Thermodynamics Assignment - Engineering Thermodynamics Solutions
VerifiedAdded on 2023/05/30
|8
|635
|62
Homework Assignment
AI Summary
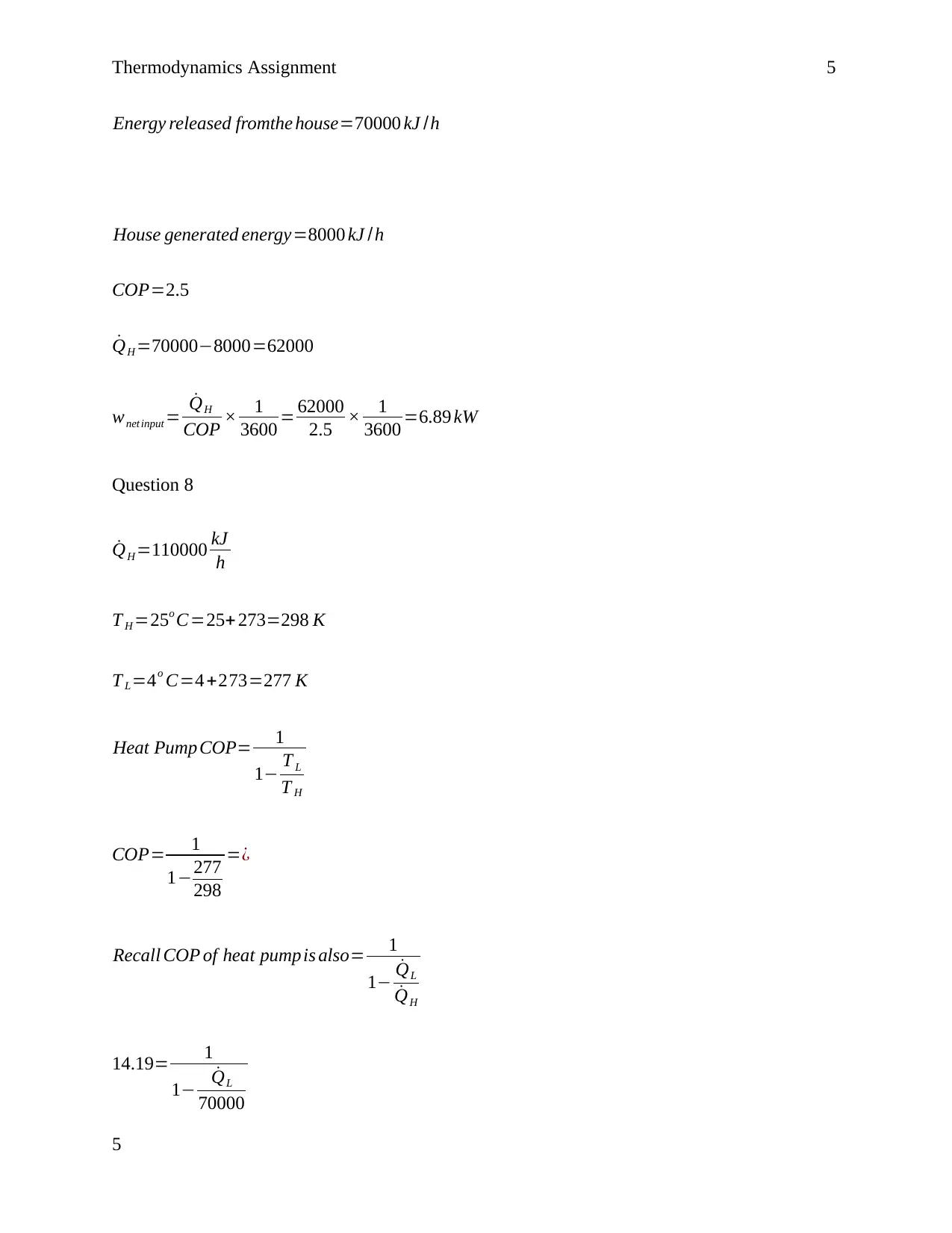
This document presents a comprehensive solution to a thermodynamics assignment, addressing various problems related to heat transfer, heat pumps, and thermodynamic cycles. The assignment covers calculations of the Coefficient of Performance (COP) for different scenarios, including refrigerators and heat pumps, and assesses the feasibility of claims based on thermodynamic principles. It involves determining heat transfer rates, net power output, and efficiency calculations for different systems, such as power plants and heating systems. The solutions include detailed calculations, formulas, and explanations to help students understand the concepts and solve similar problems. The assignment also explores the impact of heat losses and provides answers to questions about the validity of given claims based on calculated COPs. References from textbooks on thermodynamics are included to support the solutions.
1 out of 8












![[object Object]](/_next/static/media/star-bottom.7253800d.svg)