coccia 2014b).Coccia (2016c) shows
that the technologicalevolution can be also due to technologicalparasitism and symbiotic
interaction between technologies.Innovations are also due to organisationallearning,which
is a strategic process forcompetitive advantage offirms (Vera and Crossan 2004).Moreover,
managers with strategic leadership play a vitalrole for innovation processes offirms because
they inspire others with theirvision,create excitementin groups and provide incentives for
achieving goals in competitive environments (Bass and Avolio 1990).Finally,new technology
can be also due to ‘inventive analogical transfer’from experience and solutions of consequential
problems in one knowledge field – source domain – to other fields – target domains (Kalogerak
Lüthje,and Herstatt 2010,418).
Concluding observations
The high mortality rate of lung cancer is a major unsolved problem that generates a main imp
firms in drug-discovery industry – characterised by technologicaland marketdynamisms – to
develop path-breaking innovations of anticancer drugs.The inductive study here seems in general
to support the hypothesis that sources of radical and incremental innovations can be also exp
by a coevolution between relevant/consequentialproblems and problem-solving activities during
the evolution oftechnology,ceteris paribus.These findings seem to be also confirmed by other
studies,such as Coccia and Wang (2015,155ff) show that:
the sharp increase of several technological trajectories of anticancer drugs applied by nanotechnology seem
be driven by high rates of mortality of some types of cancers (e.g.pancreatic and brain) in order to find more
effective anticancer therapies that increase the progression-free survivalof patients.
These ‘technological trajectories mortality driven’ are problem-driven by high mortality in pan
and brain cancer.In short,relevant and consequentialproblems seem to be a main and general
driving force for the evolution of innovation in several industries. In fact, Roche (2015), a mult
health-care company,claims that the research process has to find:‘innovative solutions for serious,
currently unsolved medicalproblems’.Hence,leading firms in the drug-discovery industry have a
main incentive to find innovative solutions/products forunsolved problems in orderto achieve
8 M.COCCIA
associated with the main variable of speed because in this specific industry,expeditious problem
solving ofR&D lab is an importantperformance goalto supporttechnologicalinnovations with
short market life cycles (Appleyard, Brown, and Sattler 2006). Macher and Mowery (2003), in s
ductormanufacturing,also find thatthe allocation ofengineering resources to problem-solving
activities,associated with information technologyand scheduleproduction,influencesnew
process technologies and manufacturing performance (forpublic research labs cf.Coccia 2001a,
2003;Coccia and Rolfo,2002).
In general,problem-solving competence is an important factor to develop in R&D labs to sust
innovation and competitive advantage of firms.In particular,an efficient R&D management of firms
depends on the ability to speed up the activities of solving complex problems in the presence
environmentalturbulence (cf.Atuahene-Gima and Wei2011).In short,the approach ofproblem-
driven innovation here seems to be a comprehensive framework with the potentialof explaining
one of the generalsources of technicalchange over time and space.
However,innovations are due to manifold factors.For instance,the learning process between
clinicalresearch and clinicalpractice in drug discovery industry is also a vitalfactor that sup-
ports innovative products with the accumulation and advancement oftechnicalknowledge in
specific research fields (cf.Gelijns and Rosenberg 1995b,4ff; Gershon 1998;Kim and Nelson
2000;Morlacchiand Nelson 2011;Gelijns and Rosenberg 1995b,67).Anotherfactorfor the
developmentof innovation isthe dynamic capability:‘the firm’sability to integrate,build,
and reconfigure internaland externalcompetences to address rapidly changing environments’
(Teece,Pisano,and Shuen 1997,516;Helfat et al.2007,4; Coccia 2014b).Coccia (2016c) shows
that the technologicalevolution can be also due to technologicalparasitism and symbiotic
interaction between technologies.Innovations are also due to organisationallearning,which
is a strategic process forcompetitive advantage offirms (Vera and Crossan 2004).Moreover,
managers with strategic leadership play a vitalrole for innovation processes offirms because
they inspire others with theirvision,create excitementin groups and provide incentives for
achieving goals in competitive environments (Bass and Avolio 1990).Finally,new technology
can be also due to ‘inventive analogical transfer’from experience and solutions of consequential
problems in one knowledge field – source domain – to other fields – target domains (Kalogerak
Lüthje,and Herstatt 2010,418).
Concluding observations
The high mortality rate of lung cancer is a major unsolved problem that generates a main imp
firms in drug-discovery industry – characterised by technologicaland marketdynamisms – to
develop path-breaking innovations of anticancer drugs.The inductive study here seems in general
to support the hypothesis that sources of radical and incremental innovations can be also exp
by a coevolution between relevant/consequentialproblems and problem-solving activities during
the evolution oftechnology,ceteris paribus.These findings seem to be also confirmed by other
studies,such as Coccia and Wang (2015,155ff) show that:
the sharp increase of several technological trajectories of anticancer drugs applied by nanotechnology seem
be driven by high rates of mortality of some types of cancers (e.g.pancreatic and brain) in order to find more
effective anticancer therapies that increase the progression-free survivalof patients.
These ‘technological trajectories mortality driven’ are problem-driven by high mortality in pan
and brain cancer.In short,relevant and consequentialproblems seem to be a main and general
driving force for the evolution of innovation in several industries. In fact, Roche (2015), a mult
health-care company,claims that the research process has to find:‘innovative solutions for serious,
currently unsolved medicalproblems’.Hence,leading firms in the drug-discovery industry have a
main incentive to find innovative solutions/products forunsolved problems in orderto achieve
8 M.COCCIA
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

the goal of a (temporary)profit monopolyin a Schumpeterian world ofinnovation-based
competition.
The hypothesis of problem-driven innovation can explain a criticaldriving force of severalinno-
vations in drug discovery industry and has also the potentialto explain one of the generalsources
of the technologicalchange.Overall,the conceptualframework here contributesto integrate
currentapproaches ofthe sources ofinnovation in economics and managementof technology
(Dixon 1997;Ruttan 1997;von Tunzelmann et al.2008).In particular,
(1) The conceptualframework assigns a centralrole to relevantproblems and theirsolution to
explain the emergence of path-breaking innovations that sustain the industrialchange;
(2) The conceptual framework here is able to explain the development of technological traject
by solutions of consequential problems of initial radical innovation, based on learning proc
and act of insights in R&D labs of firms;
(3) Finally,the conceptualframework here is also able to show the vitalfunction of the problem-
solving activity in the R&D management directed to solve problems that induces radicaland
incrementalinnovation for sustaining and safeguarding extant competitive advantage of firm
in environment characterised by technologicaland market dynamism.
Hence, the conceptual framework here, substantiated in drug discovery industry, has sever
ponents of generalisation that could easily be extended to explain the evolution of new techno
across several industries for supporting industrialand corporate change.
However, these results are of course tentative because this study provides a preliminary an
some sources of specific radical/incremental innovations in markets with high technological co
tition.In fact,identifying the determinants ofradicalinnovations in drug discovery industry is a
complex and problematic matter,since we know that other things are often not equal.To conclude,
this study shows that the co-evolution of consequentialproblems and problem solving activities in
R&D labs of firms can be a main source of innovation, but Wright (1997, 1562) properly claims
world of technologicalchange,bounded rationality is the rule.’
Notes
1. This research began in 2014 at the UNU-MERIT (The Netherlands) and is further developed in 2015 and 2
Arizona State University while Iam a visiting scholar funded by NationalResearch Councilof Italy.This paper
benefited from helpfulcomments and suggestions by Christopher S.Hayter and two anonymous referees.The
author declares that he has no relevant or materialfinancialinterests that relate to the research discussed in
this paper.
2. Cf.Coccia (2004,2014b,2014e,2006b,2010b,2015b,2015c,2016b) and Coccia,Finardi,and Margon (2012).
3. Dosi (1982, 152, original emphasis) posits that ‘“technological paradigm” as “model” and a “pattern” of
selected technologicalproblems based on selected principles derived from naturalsciences and on selected
material technologies’(cf.Dosi,1988).
4. Age-standardized rate (W) is the rate that a population would have if it had a standard age structure.Standard-
ization is necessary when comparing several populations that differ with respect to age because age has
fulinfluence on the risk of cancer (GLOBOCAN,2012,http://globocan.iarc.fr/ –accessed February 2015).
5. Cf.Afshar (2003) and Fraser and Pai(2014).For countries with high R&D investment,see Coccia (2005,2007,
2008a,2008b,2009c,2009d,2010a,2010c,2013b,2015b);Rolfo and Coccia (2005).
6. An exon is the portion of a gene that codes for amino acids.
7. ‘A characteristic that is objectively measured and evaluated as an indicator of normal biological processe
genic processes, or pharmacologic responses to therapeutic intervention’ (National Institute of Health, as
by Amir-Aslani and Mangematin 2010,204)
8. The literature is vast and not fully cited here,but a good list of references is found in Dempke,Sutob,and Reck
(2010,262–263,271–274) and Coccia (2012d,2014d).
9. The evolution of technological paradigms is also based on developing new technological trajectories by ‘
tive analogicaltransfer’from experience and solutions in one knowledge field—source domain e.g.a type of
cancer—to solve new problems in other fields -target domains e.g. other cancers (cf. Kalogerakis, Lüthje,
statt 2010,418).
TECHNOLOGY ANALYSIS & STRATEGIC MANAGEMENT9
competition.
The hypothesis of problem-driven innovation can explain a criticaldriving force of severalinno-
vations in drug discovery industry and has also the potentialto explain one of the generalsources
of the technologicalchange.Overall,the conceptualframework here contributesto integrate
currentapproaches ofthe sources ofinnovation in economics and managementof technology
(Dixon 1997;Ruttan 1997;von Tunzelmann et al.2008).In particular,
(1) The conceptualframework assigns a centralrole to relevantproblems and theirsolution to
explain the emergence of path-breaking innovations that sustain the industrialchange;
(2) The conceptual framework here is able to explain the development of technological traject
by solutions of consequential problems of initial radical innovation, based on learning proc
and act of insights in R&D labs of firms;
(3) Finally,the conceptualframework here is also able to show the vitalfunction of the problem-
solving activity in the R&D management directed to solve problems that induces radicaland
incrementalinnovation for sustaining and safeguarding extant competitive advantage of firm
in environment characterised by technologicaland market dynamism.
Hence, the conceptual framework here, substantiated in drug discovery industry, has sever
ponents of generalisation that could easily be extended to explain the evolution of new techno
across several industries for supporting industrialand corporate change.
However, these results are of course tentative because this study provides a preliminary an
some sources of specific radical/incremental innovations in markets with high technological co
tition.In fact,identifying the determinants ofradicalinnovations in drug discovery industry is a
complex and problematic matter,since we know that other things are often not equal.To conclude,
this study shows that the co-evolution of consequentialproblems and problem solving activities in
R&D labs of firms can be a main source of innovation, but Wright (1997, 1562) properly claims
world of technologicalchange,bounded rationality is the rule.’
Notes
1. This research began in 2014 at the UNU-MERIT (The Netherlands) and is further developed in 2015 and 2
Arizona State University while Iam a visiting scholar funded by NationalResearch Councilof Italy.This paper
benefited from helpfulcomments and suggestions by Christopher S.Hayter and two anonymous referees.The
author declares that he has no relevant or materialfinancialinterests that relate to the research discussed in
this paper.
2. Cf.Coccia (2004,2014b,2014e,2006b,2010b,2015b,2015c,2016b) and Coccia,Finardi,and Margon (2012).
3. Dosi (1982, 152, original emphasis) posits that ‘“technological paradigm” as “model” and a “pattern” of
selected technologicalproblems based on selected principles derived from naturalsciences and on selected
material technologies’(cf.Dosi,1988).
4. Age-standardized rate (W) is the rate that a population would have if it had a standard age structure.Standard-
ization is necessary when comparing several populations that differ with respect to age because age has
fulinfluence on the risk of cancer (GLOBOCAN,2012,http://globocan.iarc.fr/ –accessed February 2015).
5. Cf.Afshar (2003) and Fraser and Pai(2014).For countries with high R&D investment,see Coccia (2005,2007,
2008a,2008b,2009c,2009d,2010a,2010c,2013b,2015b);Rolfo and Coccia (2005).
6. An exon is the portion of a gene that codes for amino acids.
7. ‘A characteristic that is objectively measured and evaluated as an indicator of normal biological processe
genic processes, or pharmacologic responses to therapeutic intervention’ (National Institute of Health, as
by Amir-Aslani and Mangematin 2010,204)
8. The literature is vast and not fully cited here,but a good list of references is found in Dempke,Sutob,and Reck
(2010,262–263,271–274) and Coccia (2012d,2014d).
9. The evolution of technological paradigms is also based on developing new technological trajectories by ‘
tive analogicaltransfer’from experience and solutions in one knowledge field—source domain e.g.a type of
cancer—to solve new problems in other fields -target domains e.g. other cancers (cf. Kalogerakis, Lüthje,
statt 2010,418).
TECHNOLOGY ANALYSIS & STRATEGIC MANAGEMENT9
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10. Cf.also Coccia 2001b,2006a,2008,2009a,2009e;Coccia and Cadario 2014;Coccia and Rolfo 2007,2013;Coccia,
Falavigna, and Manello 2015; for public research labs see also Coccia 2001a, 2003; Coccia and Rolfo 199
Notes on contributor
Mario Coccia is a Senior researcher at the NationalResearch Councilof Italy and Visiting Scholar at the Arizona State
University (Center for social dynamics and complexity). He has been Research Fellow at the Max Planck Institu
omics,Visiting Professor at the Polytechnics of Torino and University of Piemonte Orientale (Italy).He has conducted
research work at the Georgia Institute of Technology,Yale University,United Nations University – MERIT,University of
Maryland,Bureau d’Économie Théorique et Appliquée,University of Toronto,RAND Corporations and University of Bie-
lefeld. He has written extensively more than 280 papers in economics of science and technology, R&D manage
related disciplines.
ORCID
Coccia Mario http://orcid.org/0000-0003-1957-6731
References
Ackoff,R.L.1971.“Towards a System of Systems Concepts.” Management Science 17 (11):661–671.
Afshar,M. 2003.“From Genes to Products:Innovations in Drug Discovery.” Drug Discovery Today 8 (9):392–394.
Ambartsoumian,V.,J. Dhaliwal,E.Lee,T.Meservy,and C.Zhang.2011.“Implementing Quality Gates Throughout the
Enterprise it Production Process.” Journalof Information Technology Management 22 XXII (1):28–38.
Amir-Aslani,A.,and V.Mangematin.2010.“The Future of Drug Discovery and Development:Shifting Emphasis Towards
Personalized Medicine.” Technology Forecasting & SocialChange 77 (2):203–217.
Appleyard,M. M.,C.Brown,and L.Sattler.2006.“An International Investigation of Problem-Solving Performance in the
Semiconductor Industry.” Journalof Product Innovation Management 23 (2):147–167.
Arthur, W. B. 1989. “Competing Technologies, Increasing Returns, and Lock-In by Historical Events.” The Econo
99 (394):116–131.
AstraZeneca.2015a.Accessed April,2015.http://www.astrazeneca.com/Research/areas-of-interest.
AstraZeneca.2015b.“Openinnovation.” Accessed October1. http://openinnovation.astrazeneca.com/what-we-offer/
compound/azd9291/.
Atuahene-Gima, K., and Y. Wei. 2011. “The Vital Role of Problem-Solving Competence in New Product Success.
Product Innovation Management 28 (1):81–98.
Bass, B. M., and B. J. Avolio. 1990. “The Implications of Transactional and Transformational Leadership for Indiv
and Organizational Development.” In Research in Organizational Change and Development, edited by B. M.
L.Cummings,4 Vols,231–272.Greenwich,CT:JAI Press.
Boehringer-Ingelheim.2015.Accessed April,2015.https://www.boehringer-ingelheim.com/research_development/
drug_discovery/drug_discovery_process.html.
Bogers, M., and W. Horst. 2014. “Collaborative Prototyping: Cross-Fertilization of Knowledge in Prototype-Drive
Solving.” Journalof Product Innovation Management 31 (4):744–764.
Brugger, W., J. H. Kim, O. Hansen, and N. Triller. 2009. “Molecular Markers and Clinical Outcome with Erlotinib:
the Phase III Placebo-Controlled SATURN Study of Maintenance Therapy for Advanced NSCLC.” WCLC 2009:
Francisco,CA.
Buonanno,P.,and M.Ranzani.2013.“Thank you for not Smoking:Evidence from the Italian Smoking Ban.” Health Policy
109 (2):192–199.
Calabrese,G.,M.Coccia,and S.Rolfo.2005.“Strategy and Market Management of New Product Development:Evidence
from Italian SMEs.” InternationalJournalof Product Development 2 (1/2):170–189.
Cavallo, E., E. Ferrari, L. Bollani, and M. Coccia. 2014a. “Attitudes and Behaviour of Adopters of Technological I
in AgriculturalTractors:A Case Study in Italian AgriculturalSystem.” AgriculturalSystems 130:44–54.
Cavallo,E., E. Ferrari,L. Bollani,and M. Coccia.2014b.“Strategic ManagementImplicationsfor the Adoption of
TechnologicalInnovationsin AgriculturalTractor:The Role of Scale Factorsand EnvironmentalAttitude.”
Technology Analysis & Strategic Management 26 (7):765–779.
Cavallo,E.,E. Ferrari,and M. Coccia.2015.“Likely TechnologicalTrajectories in AgriculturalTractors by Analysing
Innovative Attitudes of Farmers.” InternationalJournalof Technology,Policy and Management 15 (2):158–177.
Christensen, C. 1997. The Innovator’s Dilemma: When New Technologies Cause Great Firms to Fail. Cambridge
Business SchoolPress.
Clovis Oncology.2014.“Phase 1 Evaluation of CO-1686,an Irreversible,Mutant-Selective Inhibitor of EGFR Mutations
(activating and T790M).” Paper presented at the 4th European Lung Cancer Conference,Geneva, Switzerland, March.
10 M.COCCIA
Falavigna, and Manello 2015; for public research labs see also Coccia 2001a, 2003; Coccia and Rolfo 199
Notes on contributor
Mario Coccia is a Senior researcher at the NationalResearch Councilof Italy and Visiting Scholar at the Arizona State
University (Center for social dynamics and complexity). He has been Research Fellow at the Max Planck Institu
omics,Visiting Professor at the Polytechnics of Torino and University of Piemonte Orientale (Italy).He has conducted
research work at the Georgia Institute of Technology,Yale University,United Nations University – MERIT,University of
Maryland,Bureau d’Économie Théorique et Appliquée,University of Toronto,RAND Corporations and University of Bie-
lefeld. He has written extensively more than 280 papers in economics of science and technology, R&D manage
related disciplines.
ORCID
Coccia Mario http://orcid.org/0000-0003-1957-6731
References
Ackoff,R.L.1971.“Towards a System of Systems Concepts.” Management Science 17 (11):661–671.
Afshar,M. 2003.“From Genes to Products:Innovations in Drug Discovery.” Drug Discovery Today 8 (9):392–394.
Ambartsoumian,V.,J. Dhaliwal,E.Lee,T.Meservy,and C.Zhang.2011.“Implementing Quality Gates Throughout the
Enterprise it Production Process.” Journalof Information Technology Management 22 XXII (1):28–38.
Amir-Aslani,A.,and V.Mangematin.2010.“The Future of Drug Discovery and Development:Shifting Emphasis Towards
Personalized Medicine.” Technology Forecasting & SocialChange 77 (2):203–217.
Appleyard,M. M.,C.Brown,and L.Sattler.2006.“An International Investigation of Problem-Solving Performance in the
Semiconductor Industry.” Journalof Product Innovation Management 23 (2):147–167.
Arthur, W. B. 1989. “Competing Technologies, Increasing Returns, and Lock-In by Historical Events.” The Econo
99 (394):116–131.
AstraZeneca.2015a.Accessed April,2015.http://www.astrazeneca.com/Research/areas-of-interest.
AstraZeneca.2015b.“Openinnovation.” Accessed October1. http://openinnovation.astrazeneca.com/what-we-offer/
compound/azd9291/.
Atuahene-Gima, K., and Y. Wei. 2011. “The Vital Role of Problem-Solving Competence in New Product Success.
Product Innovation Management 28 (1):81–98.
Bass, B. M., and B. J. Avolio. 1990. “The Implications of Transactional and Transformational Leadership for Indiv
and Organizational Development.” In Research in Organizational Change and Development, edited by B. M.
L.Cummings,4 Vols,231–272.Greenwich,CT:JAI Press.
Boehringer-Ingelheim.2015.Accessed April,2015.https://www.boehringer-ingelheim.com/research_development/
drug_discovery/drug_discovery_process.html.
Bogers, M., and W. Horst. 2014. “Collaborative Prototyping: Cross-Fertilization of Knowledge in Prototype-Drive
Solving.” Journalof Product Innovation Management 31 (4):744–764.
Brugger, W., J. H. Kim, O. Hansen, and N. Triller. 2009. “Molecular Markers and Clinical Outcome with Erlotinib:
the Phase III Placebo-Controlled SATURN Study of Maintenance Therapy for Advanced NSCLC.” WCLC 2009:
Francisco,CA.
Buonanno,P.,and M.Ranzani.2013.“Thank you for not Smoking:Evidence from the Italian Smoking Ban.” Health Policy
109 (2):192–199.
Calabrese,G.,M.Coccia,and S.Rolfo.2005.“Strategy and Market Management of New Product Development:Evidence
from Italian SMEs.” InternationalJournalof Product Development 2 (1/2):170–189.
Cavallo, E., E. Ferrari, L. Bollani, and M. Coccia. 2014a. “Attitudes and Behaviour of Adopters of Technological I
in AgriculturalTractors:A Case Study in Italian AgriculturalSystem.” AgriculturalSystems 130:44–54.
Cavallo,E., E. Ferrari,L. Bollani,and M. Coccia.2014b.“Strategic ManagementImplicationsfor the Adoption of
TechnologicalInnovationsin AgriculturalTractor:The Role of Scale Factorsand EnvironmentalAttitude.”
Technology Analysis & Strategic Management 26 (7):765–779.
Cavallo,E.,E. Ferrari,and M. Coccia.2015.“Likely TechnologicalTrajectories in AgriculturalTractors by Analysing
Innovative Attitudes of Farmers.” InternationalJournalof Technology,Policy and Management 15 (2):158–177.
Christensen, C. 1997. The Innovator’s Dilemma: When New Technologies Cause Great Firms to Fail. Cambridge
Business SchoolPress.
Clovis Oncology.2014.“Phase 1 Evaluation of CO-1686,an Irreversible,Mutant-Selective Inhibitor of EGFR Mutations
(activating and T790M).” Paper presented at the 4th European Lung Cancer Conference,Geneva, Switzerland, March.
10 M.COCCIA

ClovisOncology.2015.Accessed February,2015.http://www.clovisoncology.com/products-companion-diagnostics/
rociletinib/.
Coccia M.2001a.“A Tool for Measuring the Performance in Research Organizations.” In Technology Management
Knowledge Era,edited by D.F.Kocaoglu and T.R.Anderson,160–168.Piscatawey,NJ: IEEE Operations Center.
Coccia,M. 2001b.“Satisfaction,Work Involvement and R&D Performance.” InternationalJournalof Human Resources
Development and Management 1 (2–4):268–282.
Coccia M. 2003. “Metrics of R&D Performance and Management of Public Research Institute.” Proceedings of IE
Piscataway,231–236.
Coccia, M. 2004. “Spatial Metrics of the Technological Transfer: Analysis and Strategic Management.” Technolo
& Strategic Management 16 (1):31–51.
Coccia,M. 2005.“Countrymetrics:Valutazione Della Performance Economica e tecnologica dei paesi e posizionament
dell’Italia.” Rivista Internazionale diScienze SocialiCXIII(3/2005):377–412.
Coccia,M. 2006a.“Analysis and Classification ofPublic Research Institutes.” World Review ofScience,Technology and
Sustainable Development 3 (1):1–16.
Coccia,M. 2006b.“Classifications ofInnovations:Survey and Future Directions.”Working PaperCerisdel Consiglio
Nazionale delle Ricerche,Anno VIII,n. 2.ISSN (Print):1591-0709.
Coccia, M. 2007.“A New Taxonomy of Country Performance and Risk Based on Economic and Technological Indica
Journalof Applied Economics 10 (1):29–42.
Coccia, M. 2008. “New Organizational Behaviour of Public Research Institutions: Lessons Learned from Italian C
InternationalJournalof Business Innovation and Research 2(4):402–419.
Coccia,M. 2008a.“Science,Funding and Economic Growth:Analysis and Science Policy Implications.” World Review of
Science,Technology and Sustainable Development 5 (1):1–27.
Coccia M.2008b.“Investimento pubblico e privato in R&S:complementarietà ed interazione con la crescita della
produttività.” Economia e Politica Industriale 34 (3):127–154.
Coccia, M. 2009a.“Bureaucratization in Public Research Institutions.” Minerva, A Review of Science, Learning and
(1):31–50.
Coccia,M. 2009b.“Measuring the Impact of Sustainable TechnologicalInnovation.” InternationalJournalof Technology
Intelligence and Planning 5 (3):276–288.
Coccia,M. 2009c.“Whatis the OptimalRate ofR&D Investmentto Maximize Productivity Growth?”Technological
Forecasting & SocialChange 76 (3):433–446.
Coccia M.2009d.“A new Approach for Measuring and Analyzing Patterns ofRegionalEconomic Growth:Empirical
Analysis in Italy.” Italian Journalof RegionalScience- Scienze Regionali8 (2):71–95.
Coccia M.2009e.“Research Performance and Bureaucracy Within Public Research Labs.” Scientometrics 79 (1):93–107.
Coccia,M. 2010a.“Foresight of TechnologicalDeterminants and Primary Energy Resources of Future Economic Long
Waves.” InternationalJournalof Foresight and Innovation Policy 6 (4):225–232.
Coccia, M. 2010b. “Energy Metrics for Driving Competitiveness of Countries: Energy Weakness Magnitude, GDP
and Barrels Per Capita.” Energy Policy 38 (3):1330–1339.
Coccia,M.2010c.“Public and Private R&D Investments as Complementary Inputs for Productivity Growth.” Interna
Journalof Technology,Policy and Management 10 (1/2):73–91.
Coccia,M. 2012a.“Cartilage Tissue Engineering with Chondrogenic Cells Versus ArtificialJoint Replacement:The
Insurgence of New TechnologicalParadigms.” Health and Technology 2 (4):235–247.
Coccia, M. 2012b. “Converging Genetics, Genomics and Nanotechnologies for Groundbreaking Pathways in Bio
and Nanomedicine.” Int.J. Healthcare Technology and Management 13 (4):184–197.
Coccia, M. 2012c.“Driving Forces of Technological Change in Medicine: Radical Innovations Induced by Side Effect
their Impact on Society and Healthcare.” Technology in Society 34 (4),271–283.
Coccia,M. 2012d.“Evolutionary Growth of Knowledge in Path-Breaking Targeted Therapies for Lung Cancer:Radical
Innovations and Structure of the New TechnologicalParadigm.” InternationalJournalof Behaviouraland Healthcare
Research 3 (3–4):273–290.
Coccia,M. 2012e.“Evolutionary Trajectories ofthe Nanotechnology Research Across Worldwide Economic Players.”
Technology Analysis & Strategic Management 24 (10),1029–1050.
Coccia, M. 2013a. “The Effect of Country Wealth on Incidence of Breast Cancer.” Breast Cancer Research and T
(2):225–229.
Coccia, M. 2013b. “What are the Likely Interactions Among Innovation, Government Debt, and Employment? ” I
The European Journalof SocialScience Research 26 (4):456–471.
Coccia,M. 2014a.“Converging Scientific Fields and New TechnologicalParadigms as Main Drivers of the Division of
Scientific Labour in Drug Discovery Process:The Effects on Strategic Management of the R&D Corporate Change.”
Technology Analysis & Strategic Management 26 (7):733–749.
Coccia,M. 2014b. “Driving Forces of Technological Change: The Relation Between Population Growth and Techn
Innovation-Analysis of the OptimalInteraction Across Countries.” TechnologicalForecasting & SocialChange 82 (2):
52–65.
TECHNOLOGY ANALYSIS & STRATEGIC MANAGEMENT11
rociletinib/.
Coccia M.2001a.“A Tool for Measuring the Performance in Research Organizations.” In Technology Management
Knowledge Era,edited by D.F.Kocaoglu and T.R.Anderson,160–168.Piscatawey,NJ: IEEE Operations Center.
Coccia,M. 2001b.“Satisfaction,Work Involvement and R&D Performance.” InternationalJournalof Human Resources
Development and Management 1 (2–4):268–282.
Coccia M. 2003. “Metrics of R&D Performance and Management of Public Research Institute.” Proceedings of IE
Piscataway,231–236.
Coccia, M. 2004. “Spatial Metrics of the Technological Transfer: Analysis and Strategic Management.” Technolo
& Strategic Management 16 (1):31–51.
Coccia,M. 2005.“Countrymetrics:Valutazione Della Performance Economica e tecnologica dei paesi e posizionament
dell’Italia.” Rivista Internazionale diScienze SocialiCXIII(3/2005):377–412.
Coccia,M. 2006a.“Analysis and Classification ofPublic Research Institutes.” World Review ofScience,Technology and
Sustainable Development 3 (1):1–16.
Coccia,M. 2006b.“Classifications ofInnovations:Survey and Future Directions.”Working PaperCerisdel Consiglio
Nazionale delle Ricerche,Anno VIII,n. 2.ISSN (Print):1591-0709.
Coccia, M. 2007.“A New Taxonomy of Country Performance and Risk Based on Economic and Technological Indica
Journalof Applied Economics 10 (1):29–42.
Coccia, M. 2008. “New Organizational Behaviour of Public Research Institutions: Lessons Learned from Italian C
InternationalJournalof Business Innovation and Research 2(4):402–419.
Coccia,M. 2008a.“Science,Funding and Economic Growth:Analysis and Science Policy Implications.” World Review of
Science,Technology and Sustainable Development 5 (1):1–27.
Coccia M.2008b.“Investimento pubblico e privato in R&S:complementarietà ed interazione con la crescita della
produttività.” Economia e Politica Industriale 34 (3):127–154.
Coccia, M. 2009a.“Bureaucratization in Public Research Institutions.” Minerva, A Review of Science, Learning and
(1):31–50.
Coccia,M. 2009b.“Measuring the Impact of Sustainable TechnologicalInnovation.” InternationalJournalof Technology
Intelligence and Planning 5 (3):276–288.
Coccia,M. 2009c.“Whatis the OptimalRate ofR&D Investmentto Maximize Productivity Growth?”Technological
Forecasting & SocialChange 76 (3):433–446.
Coccia M.2009d.“A new Approach for Measuring and Analyzing Patterns ofRegionalEconomic Growth:Empirical
Analysis in Italy.” Italian Journalof RegionalScience- Scienze Regionali8 (2):71–95.
Coccia M.2009e.“Research Performance and Bureaucracy Within Public Research Labs.” Scientometrics 79 (1):93–107.
Coccia,M. 2010a.“Foresight of TechnologicalDeterminants and Primary Energy Resources of Future Economic Long
Waves.” InternationalJournalof Foresight and Innovation Policy 6 (4):225–232.
Coccia, M. 2010b. “Energy Metrics for Driving Competitiveness of Countries: Energy Weakness Magnitude, GDP
and Barrels Per Capita.” Energy Policy 38 (3):1330–1339.
Coccia,M.2010c.“Public and Private R&D Investments as Complementary Inputs for Productivity Growth.” Interna
Journalof Technology,Policy and Management 10 (1/2):73–91.
Coccia,M. 2012a.“Cartilage Tissue Engineering with Chondrogenic Cells Versus ArtificialJoint Replacement:The
Insurgence of New TechnologicalParadigms.” Health and Technology 2 (4):235–247.
Coccia, M. 2012b. “Converging Genetics, Genomics and Nanotechnologies for Groundbreaking Pathways in Bio
and Nanomedicine.” Int.J. Healthcare Technology and Management 13 (4):184–197.
Coccia, M. 2012c.“Driving Forces of Technological Change in Medicine: Radical Innovations Induced by Side Effect
their Impact on Society and Healthcare.” Technology in Society 34 (4),271–283.
Coccia,M. 2012d.“Evolutionary Growth of Knowledge in Path-Breaking Targeted Therapies for Lung Cancer:Radical
Innovations and Structure of the New TechnologicalParadigm.” InternationalJournalof Behaviouraland Healthcare
Research 3 (3–4):273–290.
Coccia,M. 2012e.“Evolutionary Trajectories ofthe Nanotechnology Research Across Worldwide Economic Players.”
Technology Analysis & Strategic Management 24 (10),1029–1050.
Coccia, M. 2013a. “The Effect of Country Wealth on Incidence of Breast Cancer.” Breast Cancer Research and T
(2):225–229.
Coccia, M. 2013b. “What are the Likely Interactions Among Innovation, Government Debt, and Employment? ” I
The European Journalof SocialScience Research 26 (4):456–471.
Coccia,M. 2014a.“Converging Scientific Fields and New TechnologicalParadigms as Main Drivers of the Division of
Scientific Labour in Drug Discovery Process:The Effects on Strategic Management of the R&D Corporate Change.”
Technology Analysis & Strategic Management 26 (7):733–749.
Coccia,M. 2014b. “Driving Forces of Technological Change: The Relation Between Population Growth and Techn
Innovation-Analysis of the OptimalInteraction Across Countries.” TechnologicalForecasting & SocialChange 82 (2):
52–65.
TECHNOLOGY ANALYSIS & STRATEGIC MANAGEMENT11
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 15
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





