Graded Chemistry Assignment: Heat, Energy, and Thermodynamics Problems
VerifiedAdded on 2022/12/29
|8
|1147
|93
Homework Assignment
AI Summary
This chemistry assignment solution addresses fundamental concepts in thermodynamics, including heat transfer, energy calculations, and temperature conversions. The assignment explores scenarios such as mixing cold milk with hot coffee, applying the law of conservation of energy, and converting between Celsius and Kelvin scales. It delves into endothermic and exothermic processes, calculating heat absorbed and released by various substances using specific heat capacity and enthalpy values. The solution includes problems involving silver and gold bars, ammonia and lead, and comparisons of substances with different specific heat capacities to determine which resists temperature change the most and which requires the least energy to raise its temperature. The assignment also analyzes which substance would have a higher final temperature when subjected to equal amounts of energy, providing detailed calculations and explanations throughout.

Running Head: ASSIGNMENT 1
GRADED ASSIGNMENT
Name of the Student
School Affiliation.
GRADED ASSIGNMENT
Name of the Student
School Affiliation.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ASSIGNMENT 2
Graded Assignment
1. Which of the following statements is true concerning what happens when cold milk is poured
into hot coffee?
a. The cold milk transfers its cold to the coffee, cooling the coffee down.
b. The cold milk transfers its heat to the coffee, warming the coffee up.
c. The warm coffee absorbs the cold from the milk, cooling the coffee down.
d. The warm coffee transfers its heat to the milk, cooling the coffee down.
The correct answer is (d). The warm coffee transfers its heat to the milk, cooling
the coffee down.
The coffee will transfer its energy to the milk, milk will, therefore, gain heat energy.
Both teaching equilibrium by attaining the same temperature.
The law of conservation of energy states that ‘'Energy is conserved, it cannot be created
or destroyed but can be only be changed from one form to another. If no energy is
allowed to enter or leave the system, the total energy of the system remains constant’’
2. Convert 60ºC to Kelvin.
273.15 K is equivalent to 0ºC
Formula: T (K) = T (ºC) + 273.15
Temperature (K) = 60 (ºC) + 273.15 = 333.15
Temperature= 333.15 K
Graded Assignment
1. Which of the following statements is true concerning what happens when cold milk is poured
into hot coffee?
a. The cold milk transfers its cold to the coffee, cooling the coffee down.
b. The cold milk transfers its heat to the coffee, warming the coffee up.
c. The warm coffee absorbs the cold from the milk, cooling the coffee down.
d. The warm coffee transfers its heat to the milk, cooling the coffee down.
The correct answer is (d). The warm coffee transfers its heat to the milk, cooling
the coffee down.
The coffee will transfer its energy to the milk, milk will, therefore, gain heat energy.
Both teaching equilibrium by attaining the same temperature.
The law of conservation of energy states that ‘'Energy is conserved, it cannot be created
or destroyed but can be only be changed from one form to another. If no energy is
allowed to enter or leave the system, the total energy of the system remains constant’’
2. Convert 60ºC to Kelvin.
273.15 K is equivalent to 0ºC
Formula: T (K) = T (ºC) + 273.15
Temperature (K) = 60 (ºC) + 273.15 = 333.15
Temperature= 333.15 K

ASSIGNMENT 3
3. What is the importance of the absolute zero?
Absolute zero is the lowest possible temperature (0 K) which is theoretical. It is
important in thermodynamics because of molecular motions theoretically.
4. For each of the following, indicate if the statement is describing an endothermic or
exothermic situation.
a. Endothermic energy is absorbed from the surrounding.
b. Exothermic (- H)
c. Endothermic products have more energy than reactants.
d. Endothermic (+ H)
e. Exothermic have more energy than the products.
f. Exothermic energy is released to the surrounding.
5. Solve the following problems.
a. A silver bar with the mass 150 grams is heated 24.5ºC to 60.5 ºC. how much heat
energy does silver bar absorb? The specific heat of silver is 0.235 J/g/ºC
Heat absorbed= mass (m) *specific heat capacity * temperature change
Heat absorbed= 150 (g) * 0.235 (J/g/ºC) * (60.5-24.5) ºC=1269.0 J
Heat absorbed=1269.0 Joules
b. A sample of gold absorbs 34.6 J of energy as its temperature is increased by 62.0
ºC .what is the mass of the gold sample. The specific heat of gold is 0.129 J/g/ºC
Heat absorbed= mass (m) *specific heat capacity * temperature change
3. What is the importance of the absolute zero?
Absolute zero is the lowest possible temperature (0 K) which is theoretical. It is
important in thermodynamics because of molecular motions theoretically.
4. For each of the following, indicate if the statement is describing an endothermic or
exothermic situation.
a. Endothermic energy is absorbed from the surrounding.
b. Exothermic (- H)
c. Endothermic products have more energy than reactants.
d. Endothermic (+ H)
e. Exothermic have more energy than the products.
f. Exothermic energy is released to the surrounding.
5. Solve the following problems.
a. A silver bar with the mass 150 grams is heated 24.5ºC to 60.5 ºC. how much heat
energy does silver bar absorb? The specific heat of silver is 0.235 J/g/ºC
Heat absorbed= mass (m) *specific heat capacity * temperature change
Heat absorbed= 150 (g) * 0.235 (J/g/ºC) * (60.5-24.5) ºC=1269.0 J
Heat absorbed=1269.0 Joules
b. A sample of gold absorbs 34.6 J of energy as its temperature is increased by 62.0
ºC .what is the mass of the gold sample. The specific heat of gold is 0.129 J/g/ºC
Heat absorbed= mass (m) *specific heat capacity * temperature change
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
ASSIGNMENT 4
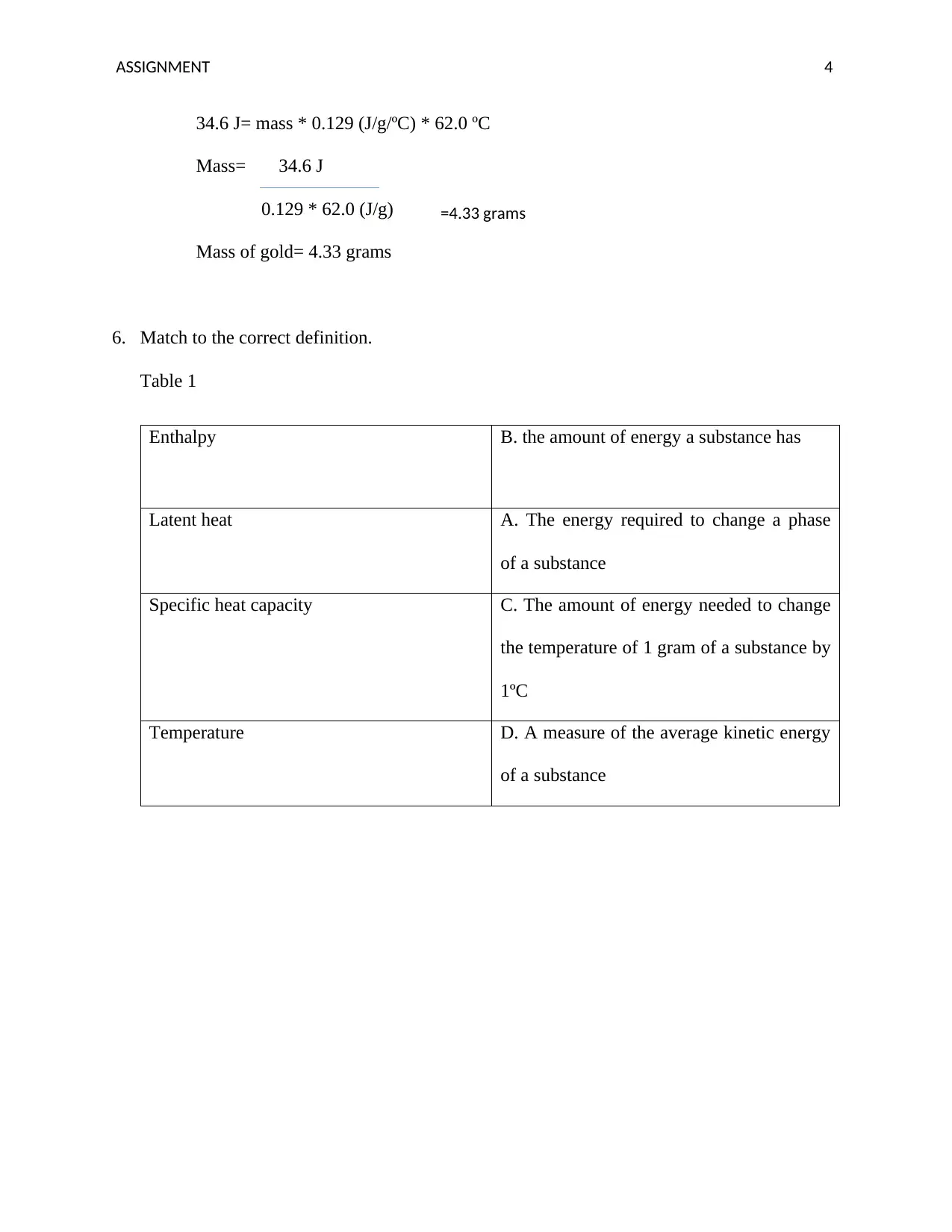
34.6 J= mass * 0.129 (J/g/ºC) * 62.0 ºC
Mass= 34.6 J
0.129 * 62.0 (J/g)
Mass of gold= 4.33 grams
6. Match to the correct definition.
Table 1
Enthalpy B. the amount of energy a substance has
Latent heat A. The energy required to change a phase
of a substance
Specific heat capacity C. The amount of energy needed to change
the temperature of 1 gram of a substance by
1ºC
Temperature D. A measure of the average kinetic energy
of a substance
=4.33 grams
34.6 J= mass * 0.129 (J/g/ºC) * 62.0 ºC
Mass= 34.6 J
0.129 * 62.0 (J/g)
Mass of gold= 4.33 grams
6. Match to the correct definition.
Table 1
Enthalpy B. the amount of energy a substance has
Latent heat A. The energy required to change a phase
of a substance
Specific heat capacity C. The amount of energy needed to change
the temperature of 1 gram of a substance by
1ºC
Temperature D. A measure of the average kinetic energy
of a substance
=4.33 grams
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
ASSIGNMENT 5
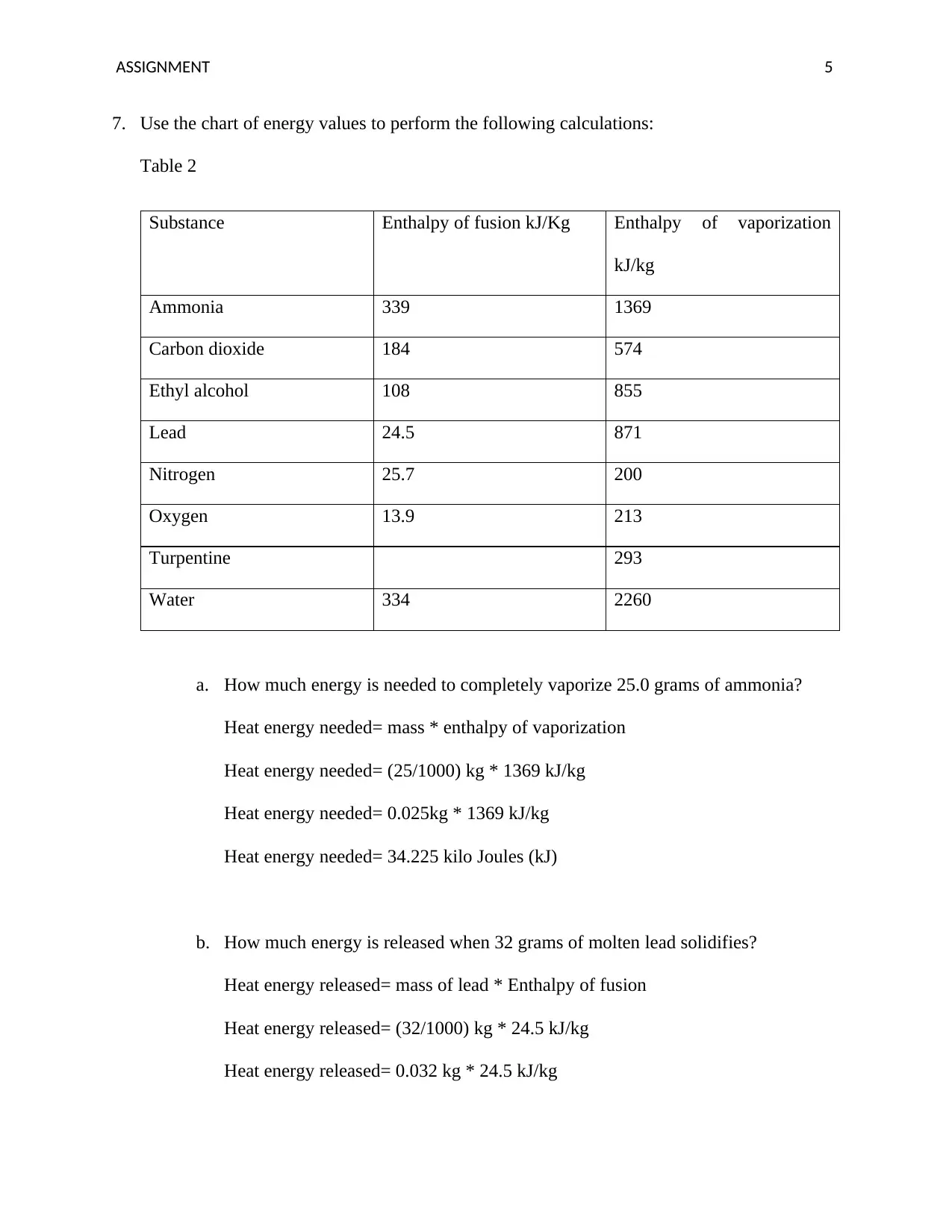
7. Use the chart of energy values to perform the following calculations:
Table 2
Substance Enthalpy of fusion kJ/Kg Enthalpy of vaporization
kJ/kg
Ammonia 339 1369
Carbon dioxide 184 574
Ethyl alcohol 108 855
Lead 24.5 871
Nitrogen 25.7 200
Oxygen 13.9 213
Turpentine 293
Water 334 2260
a. How much energy is needed to completely vaporize 25.0 grams of ammonia?
Heat energy needed= mass * enthalpy of vaporization
Heat energy needed= (25/1000) kg * 1369 kJ/kg
Heat energy needed= 0.025kg * 1369 kJ/kg
Heat energy needed= 34.225 kilo Joules (kJ)
b. How much energy is released when 32 grams of molten lead solidifies?
Heat energy released= mass of lead * Enthalpy of fusion
Heat energy released= (32/1000) kg * 24.5 kJ/kg
Heat energy released= 0.032 kg * 24.5 kJ/kg
7. Use the chart of energy values to perform the following calculations:
Table 2
Substance Enthalpy of fusion kJ/Kg Enthalpy of vaporization
kJ/kg
Ammonia 339 1369
Carbon dioxide 184 574
Ethyl alcohol 108 855
Lead 24.5 871
Nitrogen 25.7 200
Oxygen 13.9 213
Turpentine 293
Water 334 2260
a. How much energy is needed to completely vaporize 25.0 grams of ammonia?
Heat energy needed= mass * enthalpy of vaporization
Heat energy needed= (25/1000) kg * 1369 kJ/kg
Heat energy needed= 0.025kg * 1369 kJ/kg
Heat energy needed= 34.225 kilo Joules (kJ)
b. How much energy is released when 32 grams of molten lead solidifies?
Heat energy released= mass of lead * Enthalpy of fusion
Heat energy released= (32/1000) kg * 24.5 kJ/kg
Heat energy released= 0.032 kg * 24.5 kJ/kg

ASSIGNMENT 6
Heat energy released= 0.784 kilo Joules (kJ)
8. Substance A has a specific heat capacity of 2.11 J/g/ºC and substance B has a specific heat
capacity of 0.38 J/g/ºC
a. Which substance will resist temperature change the most?
Answer: Substance A will resist temperature change the most.
Reason: substance A has a higher specific heat capacity value than B. 1 gram of A
requires 2.11 J of heat energy to rise in temperature by 1 ºC. Therefore, it requires
(2.11-0.38) = 1.73 J/g/ºC a lot of energy to raise the temperature by one degree for
substance A the B.
b. Which substance will require the least amount of energy to raise its temperature by
15ºC?
Let us assume that each substance is 1 gram in mass;
Heat energy= mass * specific heat capacity * change in temperature
For substance A:
Heat energy needed= 1 g * 2.11 J/g/ºC * 15ºC
Heat energy needed=31.65 Joules
For substance B:
Heat energy needed= 1 g * 0.38 J/g/ºC * 15ºC
Heat energy needed= 5.7 Joules
Heat energy released= 0.784 kilo Joules (kJ)
8. Substance A has a specific heat capacity of 2.11 J/g/ºC and substance B has a specific heat
capacity of 0.38 J/g/ºC
a. Which substance will resist temperature change the most?
Answer: Substance A will resist temperature change the most.
Reason: substance A has a higher specific heat capacity value than B. 1 gram of A
requires 2.11 J of heat energy to rise in temperature by 1 ºC. Therefore, it requires
(2.11-0.38) = 1.73 J/g/ºC a lot of energy to raise the temperature by one degree for
substance A the B.
b. Which substance will require the least amount of energy to raise its temperature by
15ºC?
Let us assume that each substance is 1 gram in mass;
Heat energy= mass * specific heat capacity * change in temperature
For substance A:
Heat energy needed= 1 g * 2.11 J/g/ºC * 15ºC
Heat energy needed=31.65 Joules
For substance B:
Heat energy needed= 1 g * 0.38 J/g/ºC * 15ºC
Heat energy needed= 5.7 Joules
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

ASSIGNMENT 7
Substance A, therefore, needs a lot of heat energy to raise its 1 gram by 15ºC
c. If 350 J of energy was applied to both samples of equal masses, which would have a
higher final temperature?
Let us assume both substances have an equal mass of 1 gram;
Heat energy= mass * specific heat capacity * change in temperature
For substance A:
350 J= 1 g * 2.11 J/g/ºC * temperature change (ºC)
Temp. Change= (350 J/2.11J/g/ºC) = 165.88ºC
Temp. Change=165.88ºC
For substance B:
350 J= 1 g * 0.38 J/g/ºC * temperature change (ºC)
Temp. Change= (350 J/0.38 J/g/ºC) = 921.05ºC
Temp. Change= 921.05ºC
Assuming both were having the same temperature of 20ºC before supplied with heat
energy then substance A and B final temperature will be;
Substance A: 20ºC + 165.88ºC= 185.88ºC
Substance B: 20ºC + 921.05ºC= 941.05ºC
Therefore substance B will attain a higher final temperature of 941.05ºC than
substance A with a final temperature of 185.88ºC. This is because B has a lower
specific heat capacity than A.
Substance A, therefore, needs a lot of heat energy to raise its 1 gram by 15ºC
c. If 350 J of energy was applied to both samples of equal masses, which would have a
higher final temperature?
Let us assume both substances have an equal mass of 1 gram;
Heat energy= mass * specific heat capacity * change in temperature
For substance A:
350 J= 1 g * 2.11 J/g/ºC * temperature change (ºC)
Temp. Change= (350 J/2.11J/g/ºC) = 165.88ºC
Temp. Change=165.88ºC
For substance B:
350 J= 1 g * 0.38 J/g/ºC * temperature change (ºC)
Temp. Change= (350 J/0.38 J/g/ºC) = 921.05ºC
Temp. Change= 921.05ºC
Assuming both were having the same temperature of 20ºC before supplied with heat
energy then substance A and B final temperature will be;
Substance A: 20ºC + 165.88ºC= 185.88ºC
Substance B: 20ºC + 921.05ºC= 941.05ºC
Therefore substance B will attain a higher final temperature of 941.05ºC than
substance A with a final temperature of 185.88ºC. This is because B has a lower
specific heat capacity than A.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ASSIGNMENT 8
References
1. ACREE, W. E., Professor (Ed.). (2006). The Journal of Chemical Thermodynamics.
The Journal of Chemical Thermodynamics, 38(1), Iii-Vii.
doi:10.1016/j.jct.2005.11.001
2. NAVE R. (n.d.). Thermal Equilibrium. Retrieved June 10, 2019. Retrieved from
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/thereq.html
3. Theodore, L., Ricci, F., & Vliet, T. V. (2009). Thermodynamics for the practicing
engineer. Hoboken (N.J.): Wiley.
References
1. ACREE, W. E., Professor (Ed.). (2006). The Journal of Chemical Thermodynamics.
The Journal of Chemical Thermodynamics, 38(1), Iii-Vii.
doi:10.1016/j.jct.2005.11.001
2. NAVE R. (n.d.). Thermal Equilibrium. Retrieved June 10, 2019. Retrieved from
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/thereq.html
3. Theodore, L., Ricci, F., & Vliet, T. V. (2009). Thermodynamics for the practicing
engineer. Hoboken (N.J.): Wiley.
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
