Biology Report: Temperature Effects on Catechol Oxidase in Potatoes
VerifiedAdded on 2021/04/17
|10
|2609
|49
Report
AI Summary
This biology report details an experiment investigating the effect of temperature on the activity of catechol oxidase extracted from potatoes. The experiment involved preparing potato extract and exposing it to varying temperatures (30°C to 80°C) along with catechol oxidase and distilled water. The rate of the reaction was determined by measuring the absorptivity of the solutions using a spectrophotometer, with the intensity of the brown color indicating the reaction rate. The results showed that the enzyme's activity peaked at 40°C, with the absorptivity values decreasing at temperatures above and below this point. The report discusses the properties of enzymes, including their protein structure and sensitivity to temperature, and the advantages of the wound response mechanism. The findings support the theoretical understanding that enzymes have an optimal temperature for activity, and that extreme temperatures can lead to denaturation and reduced function. The report also addresses potential sources of error in the experiment and concludes that catechol oxidase exhibits the highest reaction rate at the optimal temperature of approximately 40°C.

Running head: EFFICACY OF CATECHOL OXIDASE ON POTATOES 1
Efficacy of Catechol Oxidase on Potatoes at Different Temperatures
Name
Institutional Affiliation
Efficacy of Catechol Oxidase on Potatoes at Different Temperatures
Name
Institutional Affiliation
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

EFFICACY OF CATECHOL OXIDASE ON POTATOES 2
Efficacy of catechol oxidase on potatoes at different temperatures
Abstract
Catechol oxidase is an enzyme that catalyzes the reaction between oxygen and catechol
to produce benzoquinone and water. Catechol is a colorless compound of phenol found in plant
cells and is released by broken cells but after action of catechol oxidase is converted to
benzoquinone, a pigmented product (Arthur Jr & McLemore, 1956). This is the pigment
responsible for the darkening of cut surfaces of fruits and vegetables on exposure to air. Action
of catechol oxidase, as with other enzymatic activity, is influenced by factors like temperature,
pH, and specific chemical, which are called inhibitors, which might change its three-dimensional
shape (Chen & Tan, 1995). Catechol oxidase is also found in plants but under normal conditions,
these two compounds do not come into contact with each other.
The purpose of this experiment is to discuss the effect of varying temperature on action
of catechol oxidase and describe the general properties of enzymes. The presence of the enzyme
was expected to cause a change in color from transparent to brown (Manohan & Wai, 2012). In
the experiment, three different test tubes with similar concentrations of distilled water and
catechol oxidase were prepared, with two of them being placed in water baths to cause a variance
in temperature, and equal amounts of potato extract being added to them. This was to test the
action of the enzyme at different temperatures by checking the rate at which the solution changed
color to brown. Absorptivity of the six resultant liquids was measured to determine intensity of
the brown color. Highest reactions are expected to be at between 32 and 37°.
Introduction
An enzyme is an organic compound that accelerates the rate of reactions for specific
substrates without itself being consumed, with a power to chemically recognize and even change
Efficacy of catechol oxidase on potatoes at different temperatures
Abstract
Catechol oxidase is an enzyme that catalyzes the reaction between oxygen and catechol
to produce benzoquinone and water. Catechol is a colorless compound of phenol found in plant
cells and is released by broken cells but after action of catechol oxidase is converted to
benzoquinone, a pigmented product (Arthur Jr & McLemore, 1956). This is the pigment
responsible for the darkening of cut surfaces of fruits and vegetables on exposure to air. Action
of catechol oxidase, as with other enzymatic activity, is influenced by factors like temperature,
pH, and specific chemical, which are called inhibitors, which might change its three-dimensional
shape (Chen & Tan, 1995). Catechol oxidase is also found in plants but under normal conditions,
these two compounds do not come into contact with each other.
The purpose of this experiment is to discuss the effect of varying temperature on action
of catechol oxidase and describe the general properties of enzymes. The presence of the enzyme
was expected to cause a change in color from transparent to brown (Manohan & Wai, 2012). In
the experiment, three different test tubes with similar concentrations of distilled water and
catechol oxidase were prepared, with two of them being placed in water baths to cause a variance
in temperature, and equal amounts of potato extract being added to them. This was to test the
action of the enzyme at different temperatures by checking the rate at which the solution changed
color to brown. Absorptivity of the six resultant liquids was measured to determine intensity of
the brown color. Highest reactions are expected to be at between 32 and 37°.
Introduction
An enzyme is an organic compound that accelerates the rate of reactions for specific
substrates without itself being consumed, with a power to chemically recognize and even change

EFFICACY OF CATECHOL OXIDASE ON POTATOES 3
specific reactants (Nagai & Suzuki, 2001). Enzymes, however do not have control over the
direction of the reaction taking place. Most are partially or completely composed of protein, and
they catalyze reactions by lowering the activation energy at which reactions occur and offering
favorable microclimates for the reaction to occur (Chen & Tan, 1995). Catechol oxidase is an
example of an enzyme.
Catechol oxidases are plant enzymes containing a dinuclear copper center, also called
polyphenol oxidases. Any fruits and vegetables which turn brown on exposure to air contain this
enzyme. When catechol is exposed after rupturing of a plant cell, a wound response mechanism
of the plant catalyzes oxidation of ortho diphenols to their corresponding ortho-quinones and the
reduction of oxygen to water (Duangmal & Apenten, 1999). The colorless diphenol molecules
change into colored quinine molecules which cross to for complex molecules called melanins.
This is the observed brown color. The reaction occurs according to the equation below;
Catechol +1/2 O2 ====== benzoquinone + H2O
This wound response mechanism is advantageous. This enzyme is introduced to plucked
ripe tea leaves to make the black tea that is the final product. According to Eicken et al (1998),
he states that it is important for the more poisonous and soluble ortho diphenols to be converted
to the less potent and insoluble ortho quinones. Polyphenol oxidases (PPOs) have therefore been
proposed as a means of bioremediation. This includes processes like removal of phenolic
contaminants, enzymatic waste water treatment, and industrial waste treatment. Phenols are
common pollutants in industrial waste by companies like agrochemicals, pharmaceuticals, dye
factories and wood pulp industries, and it is very important that they be removed from waste
(Gómez-López, 2002). Though a convincing description of the use of this method to get rid of
specific reactants (Nagai & Suzuki, 2001). Enzymes, however do not have control over the
direction of the reaction taking place. Most are partially or completely composed of protein, and
they catalyze reactions by lowering the activation energy at which reactions occur and offering
favorable microclimates for the reaction to occur (Chen & Tan, 1995). Catechol oxidase is an
example of an enzyme.
Catechol oxidases are plant enzymes containing a dinuclear copper center, also called
polyphenol oxidases. Any fruits and vegetables which turn brown on exposure to air contain this
enzyme. When catechol is exposed after rupturing of a plant cell, a wound response mechanism
of the plant catalyzes oxidation of ortho diphenols to their corresponding ortho-quinones and the
reduction of oxygen to water (Duangmal & Apenten, 1999). The colorless diphenol molecules
change into colored quinine molecules which cross to for complex molecules called melanins.
This is the observed brown color. The reaction occurs according to the equation below;
Catechol +1/2 O2 ====== benzoquinone + H2O
This wound response mechanism is advantageous. This enzyme is introduced to plucked
ripe tea leaves to make the black tea that is the final product. According to Eicken et al (1998),
he states that it is important for the more poisonous and soluble ortho diphenols to be converted
to the less potent and insoluble ortho quinones. Polyphenol oxidases (PPOs) have therefore been
proposed as a means of bioremediation. This includes processes like removal of phenolic
contaminants, enzymatic waste water treatment, and industrial waste treatment. Phenols are
common pollutants in industrial waste by companies like agrochemicals, pharmaceuticals, dye
factories and wood pulp industries, and it is very important that they be removed from waste
(Gómez-López, 2002). Though a convincing description of the use of this method to get rid of
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

EFFICACY OF CATECHOL OXIDASE ON POTATOES 4
industrial waste has yet to be given, it is still a question for researchers who keep finding ways to
justify it.
This experiment was to examine temperature as a factor that affects functioning of the
enzyme catechol oxidase, and to learn how to extract it from a plant substrate. Since enzymes are
protein in nature, it is expected that they will be nature at high temperatures causing the rate of
reaction to reduce. In doing this experiment we sought to find out whether the enzyme will
perform equally at all temperatures or if it will perform optimally at its peak temperature of 40°
and show lower levels of reaction at temperatures above or below this value.
Korean ginseng is a medical herb that has been used for a long time to cure different
diseases and function as health food in Asia (Duangmal & Apenten, 1999). Korean Ginseng can
be used as a vegetable or it can be dried for storage. However, during its drying process, it is
presumed that its brown color occurs due to the oxidation of peroxidase and phenolic compounds
by Polyphenol oxidase (Duangmal & Apenten, 1999). The black and brown pigment can be
produced chemically in plant issues or through the process of enzyme reaction. The browning
reaction can also be linked to an economic loss due to flavor, color, and nutritional values. This
reaction results into chemical changes in a fresh Korean ginseng.
A number of studies have characterized polyphenol oxidase from different sources such
as grape, potatoes apple, and fungi such as Trichoderma reesei (Duangmal & Apenten, 1999).
Although, polyphenol oxidase from different kind of plants and fungi has been researched for
many years, Korean ginseng PPO has not been researched yet into more detail.
During the experiment, there are certain safety rules that have to be followed to protect
our skin from contamination of chemical. These safety rules include putting on gloves and
industrial waste has yet to be given, it is still a question for researchers who keep finding ways to
justify it.
This experiment was to examine temperature as a factor that affects functioning of the
enzyme catechol oxidase, and to learn how to extract it from a plant substrate. Since enzymes are
protein in nature, it is expected that they will be nature at high temperatures causing the rate of
reaction to reduce. In doing this experiment we sought to find out whether the enzyme will
perform equally at all temperatures or if it will perform optimally at its peak temperature of 40°
and show lower levels of reaction at temperatures above or below this value.
Korean ginseng is a medical herb that has been used for a long time to cure different
diseases and function as health food in Asia (Duangmal & Apenten, 1999). Korean Ginseng can
be used as a vegetable or it can be dried for storage. However, during its drying process, it is
presumed that its brown color occurs due to the oxidation of peroxidase and phenolic compounds
by Polyphenol oxidase (Duangmal & Apenten, 1999). The black and brown pigment can be
produced chemically in plant issues or through the process of enzyme reaction. The browning
reaction can also be linked to an economic loss due to flavor, color, and nutritional values. This
reaction results into chemical changes in a fresh Korean ginseng.
A number of studies have characterized polyphenol oxidase from different sources such
as grape, potatoes apple, and fungi such as Trichoderma reesei (Duangmal & Apenten, 1999).
Although, polyphenol oxidase from different kind of plants and fungi has been researched for
many years, Korean ginseng PPO has not been researched yet into more detail.
During the experiment, there are certain safety rules that have to be followed to protect
our skin from contamination of chemical. These safety rules include putting on gloves and
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

EFFICACY OF CATECHOL OXIDASE ON POTATOES 5
glasses, while scooping catechol, leave hood of it at a halfway mark, disposing off all used items
in the right place such as the blue bag, do not return the used liquid into the original container.
Materials and methods
Six test-tubes, test-tube rack, spectrophotometer, cut potatoes, ice, cheese cloth, a
calibrated 5ml pipette, medium funnel, ice cold distilled water, pipette pump, distilled water,
disposable gloves, 500ml beaker, disposable Pasteur pipettes, 3 calibrated 1ml pipettes,
disposable gloves, five water baths(40°,50°,60°,70°, and 80°), catechol, catechol oxidase, labels.
To prepare potato extract
A handful of cut potatoes were weighed and placed in a blender. An equivalent amount of
ice cold distilled water was added to the blender after which the mixture was blended well.
Method
The test tubes were first labeled according to the water baths they would be resting in. 5
ml of catechol oxidase was pipetted into each of the test tubes, after which a similar amount of
distilled water was added. Five test tubes were then placed into the five different water baths
with one being left on the test tube rack as the room temperature standard. After that the prepared
potato extract was added in equal amounts to each of the test tubes. Two drops of catechol was
added to the test tubes (this was an optional step, and catechol was handled was handled with
plenty of care because it is poisonous). Each test tube was removed from its water bath after ten
minutes and its contents put into a vial which was put in a spectrophotometer for the solution’s
absorptivity to be measured. The values read from the machine were then recorded in Table 1.
Results
The results for absorptivity increased from 0.343 at the minimum temperature of 30° to
0.451 at 40°. This, however, seemed to be an optimum temperature for the functioning of this
glasses, while scooping catechol, leave hood of it at a halfway mark, disposing off all used items
in the right place such as the blue bag, do not return the used liquid into the original container.
Materials and methods
Six test-tubes, test-tube rack, spectrophotometer, cut potatoes, ice, cheese cloth, a
calibrated 5ml pipette, medium funnel, ice cold distilled water, pipette pump, distilled water,
disposable gloves, 500ml beaker, disposable Pasteur pipettes, 3 calibrated 1ml pipettes,
disposable gloves, five water baths(40°,50°,60°,70°, and 80°), catechol, catechol oxidase, labels.
To prepare potato extract
A handful of cut potatoes were weighed and placed in a blender. An equivalent amount of
ice cold distilled water was added to the blender after which the mixture was blended well.
Method
The test tubes were first labeled according to the water baths they would be resting in. 5
ml of catechol oxidase was pipetted into each of the test tubes, after which a similar amount of
distilled water was added. Five test tubes were then placed into the five different water baths
with one being left on the test tube rack as the room temperature standard. After that the prepared
potato extract was added in equal amounts to each of the test tubes. Two drops of catechol was
added to the test tubes (this was an optional step, and catechol was handled was handled with
plenty of care because it is poisonous). Each test tube was removed from its water bath after ten
minutes and its contents put into a vial which was put in a spectrophotometer for the solution’s
absorptivity to be measured. The values read from the machine were then recorded in Table 1.
Results
The results for absorptivity increased from 0.343 at the minimum temperature of 30° to
0.451 at 40°. This, however, seemed to be an optimum temperature for the functioning of this
EFFICACY OF CATECHOL OXIDASE ON POTATOES 6
enzyme because the trend onwards shows lower and lower results for absorptivity, with the least
value being at the highest temperature of 80° (Oktay et al., 1995). Exact amounts of catechol
oxidase were put in each test tube so that enzyme concentration would not be a factor in the
difference in reactivity rates.
Temperature of vial(in degrees Celsius) Amount of 562nm light absorbed(absorption
units)
30 0.343
40 0.451
50 0.394
60 0.281
70 0.162
80 0.022
Table 1: values of temperature of vials against corresponding absorptivity.
Discussion
A change of color from colorless to varying shades of brown was observed in all the test
tubes. This shows that some of the solutions got to react in more optimal temperature conditions
than others. The vial at 40° from the values obtained had the deepest brown color which means it
had the highest conversion of catechol to benzoquinone. These observations show that higher
temperatures do not favor the functioning of this enzyme even though very low temperatures are
known to deactivate enzymes and higher temperatures in this situation would be increasing
kinetic energy for reactions (Shimizu et al., 2011). There is a drastic drop in the absorptivity
values at 80°, showing that the rate of reaction was almost zero.
enzyme because the trend onwards shows lower and lower results for absorptivity, with the least
value being at the highest temperature of 80° (Oktay et al., 1995). Exact amounts of catechol
oxidase were put in each test tube so that enzyme concentration would not be a factor in the
difference in reactivity rates.
Temperature of vial(in degrees Celsius) Amount of 562nm light absorbed(absorption
units)
30 0.343
40 0.451
50 0.394
60 0.281
70 0.162
80 0.022
Table 1: values of temperature of vials against corresponding absorptivity.
Discussion
A change of color from colorless to varying shades of brown was observed in all the test
tubes. This shows that some of the solutions got to react in more optimal temperature conditions
than others. The vial at 40° from the values obtained had the deepest brown color which means it
had the highest conversion of catechol to benzoquinone. These observations show that higher
temperatures do not favor the functioning of this enzyme even though very low temperatures are
known to deactivate enzymes and higher temperatures in this situation would be increasing
kinetic energy for reactions (Shimizu et al., 2011). There is a drastic drop in the absorptivity
values at 80°, showing that the rate of reaction was almost zero.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
EFFICACY OF CATECHOL OXIDASE ON POTATOES 7
` Enzymes are biological catalysts, and this is why it was possible to obtain catechol
oxidase from potatoes, which speed up cell reactions (Kavino, et al., 2007). They are either in
part or in whole composed of proteins which generally get denatured at very high temperatures
and are very specific in functions. This is why the cooler the temperature of the mixtures, the
quicker the reactions were. The results in the table therefore bear a resemblance to what is
suggested by primary literature, which says that the optimum temperature for potatoes is about
40°. The brown colour was also achieved faster at lower temperatures. Theoretically, also, the
enzyme had lost most of its functioning to denaturing at between 70 to 80° and this can be seen
in the results found which show very low values for absorptivity at those temperatures.
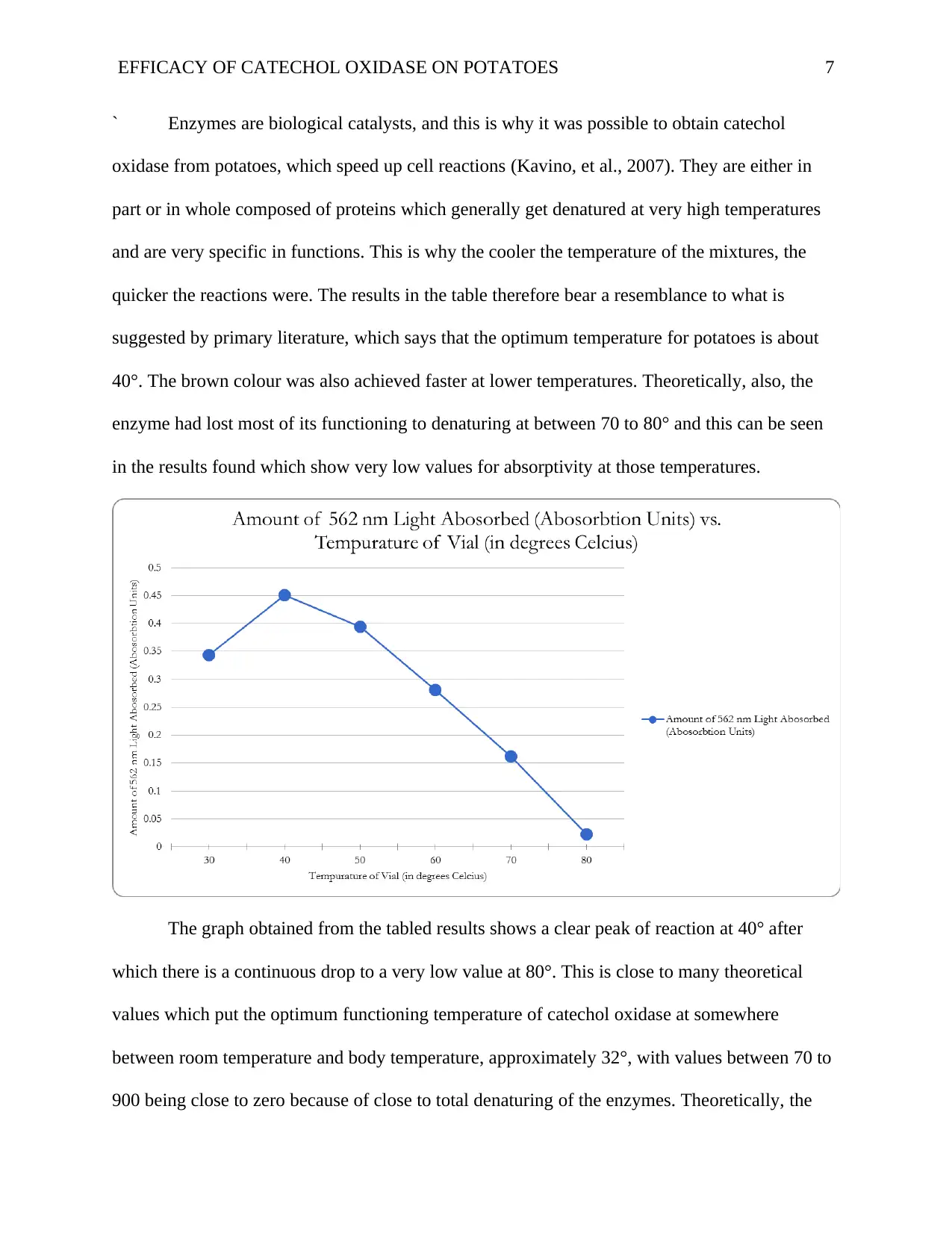
The graph obtained from the tabled results shows a clear peak of reaction at 40° after
which there is a continuous drop to a very low value at 80°. This is close to many theoretical
values which put the optimum functioning temperature of catechol oxidase at somewhere
between room temperature and body temperature, approximately 32°, with values between 70 to
900 being close to zero because of close to total denaturing of the enzymes. Theoretically, the
` Enzymes are biological catalysts, and this is why it was possible to obtain catechol
oxidase from potatoes, which speed up cell reactions (Kavino, et al., 2007). They are either in
part or in whole composed of proteins which generally get denatured at very high temperatures
and are very specific in functions. This is why the cooler the temperature of the mixtures, the
quicker the reactions were. The results in the table therefore bear a resemblance to what is
suggested by primary literature, which says that the optimum temperature for potatoes is about
40°. The brown colour was also achieved faster at lower temperatures. Theoretically, also, the
enzyme had lost most of its functioning to denaturing at between 70 to 80° and this can be seen
in the results found which show very low values for absorptivity at those temperatures.
The graph obtained from the tabled results shows a clear peak of reaction at 40° after
which there is a continuous drop to a very low value at 80°. This is close to many theoretical
values which put the optimum functioning temperature of catechol oxidase at somewhere
between room temperature and body temperature, approximately 32°, with values between 70 to
900 being close to zero because of close to total denaturing of the enzymes. Theoretically, the
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

EFFICACY OF CATECHOL OXIDASE ON POTATOES 8
enzymes are also completely deactivated at freezing point and there is no mobility or active
energy to support any reaction.
Errors in this equation would arise from possible inconsistencies in the potato extract
used in the experiment. It is also not easy to maintain the specific temperatures of a water bath
under normal conditions (Lee et al., 2009). Another source of error would be human error when
making readings and faulty measuring devices.
Conclusion
The experiment shows that catechol oxidase has highest reaction rate at optimal
temperature of about 40°. It also explained the process of how a cut plant cell changes color to
brown or black, and why this is an advantageous reaction for them. Temperature was studied as
an important aspect for the function of not only catechol oxidase but also other enzymes as they
all have the same inherent protein structure.
enzymes are also completely deactivated at freezing point and there is no mobility or active
energy to support any reaction.
Errors in this equation would arise from possible inconsistencies in the potato extract
used in the experiment. It is also not easy to maintain the specific temperatures of a water bath
under normal conditions (Lee et al., 2009). Another source of error would be human error when
making readings and faulty measuring devices.
Conclusion
The experiment shows that catechol oxidase has highest reaction rate at optimal
temperature of about 40°. It also explained the process of how a cut plant cell changes color to
brown or black, and why this is an advantageous reaction for them. Temperature was studied as
an important aspect for the function of not only catechol oxidase but also other enzymes as they
all have the same inherent protein structure.

EFFICACY OF CATECHOL OXIDASE ON POTATOES 9
References
Oktay, M., Küfreviolu, I., Kocaçalişkan, I., & Şaklrolu, H. (1995). Polyphenoloxidase from
Amasya apple. Journal of Food Science, 60(3), 494-496.
Duangmal, K., & Apenten, R. K. O. (1999). A comparative study of polyphenoloxidases from
taro (Colocasia esculenta) and potato (Solanum tuberosum var. Romano). Food
Chemistry, 64(3), 351-359.
Eicken, C., Zippel, F., Büldt-Karentzopoulos, K., & Krebs, B. (1998). Biochemical and
spectroscopic characterization of catechol oxidase from sweet potatoes (Ipomoea batatas)
containing a type‐3 dicopper center. FEBS letters, 436(2), 293-299.
Eicken, C., Zippel, F., Büldt-Karentzopoulos, K., & Krebs, B. (1998). Biochemical and
spectroscopic characterization of catechol oxidase from sweet potatoes (Ipomoea batatas)
containing a type‐3 dicopper center. FEBS letters, 436(2), 293-299.
Kavino, M., Harish, S., Kumar, N., Saravanakumar, D., Damodaran, T., Soorianathasundaram,
K., & Samiyappan, R. (2007). Rhizosphere and endophytic bacteria for induction of
systemic resistance of banana plantlets against bunchy top virus. Soil Biology and
Biochemistry, 39(5), 1087-1098.
Gómez-López, V. M. (2002). Some biochemical properties of polyphenol oxidase from two
varieties of avocado. Food Chemistry, 77(2), 163-169.
Nagai, T., & Suzuki, N. (2001). Partial purification of polyphenol oxidase from Chinese cabbage
Brassica rapa L. Journal of agricultural and food chemistry, 49(8), 3922-3926.
Chen, Y., & Tan, T. C. (1995). Dopamine-sensing efficacy and characteristics of pretreated plant
tissue powder sensors. Sensors and Actuators B: Chemical, 28(1), 39-48.
References
Oktay, M., Küfreviolu, I., Kocaçalişkan, I., & Şaklrolu, H. (1995). Polyphenoloxidase from
Amasya apple. Journal of Food Science, 60(3), 494-496.
Duangmal, K., & Apenten, R. K. O. (1999). A comparative study of polyphenoloxidases from
taro (Colocasia esculenta) and potato (Solanum tuberosum var. Romano). Food
Chemistry, 64(3), 351-359.
Eicken, C., Zippel, F., Büldt-Karentzopoulos, K., & Krebs, B. (1998). Biochemical and
spectroscopic characterization of catechol oxidase from sweet potatoes (Ipomoea batatas)
containing a type‐3 dicopper center. FEBS letters, 436(2), 293-299.
Eicken, C., Zippel, F., Büldt-Karentzopoulos, K., & Krebs, B. (1998). Biochemical and
spectroscopic characterization of catechol oxidase from sweet potatoes (Ipomoea batatas)
containing a type‐3 dicopper center. FEBS letters, 436(2), 293-299.
Kavino, M., Harish, S., Kumar, N., Saravanakumar, D., Damodaran, T., Soorianathasundaram,
K., & Samiyappan, R. (2007). Rhizosphere and endophytic bacteria for induction of
systemic resistance of banana plantlets against bunchy top virus. Soil Biology and
Biochemistry, 39(5), 1087-1098.
Gómez-López, V. M. (2002). Some biochemical properties of polyphenol oxidase from two
varieties of avocado. Food Chemistry, 77(2), 163-169.
Nagai, T., & Suzuki, N. (2001). Partial purification of polyphenol oxidase from Chinese cabbage
Brassica rapa L. Journal of agricultural and food chemistry, 49(8), 3922-3926.
Chen, Y., & Tan, T. C. (1995). Dopamine-sensing efficacy and characteristics of pretreated plant
tissue powder sensors. Sensors and Actuators B: Chemical, 28(1), 39-48.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

EFFICACY OF CATECHOL OXIDASE ON POTATOES 10
Manohan, D., & Wai, W. C. (2012). Characterization of polyphenol oxidase in sweet potato
(Ipomoea Batatas (L.)). J. Advancement Sci. Arts, 3(1), 14-31.
Shimizu, M. M., Melo, G. A., dos Santos, A. B., Bottcher, A., Cesarino, I., Araújo, P., ... &
Mazzafera, P. (2011). Enzyme characterisation, isolation and cDNA cloning of
polyphenol oxidase in the hearts of palm of three commercially important species. Plant
Physiology and Biochemistry, 49(9), 970-977.
Arthur Jr, J. C., & McLemore, T. A. (1956). Sweet Potato Dehydration, Properties of
Polyphenolases Causing Discoloration of Sweet Potatoes during Processing. Journal of
Agricultural and Food Chemistry, 4(6), 553-555.
Lee, C. H., Lin, H. C., Cheng, S. H., Lin, T. S., & Mou, C. Y. (2009). Hydroxo-Bridged
Dinuclear Cupric Complexes Encapsulated in Various Mesoporous Silicas to Mimic the
Catalytic Activity of Catechol Oxidases: Reactivity and Selectivity Study. The Journal of
Physical Chemistry C, 113(36), 16058-16069.
Manohan, D., & Wai, W. C. (2012). Characterization of polyphenol oxidase in sweet potato
(Ipomoea Batatas (L.)). J. Advancement Sci. Arts, 3(1), 14-31.
Shimizu, M. M., Melo, G. A., dos Santos, A. B., Bottcher, A., Cesarino, I., Araújo, P., ... &
Mazzafera, P. (2011). Enzyme characterisation, isolation and cDNA cloning of
polyphenol oxidase in the hearts of palm of three commercially important species. Plant
Physiology and Biochemistry, 49(9), 970-977.
Arthur Jr, J. C., & McLemore, T. A. (1956). Sweet Potato Dehydration, Properties of
Polyphenolases Causing Discoloration of Sweet Potatoes during Processing. Journal of
Agricultural and Food Chemistry, 4(6), 553-555.
Lee, C. H., Lin, H. C., Cheng, S. H., Lin, T. S., & Mou, C. Y. (2009). Hydroxo-Bridged
Dinuclear Cupric Complexes Encapsulated in Various Mesoporous Silicas to Mimic the
Catalytic Activity of Catechol Oxidases: Reactivity and Selectivity Study. The Journal of
Physical Chemistry C, 113(36), 16058-16069.
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.