Exploring Thermal Properties: Thermodynamics & Heat Transfer Concepts
VerifiedAdded on 2023/06/15
|8
|1747
|52
Homework Assignment
AI Summary
This assignment delves into the thermal properties of matter, applying the first law of thermodynamics to analyze thermodynamic systems. It investigates isothermal and adiabatic processes, examining how work and heat affect internal energy. The solution explains heat transfer mechanisms—conduction, convection, and radiation—detailing how they occur and are influenced by material properties and temperature differences. Examples such as boilers, condensers, and transformers are provided to illustrate practical applications. The document also addresses the behavior of gases, including the relationship between pressure, volume, and temperature, and discusses the assumptions underlying ideal gas behavior. The content is available on Desklib, a platform offering a range of study tools and solved assignments for students.

Thermal Properties of Matter 1
THERMAL PROPERTIES OF MATTER
By Name
Course
Instructor
Institution
Location
Date
THERMAL PROPERTIES OF MATTER
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Thermal Properties of Matter 2
Section A
Criteria 1.1
1. In both cases, the gas expands against the surrounding. Work is thus done on the
externals surroundings since there is a sudden decrease in the external pressure as weigh
is withdrawn from the piston and the expansion of the gas is in correlation with the
external pressure.
2. There is an isothermal expansion in the heat exchange between Gas A and the
surrounding hence a larger transfer of heat is experienced. Thermal heat must be
introduced into the systems in order to maintain the temperature of the expanding system
in compensation of the work being done isothermally on the environment. An arrow
should be pointed into the system (Ibrahim Dincer, 2011, p.289). The system in B is
highly insulated hence there is no transfer of heat as heat cannot flow out of the system.
The temperature of the system therefore decreases. There is no arrow in B.
3. There is not temperature change since the system is in an isothermal process hence ∆U=0
and W=-Q. The system therefore does not do any work on the surrounding and instead
the compensation of the work is done by heat that comes from the surrounding, work
out=-heat in (Philip J. Pritchard, 2015, p.266).
4. The system is in an adiabatic process hence Q=0 and ∆U=W, work<0 as the system does
not work on the surrounding and the energy of the internal surrounding decreases in
correspondence with a decrease in the temperature.
Criteria 1.2, 1.3, 1.4 and 3.2
a. Boiler, condenser, transformer
Section A
Criteria 1.1
1. In both cases, the gas expands against the surrounding. Work is thus done on the
externals surroundings since there is a sudden decrease in the external pressure as weigh
is withdrawn from the piston and the expansion of the gas is in correlation with the
external pressure.
2. There is an isothermal expansion in the heat exchange between Gas A and the
surrounding hence a larger transfer of heat is experienced. Thermal heat must be
introduced into the systems in order to maintain the temperature of the expanding system
in compensation of the work being done isothermally on the environment. An arrow
should be pointed into the system (Ibrahim Dincer, 2011, p.289). The system in B is
highly insulated hence there is no transfer of heat as heat cannot flow out of the system.
The temperature of the system therefore decreases. There is no arrow in B.
3. There is not temperature change since the system is in an isothermal process hence ∆U=0
and W=-Q. The system therefore does not do any work on the surrounding and instead
the compensation of the work is done by heat that comes from the surrounding, work
out=-heat in (Philip J. Pritchard, 2015, p.266).
4. The system is in an adiabatic process hence Q=0 and ∆U=W, work<0 as the system does
not work on the surrounding and the energy of the internal surrounding decreases in
correspondence with a decrease in the temperature.
Criteria 1.2, 1.3, 1.4 and 3.2
a. Boiler, condenser, transformer
Thermal Properties of Matter 3
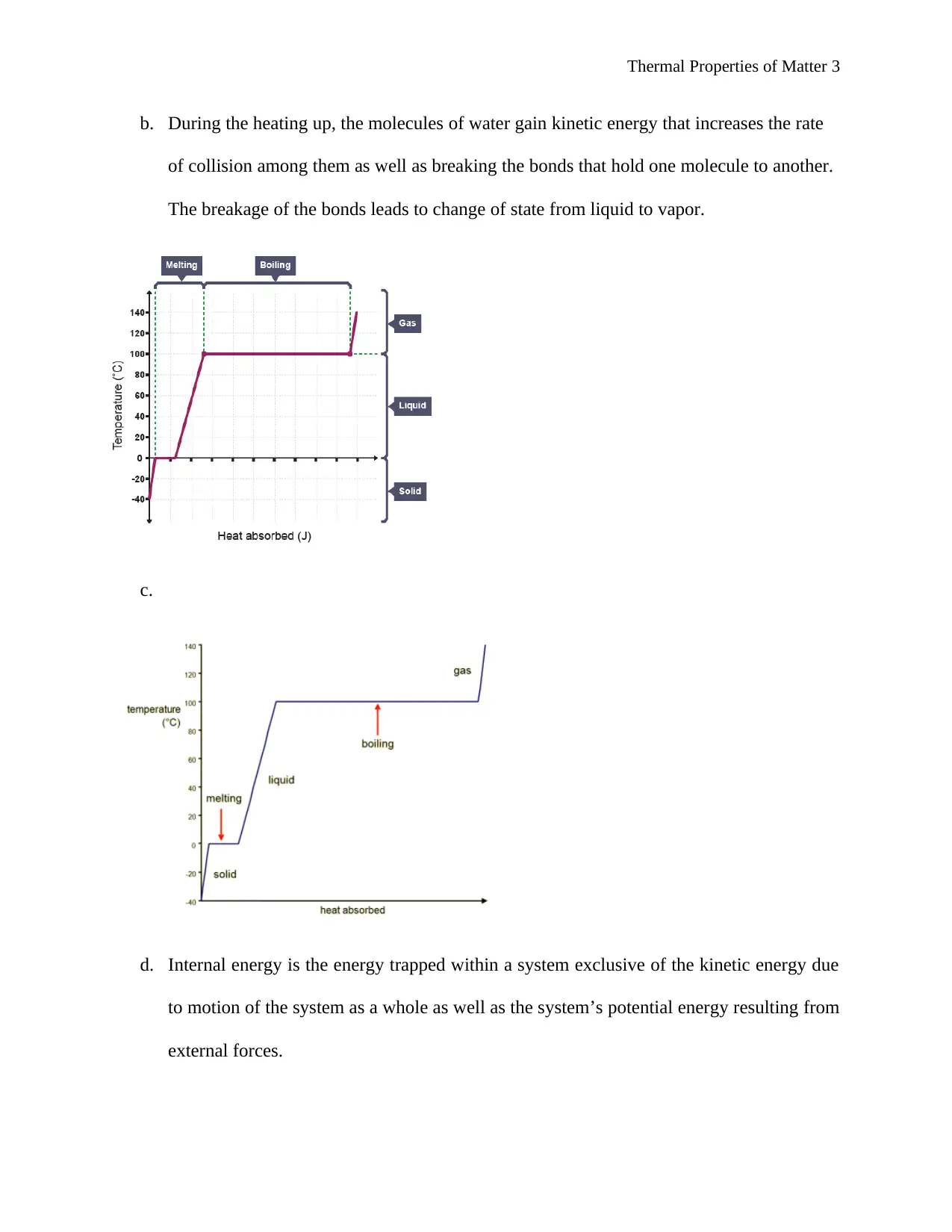
b. During the heating up, the molecules of water gain kinetic energy that increases the rate
of collision among them as well as breaking the bonds that hold one molecule to another.
The breakage of the bonds leads to change of state from liquid to vapor.
c.
d. Internal energy is the energy trapped within a system exclusive of the kinetic energy due
to motion of the system as a whole as well as the system’s potential energy resulting from
external forces.
b. During the heating up, the molecules of water gain kinetic energy that increases the rate
of collision among them as well as breaking the bonds that hold one molecule to another.
The breakage of the bonds leads to change of state from liquid to vapor.
c.
d. Internal energy is the energy trapped within a system exclusive of the kinetic energy due
to motion of the system as a whole as well as the system’s potential energy resulting from
external forces.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Thermal Properties of Matter 4
e. The pressure of a fixed mass of a gas is inversely proportional to its volume at constant
temperature. Temperature does not affect mass, increases pressure and volume. An
increase in temperature increases the rate of collision of molecules in a substance thereby
increasing the pressure. An increase in temperature expands the molecules in a substance
an increase in the volume (Peter Atkins, 2017, p.308).
f. It is assumed that:
There is negligible interaction between the molecules of the gas
The gas molecules are infinitesimally small in relation to their container
Real gases concur with the predictions of equations of ideal gases to a range of 5% at normal
pressures and temperatures with a significant deviation experienced when the temperatures are
lowered.
Criteria 3.1
Matter is composed of molecules and atoms that are always in continuous and different forms of
motion including rotation, vibration and translation depending on the nature of the molecules and
atoms. The motion among the molecules and atoms result into the creation of heat or thermal
energy and that any form of matter exhibits a form of thermal energy. The higher the speed of
motion of the atoms the higher the amount of thermal energy exhibited by matter. atoms and
molecules move from one region to another due to temperature different between the two regions
in which they move (Ibrahim Dincer, 2011, p.188). Temperature defines the average measure of
the value of the energy for all the molecules and atoms that ate present in a given system. The
transfer of heat from one region to another takes place mainly through three main ways among
them, conduction, convection and radiation which are determined by the nature of the surface of
transfer of the heat. Both convection and conduction require a medium for transfer of heat while
e. The pressure of a fixed mass of a gas is inversely proportional to its volume at constant
temperature. Temperature does not affect mass, increases pressure and volume. An
increase in temperature increases the rate of collision of molecules in a substance thereby
increasing the pressure. An increase in temperature expands the molecules in a substance
an increase in the volume (Peter Atkins, 2017, p.308).
f. It is assumed that:
There is negligible interaction between the molecules of the gas
The gas molecules are infinitesimally small in relation to their container
Real gases concur with the predictions of equations of ideal gases to a range of 5% at normal
pressures and temperatures with a significant deviation experienced when the temperatures are
lowered.
Criteria 3.1
Matter is composed of molecules and atoms that are always in continuous and different forms of
motion including rotation, vibration and translation depending on the nature of the molecules and
atoms. The motion among the molecules and atoms result into the creation of heat or thermal
energy and that any form of matter exhibits a form of thermal energy. The higher the speed of
motion of the atoms the higher the amount of thermal energy exhibited by matter. atoms and
molecules move from one region to another due to temperature different between the two regions
in which they move (Ibrahim Dincer, 2011, p.188). Temperature defines the average measure of
the value of the energy for all the molecules and atoms that ate present in a given system. The
transfer of heat from one region to another takes place mainly through three main ways among
them, conduction, convection and radiation which are determined by the nature of the surface of
transfer of the heat. Both convection and conduction require a medium for transfer of heat while
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Thermal Properties of Matter 5
radiation occurs through a vacuum. Heat is transferred from one point to another in a system
where a temperature difference exists in the given system. The temperature difference is such
that there is movement of heat from a higher to a lower system.
Conduction: Heat transfer through conduction occurs where there are collisions between the
molecules and the atoms present in a substance and the corresponding transfer of kinetic energy
(Doran, 2013, p.312). An illustration is as shown below in which there two substances of which
are at different temperatures and are separated from each other using a barrier that is finally
removed.
Upon the removal of the barrier, the hot atoms collide with the cold atoms and during such
collisions the faster atoms which are the hot atoms lose some of their speed while the slower
atoms which are the cold atoms gains some speed. This occurs by the faster atoms transferring
some of their kinetic energy to the slow atoms. The transfer of kinetic energy form the hot side
of the system to the cold side of the system is proceeds through conduction. The rate of transfer
of heat in a material depends on the nature of the material, measured by the thermal conductivity
of the material (Lienhard, 2013, p.185). The thermal conductivity of a material is a affected by
various factors among them cross sectional area, length and temperature of the material. The
amount of heat that flows through over time in a system is thus defined from the expression
Heat flow over time=thermal conductivity*(area/length)*(temperature difference)
radiation occurs through a vacuum. Heat is transferred from one point to another in a system
where a temperature difference exists in the given system. The temperature difference is such
that there is movement of heat from a higher to a lower system.
Conduction: Heat transfer through conduction occurs where there are collisions between the
molecules and the atoms present in a substance and the corresponding transfer of kinetic energy
(Doran, 2013, p.312). An illustration is as shown below in which there two substances of which
are at different temperatures and are separated from each other using a barrier that is finally
removed.
Upon the removal of the barrier, the hot atoms collide with the cold atoms and during such
collisions the faster atoms which are the hot atoms lose some of their speed while the slower
atoms which are the cold atoms gains some speed. This occurs by the faster atoms transferring
some of their kinetic energy to the slow atoms. The transfer of kinetic energy form the hot side
of the system to the cold side of the system is proceeds through conduction. The rate of transfer
of heat in a material depends on the nature of the material, measured by the thermal conductivity
of the material (Lienhard, 2013, p.185). The thermal conductivity of a material is a affected by
various factors among them cross sectional area, length and temperature of the material. The
amount of heat that flows through over time in a system is thus defined from the expression
Heat flow over time=thermal conductivity*(area/length)*(temperature difference)

Thermal Properties of Matter 6
From this expression it can be observed that the amount of heat transferred will be larger for a
given temperature difference in a system for materials having high thermal conductivities. Such
materials include copper and are said to be good conductors. On the other hand, materials having
lower thermal conductivities tend to transfer relatively little amount of heat over time and are
thus called poor conductors.
Convection: Liquids and gases carry away with them to the direction of travel thermal energy
when they are heated. This type of heat transfer is called convection where the fluid above the
hot surface expands thereby becoming less dense and finally rises. Molecules as well expand
when exposed to thermal energy (Doran, 2013, p.288). There is a proportional increase in the
volume of a fluid when the temperature increased since the increase in temperature results into
an increase in the mass of the fluid. Such an effect of the temperature increase brings about
displacement of the fluid where the immediate hot air rises pushing the cold air, which is denser
down. This series of events illustrates conventional currents and the equation for convection rate
calculation is as shown below:
Q = hc ∙ A ∙ (Ts – Tf)
where hc=coefficient of the convective heat transfer, Q=heat
transferred per unit time, Ts=surface temperature and Tf=the
temperature of the fluid (Peter Atkins, 2017, p.265). A space heater is
an ideal example of convection. As the air surrounding the space
heater near the floor gets heater, the air gains thermal energy, expands
and then rises to the top of the room thereby pushing the colder air downwards to get heated
creating convection current.
From this expression it can be observed that the amount of heat transferred will be larger for a
given temperature difference in a system for materials having high thermal conductivities. Such
materials include copper and are said to be good conductors. On the other hand, materials having
lower thermal conductivities tend to transfer relatively little amount of heat over time and are
thus called poor conductors.
Convection: Liquids and gases carry away with them to the direction of travel thermal energy
when they are heated. This type of heat transfer is called convection where the fluid above the
hot surface expands thereby becoming less dense and finally rises. Molecules as well expand
when exposed to thermal energy (Doran, 2013, p.288). There is a proportional increase in the
volume of a fluid when the temperature increased since the increase in temperature results into
an increase in the mass of the fluid. Such an effect of the temperature increase brings about
displacement of the fluid where the immediate hot air rises pushing the cold air, which is denser
down. This series of events illustrates conventional currents and the equation for convection rate
calculation is as shown below:
Q = hc ∙ A ∙ (Ts – Tf)
where hc=coefficient of the convective heat transfer, Q=heat
transferred per unit time, Ts=surface temperature and Tf=the
temperature of the fluid (Peter Atkins, 2017, p.265). A space heater is
an ideal example of convection. As the air surrounding the space
heater near the floor gets heater, the air gains thermal energy, expands
and then rises to the top of the room thereby pushing the colder air downwards to get heated
creating convection current.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Thermal Properties of Matter 7
Radiation: Radiation is heat transfer that takes place through the emission of electromagnetic
waves that carry with them thermal energy from the objecting that is emitting the energy.
Radiation takes place through a vacuum and is the result of direct random movement of
molecules and atoms in matter. Electromagnetic radiation is generated when charge electrons
and protons move about an atom. The temperature of a material determines the amount of
thermal energy emitted through radiation (Hecht, 2013, p.178). The hotter the object the higher
the radiation emitted. An example of heat radiation is the sun that transfers thermal energy across
the solar system. Objects radiate infrared waves at normal room temperature. The wavelength
and the frequency of the radiated waves is affected by the temperature of the emitting surface.
The wavelength decreases with an increase in temperature thus higher temperatures leader to
shorter wavelengths that have high frequencies. Stefan-Boltzmann law is used to determine the
thermal radiation of a substance as follows:
P = e ∙ σ ∙ A· (Tr4 – Tc4)
Where P=radiated power, Tr=radiator temperature, A=area of radiating surface, Tc=temperature
of the surrounding, e=emissivity, σ=Stefan’s constant.
Radiation: Radiation is heat transfer that takes place through the emission of electromagnetic
waves that carry with them thermal energy from the objecting that is emitting the energy.
Radiation takes place through a vacuum and is the result of direct random movement of
molecules and atoms in matter. Electromagnetic radiation is generated when charge electrons
and protons move about an atom. The temperature of a material determines the amount of
thermal energy emitted through radiation (Hecht, 2013, p.178). The hotter the object the higher
the radiation emitted. An example of heat radiation is the sun that transfers thermal energy across
the solar system. Objects radiate infrared waves at normal room temperature. The wavelength
and the frequency of the radiated waves is affected by the temperature of the emitting surface.
The wavelength decreases with an increase in temperature thus higher temperatures leader to
shorter wavelengths that have high frequencies. Stefan-Boltzmann law is used to determine the
thermal radiation of a substance as follows:
P = e ∙ σ ∙ A· (Tr4 – Tc4)
Where P=radiated power, Tr=radiator temperature, A=area of radiating surface, Tc=temperature
of the surrounding, e=emissivity, σ=Stefan’s constant.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Thermal Properties of Matter 8
References
Doran, P.M., 2013. Bioprocess Engineering Principles. 4th ed. Oxford: Academic Press.
Frederick J. Bueche, E.H., 2011. Schaum's Outline of College Physics, 11th Edition. 5th ed. New
York: McGraw Hill Professional.
Hecht, E., 2013. Fundamentals of Fluid Mechanics. 6th ed. London: John Wiley & Sons.
Ibrahim Dincer, n., 2011. Thermal Energy Storage: Systems and Applications. 2nd ed. London:
John Wiley & Sons.
Lienhard, J.H., 2013. A Heat Transfer Textbook: Fourth Edition. 4th ed. New York: Courier
Corporation.
Michael J. Moran, H.N.S.D.D.B.M.B.B., 2010. Fundamentals of Engineering Thermodynamics.
7th ed. New York: John Wiley & Sons.
Peter Atkins, J.D.P., 2017. Elements of Physical Chemistry. 4th ed. Oxford: Oxford University
Press.
Philip J. Pritchard, J.W.M., 2015. Fox and McDonald's Introduction to Fluid Mechanics, 9th
Edition. 3rd ed. Chicago: Wiley.
References
Doran, P.M., 2013. Bioprocess Engineering Principles. 4th ed. Oxford: Academic Press.
Frederick J. Bueche, E.H., 2011. Schaum's Outline of College Physics, 11th Edition. 5th ed. New
York: McGraw Hill Professional.
Hecht, E., 2013. Fundamentals of Fluid Mechanics. 6th ed. London: John Wiley & Sons.
Ibrahim Dincer, n., 2011. Thermal Energy Storage: Systems and Applications. 2nd ed. London:
John Wiley & Sons.
Lienhard, J.H., 2013. A Heat Transfer Textbook: Fourth Edition. 4th ed. New York: Courier
Corporation.
Michael J. Moran, H.N.S.D.D.B.M.B.B., 2010. Fundamentals of Engineering Thermodynamics.
7th ed. New York: John Wiley & Sons.
Peter Atkins, J.D.P., 2017. Elements of Physical Chemistry. 4th ed. Oxford: Oxford University
Press.
Philip J. Pritchard, J.W.M., 2015. Fox and McDonald's Introduction to Fluid Mechanics, 9th
Edition. 3rd ed. Chicago: Wiley.
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





