MAE 204 Thermodynamics Assignment: HW #5 Solutions and Analysis
VerifiedAdded on 2022/10/11
|6
|463
|56
Homework Assignment
AI Summary
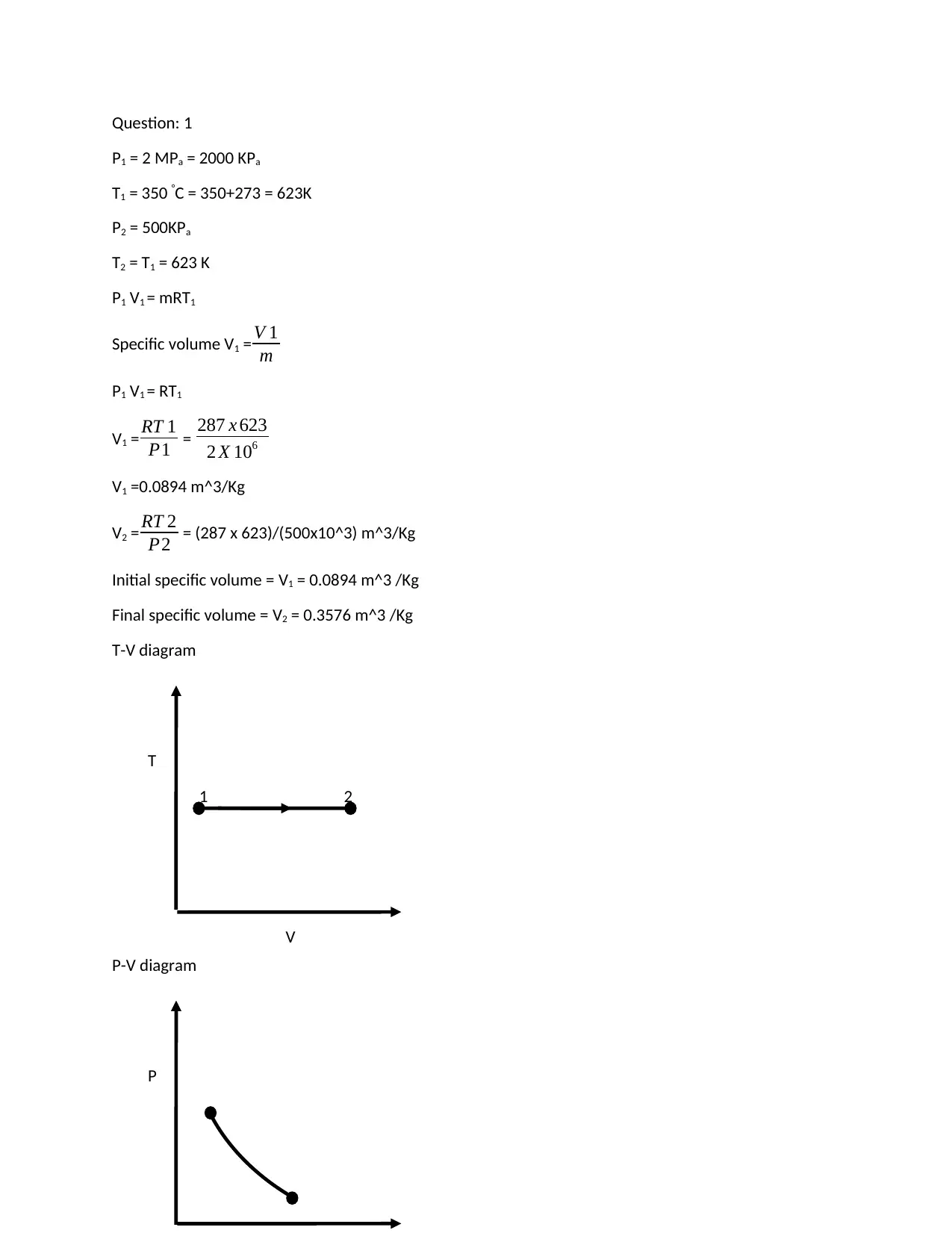
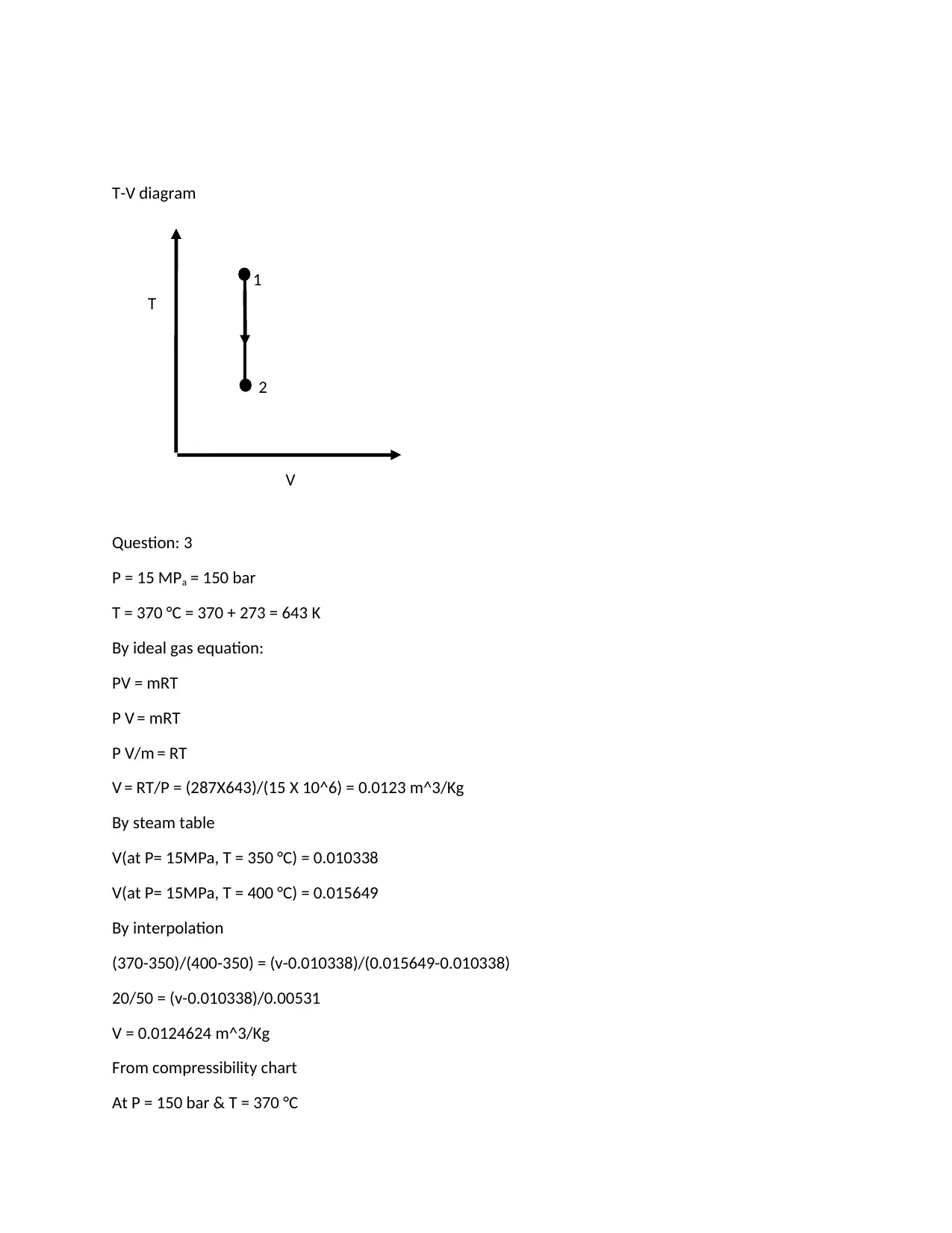
This assignment solution addresses three thermodynamics problems from MAE 204. The first problem involves an isothermal expansion of air, calculating initial and final specific volumes, work done, and heat transfer. The second problem analyzes a rigid container with R-134a undergoing cooling, determining heat transfer, the final phase, and temperature. The third problem explores the properties of a substance using the ideal gas equation, steam tables, and a compressibility chart, calculating and comparing specific volumes and determining the percentage error of the estimates. The solution demonstrates the application of thermodynamic principles and equations to solve practical engineering problems, including the use of T-V and P-V diagrams.
1 out of 6






![[object Object]](/_next/static/media/star-bottom.7253800d.svg)