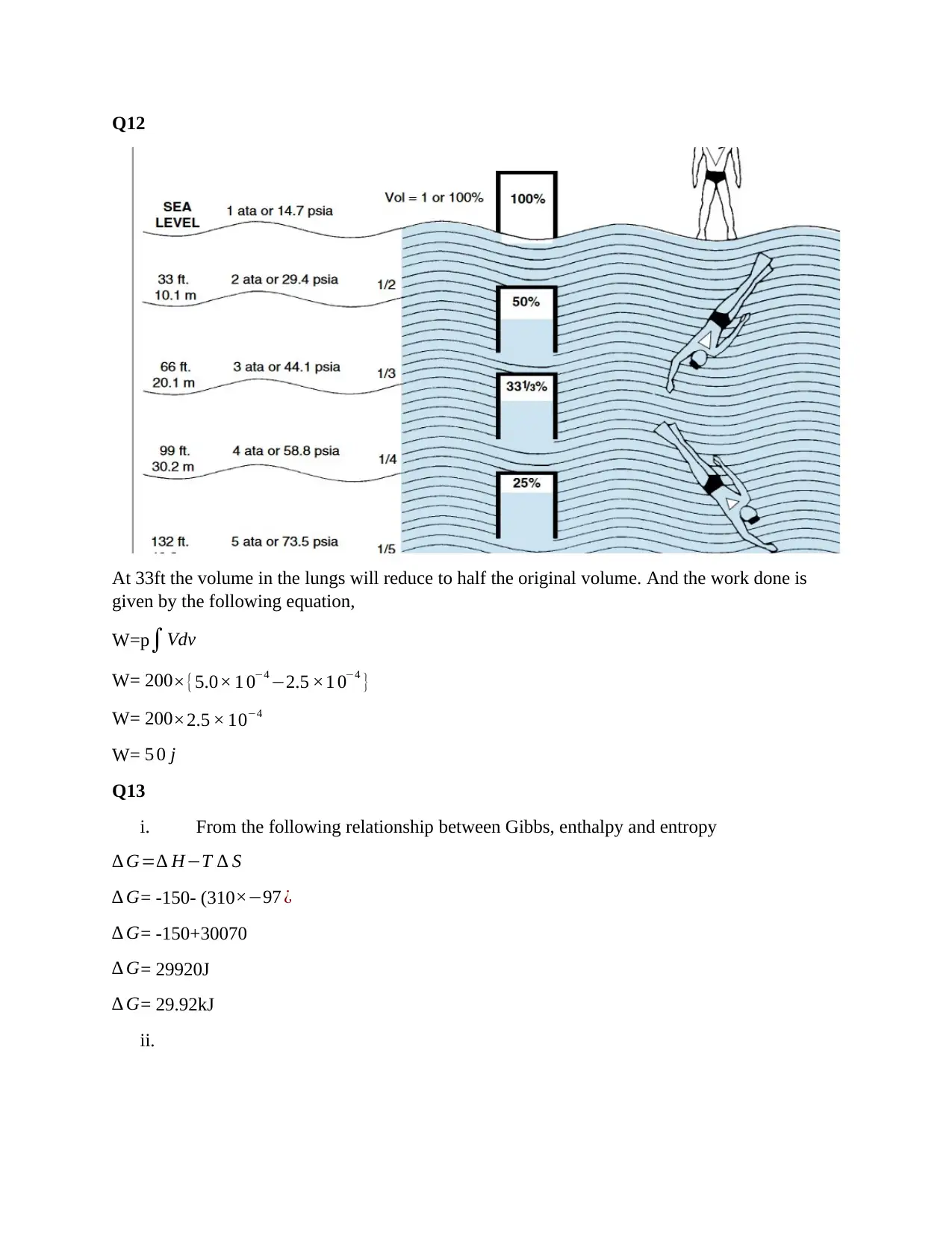
1st Year Thermodynamics Practice Questions Solution - Dr. Mark Gray
VerifiedAdded on 2022/09/05
|8
|566
|22
Homework Assignment
AI Summary
This document provides a comprehensive solution to a thermodynamics homework assignment. The solution covers several key concepts, including calculating heat capacity, enthalpy changes, and temperature changes in various scenarios. It addresses questions related to the heat transfer of bovine brain extract, temperature changes in the human body, and the molar enthalpy change of nitrous oxide. The assignment also explores concepts like Gibbs free energy, entropy, and the spontaneity of reactions. Detailed calculations and explanations are provided for each problem, including determining work done, energy changes, and the application of the second law of thermodynamics. The solution also includes multiple-choice questions on fundamental thermodynamic principles and provides a list of references for further study.
1 out of 8








![[object Object]](/_next/static/media/star-bottom.7253800d.svg)