University of Highlands & Islands Thermodynamics & Fluids Assignment
VerifiedAdded on 2022/09/09
|28
|3097
|18
Homework Assignment
AI Summary
This document presents a comprehensive solution to a Thermodynamics and Fluids assignment, likely for a Mechanical Engineering program. The assignment covers a range of topics including thermodynamic systems, open systems, and their interactions with surroundings. It includes diagrams and explanations of temperature-enthalpy diagrams showing phase changes of water, calculations involving steam properties, and sketching of processes on pressure-volume diagrams. The solution also delves into calculations of specific gas constants, mass and volume of air in a cylinder, and the mechanism of heat transfer by convection. It further explores static and dynamic pressure, hydrostatic pressure, and fluid viscosity at the molecular level. Additional problems address non-Newtonian fluids, gas density, and energy balance in a steam turbine condenser, including the use of steam tables and non-flow energy equations. The assignment concludes with explanations of perfect gases and adiabatic compression processes.

Thermodynamics and Fluid mechanics
Term Paper
Date
Number of Words
Name
Institution Affiliate
Term Paper
Date
Number of Words
Name
Institution Affiliate
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
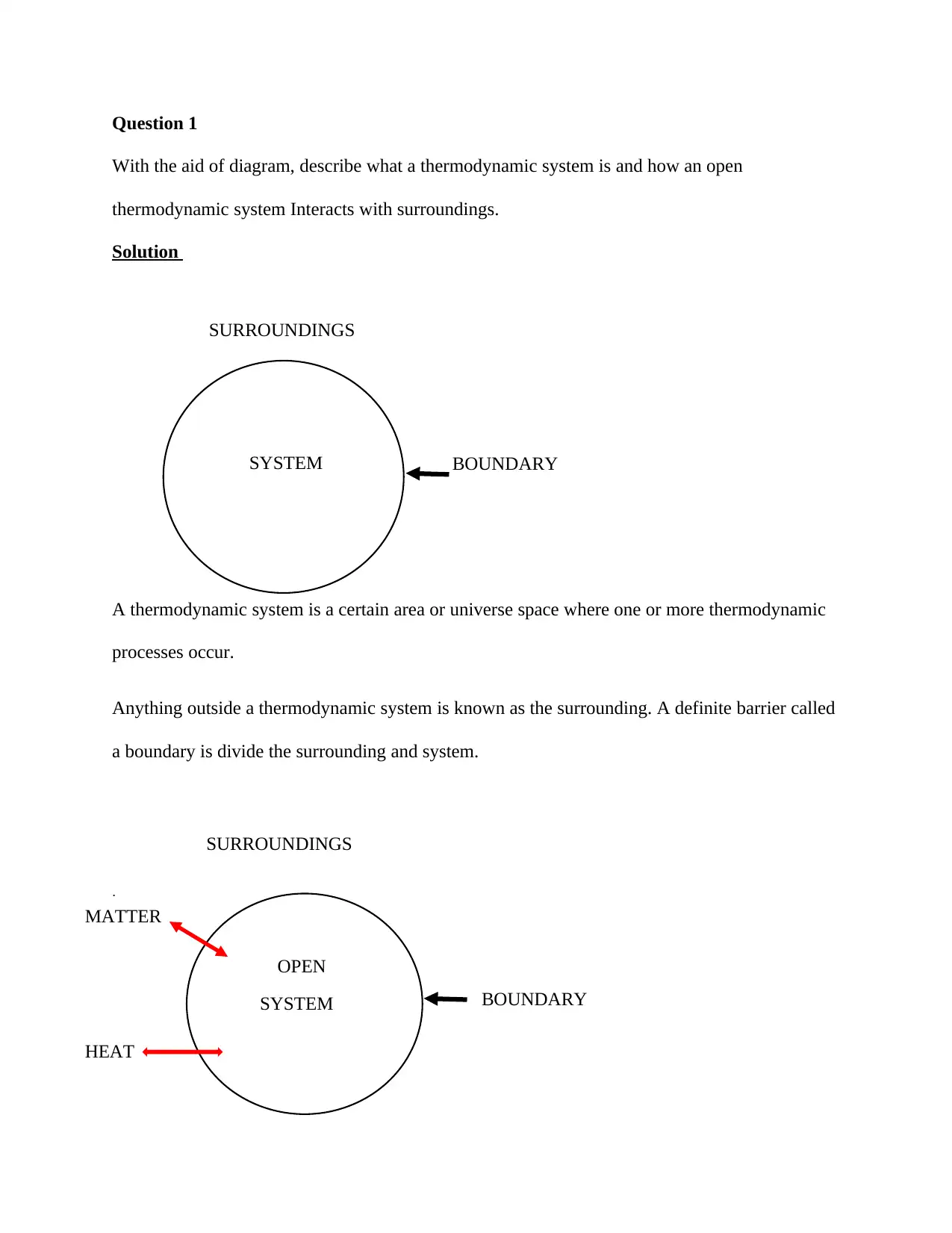
Question 1
With the aid of diagram, describe what a thermodynamic system is and how an open
thermodynamic system Interacts with surroundings.
Solution
SURROUNDINGS
A thermodynamic system is a certain area or universe space where one or more thermodynamic
processes occur.
Anything outside a thermodynamic system is known as the surrounding. A definite barrier called
a boundary is divide the surrounding and system.
.
SYSTEM BOUNDARY
OPEN
SYSTEM BOUNDARY
MATTER
HEAT
SURROUNDINGS
With the aid of diagram, describe what a thermodynamic system is and how an open
thermodynamic system Interacts with surroundings.
Solution
SURROUNDINGS
A thermodynamic system is a certain area or universe space where one or more thermodynamic
processes occur.
Anything outside a thermodynamic system is known as the surrounding. A definite barrier called
a boundary is divide the surrounding and system.
.
SYSTEM BOUNDARY
OPEN
SYSTEM BOUNDARY
MATTER
HEAT
SURROUNDINGS

An open system is a thermodynamic system that permits the flow of both energy and mass into
and out of the system.
and out of the system.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
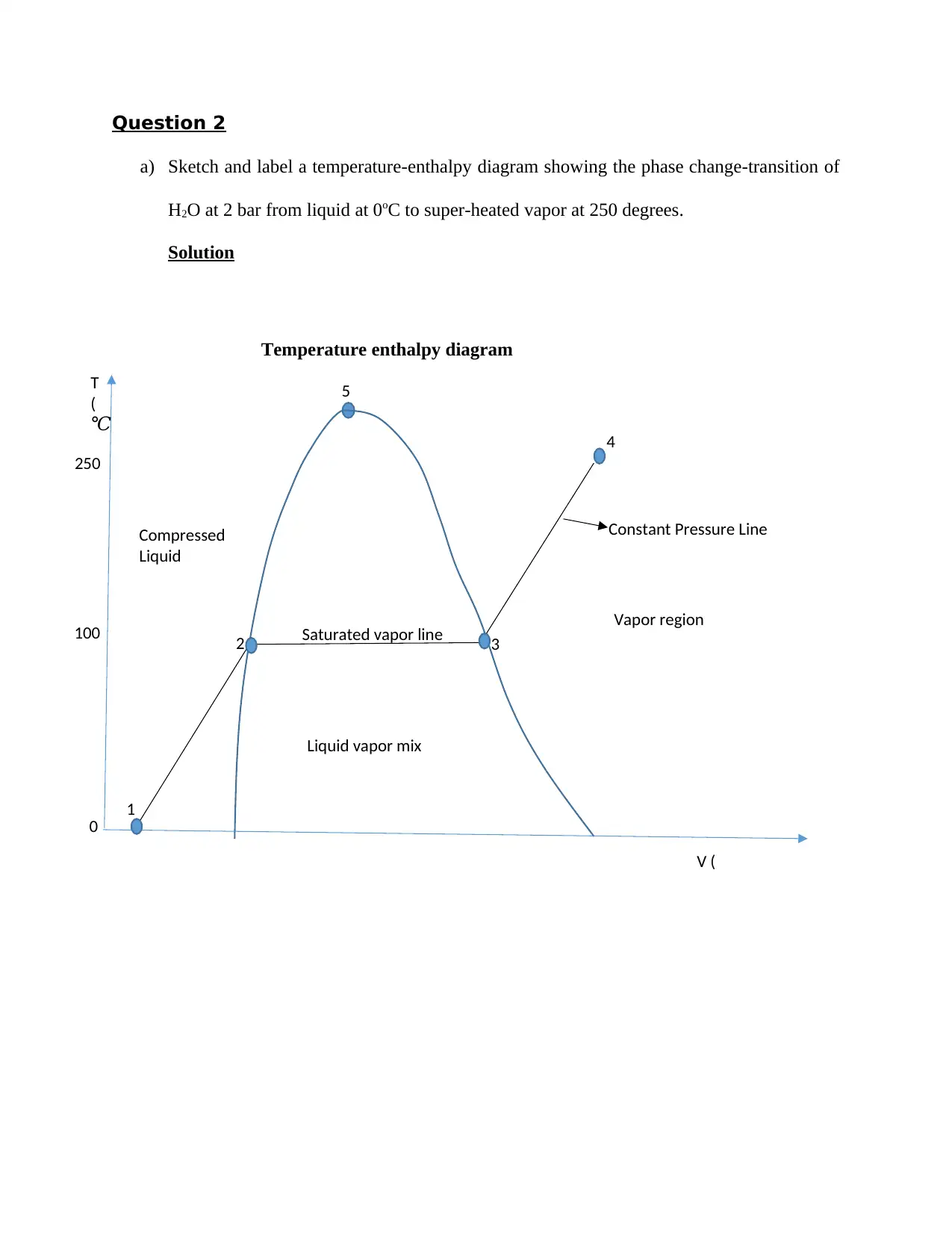
Question 2
a) Sketch and label a temperature-enthalpy diagram showing the phase change-transition of
H2O at 2 bar from liquid at 0oC to super-heated vapor at 250 degrees.
Solution
1
Saturated vapor line 3
5
4
Constant Pressure Line
2
V (
m3 K g−1 ¿
T
(
℃ ¿
0
100
250
Liquid vapor mix
Compressed
Liquid
Vapor region
Temperature enthalpy diagram
a) Sketch and label a temperature-enthalpy diagram showing the phase change-transition of
H2O at 2 bar from liquid at 0oC to super-heated vapor at 250 degrees.
Solution
1
Saturated vapor line 3
5
4
Constant Pressure Line
2
V (
m3 K g−1 ¿
T
(
℃ ¿
0
100
250
Liquid vapor mix
Compressed
Liquid
Vapor region
Temperature enthalpy diagram
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Key
1 Compressed liquid at 0 ℃
2 Saturated Liquid
3 Saturated vapor
4 Super-heated vapor
5 Critical point
Note: The temperature is held constant at 2 bars.
b) With the aid of the steam property table, calculate:
i. Specific internal energy of steam at 20 bar which has a dryness fraction of 0.75.
Solution
¿ the saturated steam table at P=20 ¯, ∧x=0.75
We find
Uf =907 KJ
kg , Ug=2600 KJ
Kg
Using the formula Ux=Uf +x ( Ug−hf )
Subsitutingthe values ofUf ∧hf ∈the above formula
Ux=907+0.75 ¿
¿ 907+1269.75
¿ 2176.75
Therefore , specific enrgy of the staem=2176.75 KJ
kg
1 Compressed liquid at 0 ℃
2 Saturated Liquid
3 Saturated vapor
4 Super-heated vapor
5 Critical point
Note: The temperature is held constant at 2 bars.
b) With the aid of the steam property table, calculate:
i. Specific internal energy of steam at 20 bar which has a dryness fraction of 0.75.
Solution
¿ the saturated steam table at P=20 ¯, ∧x=0.75
We find
Uf =907 KJ
kg , Ug=2600 KJ
Kg
Using the formula Ux=Uf +x ( Ug−hf )
Subsitutingthe values ofUf ∧hf ∈the above formula
Ux=907+0.75 ¿
¿ 907+1269.75
¿ 2176.75
Therefore , specific enrgy of the staem=2176.75 KJ
kg

ii. Specific enthalpy of steam at 25 bar, 400° C.
Solution
Given that 25¯¿ 25 atm=2525 Pa , T =400
Now , here we will use both theTemperature value∧prsusure value
because we the steam is acting beyond vapour−equilibrium
¿ the steam table
hf =535 KJ
Kg ,hg=2717 KJ
kg
using the formulah=hf + x ( hg−hf )
Substituting the values of hf ∧hg∈the formulaabove
hx=35+ 0.75 ( 2717−535 ) kJ / kg
¿ ( 535+1636.5 ) KJ /kg
¿ 2,171.5 KJ
kg
Therefore , the specific enthalpy hx=2,171.5 KJ
kg
Question 3
Solution
Given that 25¯¿ 25 atm=2525 Pa , T =400
Now , here we will use both theTemperature value∧prsusure value
because we the steam is acting beyond vapour−equilibrium
¿ the steam table
hf =535 KJ
Kg ,hg=2717 KJ
kg
using the formulah=hf + x ( hg−hf )
Substituting the values of hf ∧hg∈the formulaabove
hx=35+ 0.75 ( 2717−535 ) kJ / kg
¿ ( 535+1636.5 ) KJ /kg
¿ 2,171.5 KJ
kg
Therefore , the specific enthalpy hx=2,171.5 KJ
kg
Question 3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
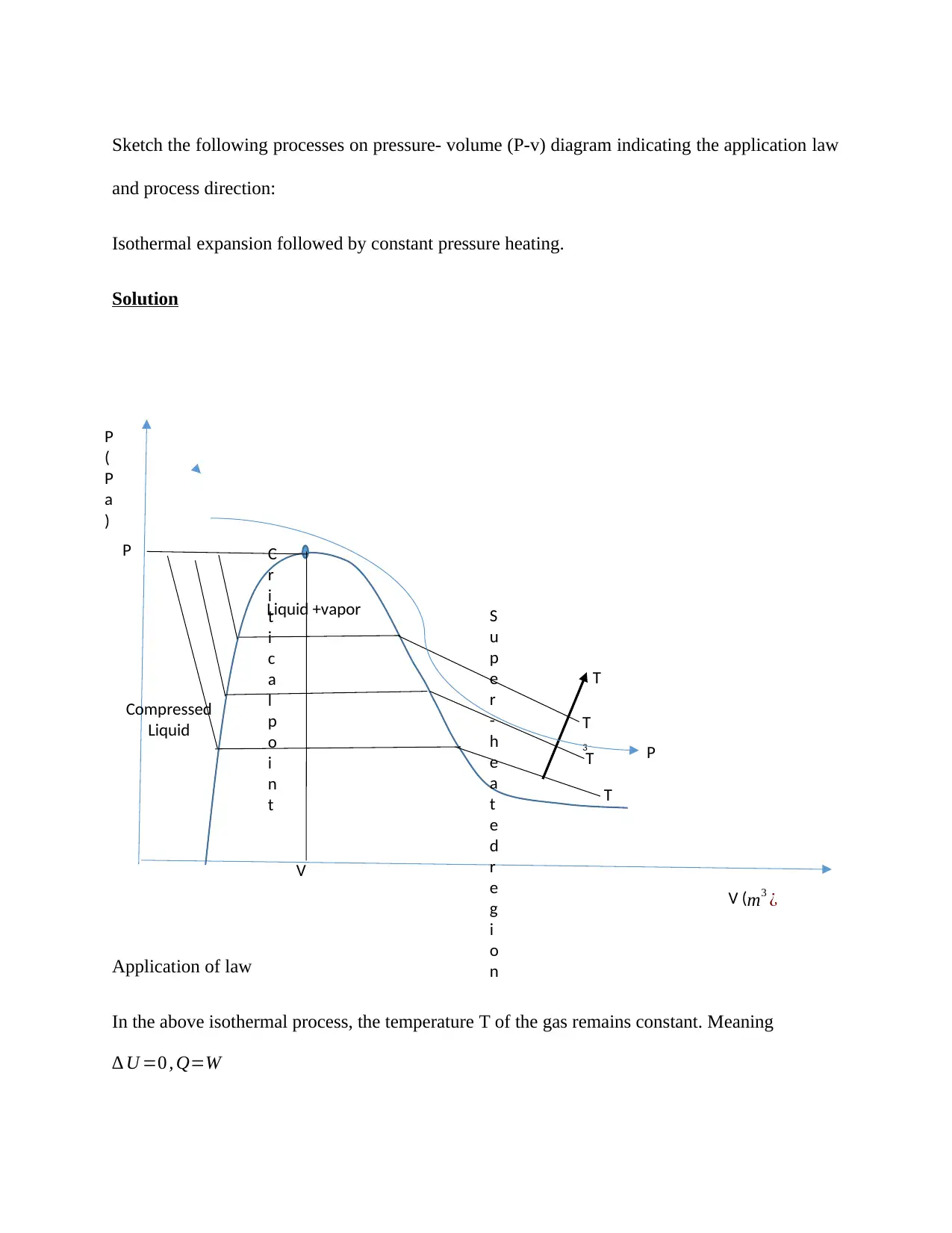
Sketch the following processes on pressure- volume (P-v) diagram indicating the application law
and process direction:
Isothermal expansion followed by constant pressure heating.
Solution
Application of law
In the above isothermal process, the temperature T of the gas remains constant. Meaning
∆ U =0 , Q=W
P
(
P
a
)
T
1
V (m3 ¿
S
u
p
e
r
-
h
e
a
t
e
d
r
e
g
i
o
n
T
3
Compressed
Liquid
C
r
i
t
i
c
a
l
p
o
i
n
t
Liquid +vapor
T
e
T
2
P
C
V
C
P
r
and process direction:
Isothermal expansion followed by constant pressure heating.
Solution
Application of law
In the above isothermal process, the temperature T of the gas remains constant. Meaning
∆ U =0 , Q=W
P
(
P
a
)
T
1
V (m3 ¿
S
u
p
e
r
-
h
e
a
t
e
d
r
e
g
i
o
n
T
3
Compressed
Liquid
C
r
i
t
i
c
a
l
p
o
i
n
t
Liquid +vapor
T
e
T
2
P
C
V
C
P
r
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Also, according to general gas law, PV=nRT.
But n=constant and T=constant, then PV=constant
But n=constant and T=constant, then PV=constant

Question 4
The cylinder of an internal combustion engine with a capacity of 1 liters contains air at a
pressure and temperature of 14 bar and 80°C respectively. Taking the molecular mass, (Mr) of
air as 29 and the universal gas constant, R0 as 8.3144 kJ kmol-1 K-1, calculate:
I. The specific gas constant, R (2 Marks)
Solution
R = Ro/mr
The specific gas constant, R = the universal gas constant/ the molecular mass
The specific gas constant, R = (8.3144 kJ kmol-1 K-1)/29
The specific gas constant, R = 0.2867 kJ kmol-1 K-1
ii. The mass of air in the cylinder
Solution
PV = mRT
Where P is pressure = 14 bar
1 bar air pressure = 101 kPa
P = 14 bar/1 bar x 101 kPa = 1414 kpa
V volume = 1 liters
1 liter = 0.001 m3
V volume = (1 liter)/ (1 liters) x 0.001 m3 = 0.001 m3
R is The specific gas constant, R = 0.2867 kJ kmol-1 K-1
The cylinder of an internal combustion engine with a capacity of 1 liters contains air at a
pressure and temperature of 14 bar and 80°C respectively. Taking the molecular mass, (Mr) of
air as 29 and the universal gas constant, R0 as 8.3144 kJ kmol-1 K-1, calculate:
I. The specific gas constant, R (2 Marks)
Solution
R = Ro/mr
The specific gas constant, R = the universal gas constant/ the molecular mass
The specific gas constant, R = (8.3144 kJ kmol-1 K-1)/29
The specific gas constant, R = 0.2867 kJ kmol-1 K-1
ii. The mass of air in the cylinder
Solution
PV = mRT
Where P is pressure = 14 bar
1 bar air pressure = 101 kPa
P = 14 bar/1 bar x 101 kPa = 1414 kpa
V volume = 1 liters
1 liter = 0.001 m3
V volume = (1 liter)/ (1 liters) x 0.001 m3 = 0.001 m3
R is The specific gas constant, R = 0.2867 kJ kmol-1 K-1
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

T is temperature = 80oC + 273 K = 353 k
m = PV/RT = (1414 kpa)( 0.001 m3)/(0.2867 kJ kmol-1 K-1)(353 k)
m = 0.01397 kg
iii. The volume of the air if it is compressed to a pressure of 23 bar and temperature
120°C.
Solution
P1V1/T1 =P2V2/T2
Pressure 1 P1 = 14 bar
Volume 1 V1 = 0.001 m3
Temperature 1 T1 = 353 k
Pressure 2 P2 = 23 bar
Volume 2 V2 = ?
Temperature T2 = 120oC = 120oC + 273 K = 393 K
(14 bar) (0.001 m3)/ (353 K) = (23 bar) (V2)/ (393 K)
V2 = [(14 bar) (0.001 m3)/ (353 K)] x (393 K)/(23bar)
V2 = 0.0006777 m3
m = PV/RT = (1414 kpa)( 0.001 m3)/(0.2867 kJ kmol-1 K-1)(353 k)
m = 0.01397 kg
iii. The volume of the air if it is compressed to a pressure of 23 bar and temperature
120°C.
Solution
P1V1/T1 =P2V2/T2
Pressure 1 P1 = 14 bar
Volume 1 V1 = 0.001 m3
Temperature 1 T1 = 353 k
Pressure 2 P2 = 23 bar
Volume 2 V2 = ?
Temperature T2 = 120oC = 120oC + 273 K = 393 K
(14 bar) (0.001 m3)/ (353 K) = (23 bar) (V2)/ (393 K)
V2 = [(14 bar) (0.001 m3)/ (353 K)] x (393 K)/(23bar)
V2 = 0.0006777 m3
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Question 5
Briefly describe the mechanism of heat transfer by convection and list one factor that influence
the rate of heat transfer by this method.1
Solution
Convection is the transfer of heat from one place to another by movement of fluids (bulk
movement of molecules within fluids) such as air or water (Gases and liquids).
Viscosity is one factor that affects convection. Currents move quickly within liquids of low
viscosity.
Question 6
1 Michael, M, & T Tsatsaronis, Engineering thermodynamics. in, 2nd ed., CRC Press, 2017, pp.
1-122.
Briefly describe the mechanism of heat transfer by convection and list one factor that influence
the rate of heat transfer by this method.1
Solution
Convection is the transfer of heat from one place to another by movement of fluids (bulk
movement of molecules within fluids) such as air or water (Gases and liquids).
Viscosity is one factor that affects convection. Currents move quickly within liquids of low
viscosity.
Question 6
1 Michael, M, & T Tsatsaronis, Engineering thermodynamics. in, 2nd ed., CRC Press, 2017, pp.
1-122.
a) Briefly describe the difference between static and dynamic pressure (in terms of their
origin)
Solution
Static pressure originates from fluid that is no moving (stationary fluid). If we have fluid
which is not moving, the pressure in the fluid is called static therefore static pressure is
quantity of pressure in a non-moving liquid. For static pressure in all directions, pressure
is the same. Dynamic pressure originated from the fluid that is in motion, dynamic
pressure depends on movement direction.
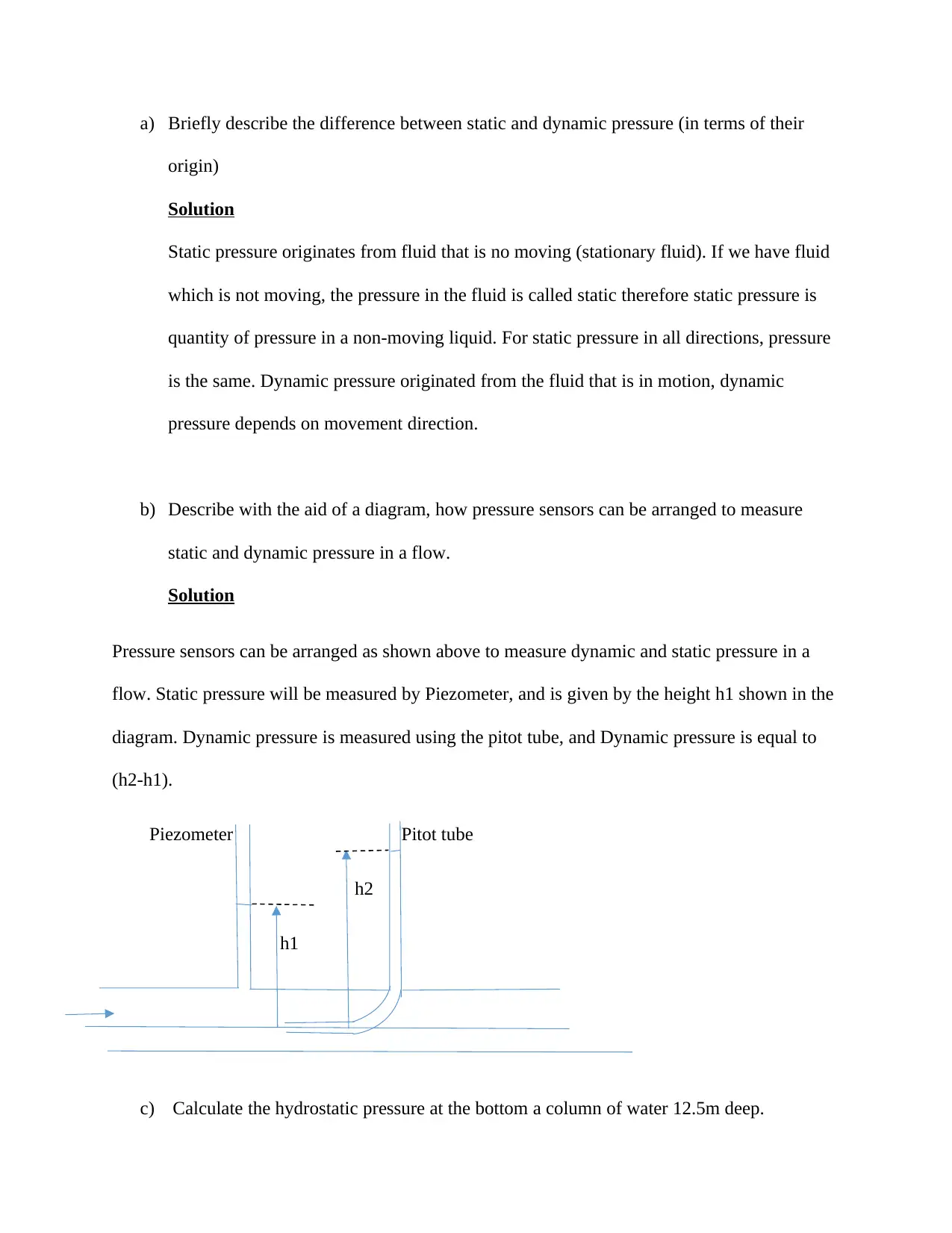
b) Describe with the aid of a diagram, how pressure sensors can be arranged to measure
static and dynamic pressure in a flow.
Solution
Pressure sensors can be arranged as shown above to measure dynamic and static pressure in a
flow. Static pressure will be measured by Piezometer, and is given by the height h1 shown in the
diagram. Dynamic pressure is measured using the pitot tube, and Dynamic pressure is equal to
(h2-h1).
Piezometer Pitot tube
h2
h1
c) Calculate the hydrostatic pressure at the bottom a column of water 12.5m deep.
origin)
Solution
Static pressure originates from fluid that is no moving (stationary fluid). If we have fluid
which is not moving, the pressure in the fluid is called static therefore static pressure is
quantity of pressure in a non-moving liquid. For static pressure in all directions, pressure
is the same. Dynamic pressure originated from the fluid that is in motion, dynamic
pressure depends on movement direction.
b) Describe with the aid of a diagram, how pressure sensors can be arranged to measure
static and dynamic pressure in a flow.
Solution
Pressure sensors can be arranged as shown above to measure dynamic and static pressure in a
flow. Static pressure will be measured by Piezometer, and is given by the height h1 shown in the
diagram. Dynamic pressure is measured using the pitot tube, and Dynamic pressure is equal to
(h2-h1).
Piezometer Pitot tube
h2
h1
c) Calculate the hydrostatic pressure at the bottom a column of water 12.5m deep.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 28
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





