University of Pharmacy: Thermosensitive Hydrogels Comprehensive Report
VerifiedAdded on 2023/01/19
|20
|5758
|20
Report
AI Summary
This report provides a comprehensive overview of thermosensitive hydrogels, focusing on their preparation, classification, characterization, and diverse applications, particularly in drug delivery systems. The introduction highlights the significance of hydrogels as controlled-release polymeric drug systems, emphasizing their biocompatibility and biodegradability. The report delves into the classification of hydrogels based on polymer composition and configuration, including homopolymeric, copolymeric, and interpenetrating polymer hydrogels. It also discusses the environmental sensitivity of hydrogels and various characterization processes, such as in vitro and in vivo tests, including injectable formulations and specific examples like tramadol release from poloxamer thermogels and tests for vulvovaginal candidiasis. Characterization methods, including solubility, swelling methods, FTR method, scanning electron microscopy, and rheology, are discussed. The report further explores the application of thermosensitive hydrogels in drug delivery, tissue engineering, and other biomedical fields, highlighting their advantages in drug delivery and sustained release. The report also touches on the need for careful analysis of hydrogel types and modification of desired features to suit drug release kinetics.

Thermosensitive hydrogels
University
Pharmacology Report
University
Pharmacology Report
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of contents
Introduction....................................................................................................................................3
Preparation.....................................................................................................................................4
Hydrogel classification..................................................................................................................5
Configuration classification.......................................................................................................5
Cross-linking type of classification...........................................................................................5
Environmental sensitivity hydrogels..........................................................................................6
Characterization process..............................................................................................................11
In vitro tests..............................................................................................................................11
Injectable formulation..........................................................................................................11
Tramadol release from poloxamer thermogels....................................................................11
In vivo tests..............................................................................................................................11
Vulvovaginal candidiasis using amphotericin tests.............................................................11
Reversible PDLLA-PEG-PDLLA copolymer thermogels...................................................12
Characterization methods.........................................................................................................12
Solubility..............................................................................................................................12
Swelling method...................................................................................................................12
FTR method..........................................................................................................................13
Scanning Electron Microscopy............................................................................................13
Light scattering....................................................................................................................13
Sol-gel analysis....................................................................................................................14
Rheology..............................................................................................................................14
Other methods......................................................................................................................14
Application of hydrogel...............................................................................................................15
References....................................................................................................................................17
2
Introduction....................................................................................................................................3
Preparation.....................................................................................................................................4
Hydrogel classification..................................................................................................................5
Configuration classification.......................................................................................................5
Cross-linking type of classification...........................................................................................5
Environmental sensitivity hydrogels..........................................................................................6
Characterization process..............................................................................................................11
In vitro tests..............................................................................................................................11
Injectable formulation..........................................................................................................11
Tramadol release from poloxamer thermogels....................................................................11
In vivo tests..............................................................................................................................11
Vulvovaginal candidiasis using amphotericin tests.............................................................11
Reversible PDLLA-PEG-PDLLA copolymer thermogels...................................................12
Characterization methods.........................................................................................................12
Solubility..............................................................................................................................12
Swelling method...................................................................................................................12
FTR method..........................................................................................................................13
Scanning Electron Microscopy............................................................................................13
Light scattering....................................................................................................................13
Sol-gel analysis....................................................................................................................14
Rheology..............................................................................................................................14
Other methods......................................................................................................................14
Application of hydrogel...............................................................................................................15
References....................................................................................................................................17
2

Introduction
Hydrogel entails a promising class of the polymeric drug system which have controlled
released rates. Among these are the thermosensitive hydrogels which have transition
temperate within the body range. Most of the compounds in this category tend to be
biocompatible and biodegradable making it a favorable thermosensitive hydrogel.
Hydrogels depict the desired forms of chemical properties which are specific to the certain
key biomedical function. It has been explored in molecular engineering among many types of
acrylic which other crosslinkers available. The most widely used hydrogels are the water-
swollen hydrogen, which is cross-linked with PEHAM which is introduced biologically he
inert hydrogel tend to be normal in the processes involving biology and the degradation
resistances process. Further it is also permeable to metabolites and non absorbent in the
body.. Its nature of being biocompatible is able to offer properties which are key in
withstanding heat without damage and cab used to prepare other forms. The swelling and the
biomedical characteristics of thermosensitive hydrogels have been studied extensively
(Kumru, Shalom, Antonietti & Schmidt, 2017).
Aqueous polymers have been used to transform the gels into environmental changes such as
the pH and temperature which result in situ hydrogel formation. This has focussed the key
interest in the development of biomedical importance and pharmaceuticals. Formation of the
hydrogels under physiological state often tends to be crucial in maintaining the desired
integrity over certain distortion of time. With regard to the simplicity of the pharmaceutical
formulation, biological compatibility with biological systems tends to be convenient. Usage
of water-based sol-gel transition such as those of low molecular weight has been used in the
in biomedical uses (Ma and Tian, 2014).
Thermosensitive hydrogels have played a crucial role in biomedical extensive research
studies and occasioned in the drug delivery system. The thermosensitive hydrogel has the
potential in various aspects such as those in drug delivery, encapsulating of cells engineering
of tissues among others. Injectable hydrogels with low solution gel transition temperature
under the normal physiological temperature have been widely been used. Incorporation in
pharmaceutical drug development t protocols he hydrogels have been shown to act on the
sustained drug depot in situ release. Thermosensitive hydrogels injected have various
3
Hydrogel entails a promising class of the polymeric drug system which have controlled
released rates. Among these are the thermosensitive hydrogels which have transition
temperate within the body range. Most of the compounds in this category tend to be
biocompatible and biodegradable making it a favorable thermosensitive hydrogel.
Hydrogels depict the desired forms of chemical properties which are specific to the certain
key biomedical function. It has been explored in molecular engineering among many types of
acrylic which other crosslinkers available. The most widely used hydrogels are the water-
swollen hydrogen, which is cross-linked with PEHAM which is introduced biologically he
inert hydrogel tend to be normal in the processes involving biology and the degradation
resistances process. Further it is also permeable to metabolites and non absorbent in the
body.. Its nature of being biocompatible is able to offer properties which are key in
withstanding heat without damage and cab used to prepare other forms. The swelling and the
biomedical characteristics of thermosensitive hydrogels have been studied extensively
(Kumru, Shalom, Antonietti & Schmidt, 2017).
Aqueous polymers have been used to transform the gels into environmental changes such as
the pH and temperature which result in situ hydrogel formation. This has focussed the key
interest in the development of biomedical importance and pharmaceuticals. Formation of the
hydrogels under physiological state often tends to be crucial in maintaining the desired
integrity over certain distortion of time. With regard to the simplicity of the pharmaceutical
formulation, biological compatibility with biological systems tends to be convenient. Usage
of water-based sol-gel transition such as those of low molecular weight has been used in the
in biomedical uses (Ma and Tian, 2014).
Thermosensitive hydrogels have played a crucial role in biomedical extensive research
studies and occasioned in the drug delivery system. The thermosensitive hydrogel has the
potential in various aspects such as those in drug delivery, encapsulating of cells engineering
of tissues among others. Injectable hydrogels with low solution gel transition temperature
under the normal physiological temperature have been widely been used. Incorporation in
pharmaceutical drug development t protocols he hydrogels have been shown to act on the
sustained drug depot in situ release. Thermosensitive hydrogels injected have various
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

advantages over other simple drug delivery system drugs environment, prolonging of drug
delivery and relative ease of application (Matanović, Kristl, and Grabnar, 2014).
The state of drug delivery systems have been adversely promulgated and developed in the
hydrogel formulation to achieve the drug delivery aspects aiding the pharmaceutical progress.
They have been utilized in the improvement of the shortcoming of convention drug system.
Hydrogel belongs to a class of networks polymeric which have the function to retain high
amounts of water and maintaining their 3 D designs shapes. Hydrogels have been sensitivity
to various stimuli on their environment including chemical substances such as the
temperature, ph, light, and pressure. Hydrogel sensitivity, the investigation has focussed on in
situ gel formation which has been widely circulated (Li & Mooney, 2016).
Advances in the drug delivery systems, the injectable hydrogels which are thermosensitive
have been shown to act and play a critical. Under different temperature environment, the
thermosensitive hydrogels are free solutions which flow freely. In vivo injection has been
utilized to convert the free-flowing solution and nonflowing stages undertaken at body
temperature. The incorporation of the pharmaceutical hydrogels has been shown to have
more impact on the sustained effects of drug release. Thermos sensitive hydrogels tend to
have more advantages due to enhancement solubility nature of the hydrophobic drugs. They
offer enhances safety to patients they do not have toxic initiators any surgical procedures, site
specify and delivery of drugs to various types such as the hydrophobic, hydrophilic drugs
proteins, nucleic acid and even peptide drugs (Vashist Vashist, Gupta & Ahmad., 2014)
Application of thermosensitive hydrogel in drug formulations systems has shown there is a
need for crucial analysis on the type of hydrogel to be used and how other desired features
could be modified to suit the drug release kinetics. Both naturally occurring and synthetic
thermosensitive polymers have been widely been used to achieve the desired hydrogel
properties so as to achieve the needed drug profile effects (Li & Guan, 2011).
Preparation
Hydrogels have been shown to be characterized in various ways. The key steps entail
procedures such as parallel cross-linking and polymerization on multifaceted monomers and
other procedures on the synthesis of molecular polymer which have groups which are
reactive and linked to active group agents. The multiple steps have often involved various
4
delivery and relative ease of application (Matanović, Kristl, and Grabnar, 2014).
The state of drug delivery systems have been adversely promulgated and developed in the
hydrogel formulation to achieve the drug delivery aspects aiding the pharmaceutical progress.
They have been utilized in the improvement of the shortcoming of convention drug system.
Hydrogel belongs to a class of networks polymeric which have the function to retain high
amounts of water and maintaining their 3 D designs shapes. Hydrogels have been sensitivity
to various stimuli on their environment including chemical substances such as the
temperature, ph, light, and pressure. Hydrogel sensitivity, the investigation has focussed on in
situ gel formation which has been widely circulated (Li & Mooney, 2016).
Advances in the drug delivery systems, the injectable hydrogels which are thermosensitive
have been shown to act and play a critical. Under different temperature environment, the
thermosensitive hydrogels are free solutions which flow freely. In vivo injection has been
utilized to convert the free-flowing solution and nonflowing stages undertaken at body
temperature. The incorporation of the pharmaceutical hydrogels has been shown to have
more impact on the sustained effects of drug release. Thermos sensitive hydrogels tend to
have more advantages due to enhancement solubility nature of the hydrophobic drugs. They
offer enhances safety to patients they do not have toxic initiators any surgical procedures, site
specify and delivery of drugs to various types such as the hydrophobic, hydrophilic drugs
proteins, nucleic acid and even peptide drugs (Vashist Vashist, Gupta & Ahmad., 2014)
Application of thermosensitive hydrogel in drug formulations systems has shown there is a
need for crucial analysis on the type of hydrogel to be used and how other desired features
could be modified to suit the drug release kinetics. Both naturally occurring and synthetic
thermosensitive polymers have been widely been used to achieve the desired hydrogel
properties so as to achieve the needed drug profile effects (Li & Guan, 2011).
Preparation
Hydrogels have been shown to be characterized in various ways. The key steps entail
procedures such as parallel cross-linking and polymerization on multifaceted monomers and
other procedures on the synthesis of molecular polymer which have groups which are
reactive and linked to active group agents. The multiple steps have often involved various
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

steps in the polymer engineering designs and forms of networks polymer having scale
molecular control such as tailored density properties, such as biodegradation of chemical
compounds l response, biological factors, and mechanical strengthened and stimuli response.
Hydrogel classification
The hydrogels have been classified into various stages linked to various natural original
forms of synthetics (Zhao et al 2013). Classification can be undertaken using polymer
composition. Preparation methods tend to lead to critical formation of critical key classess
related to the hdrogel which include polymer classes of the hydrogel. They are exemplified
by the following types;
Homopolymeric hydrogels commonly referred to as network polymers and are obtained from
single species which entail the monomers and are the basic structure which has structural unit
polymer network. Homopolymers have been shown to have crosslinking skeletal structure
design to the monomer and polymer nature. The cross-linked skeletal structure tends to have
homopolymers which have cross-linked skeletal structures depending on the polymerization
technique undertaken on the polymer (Maeda et al., 2008).
Copolymeric hydrogels originated from two different monomers. They have at least a single
component of hydrophilic arrangement undertaken at random alternating or configured along
the polymer network.
Multi polymer interpenetrating polymer hydrogel is a critical class of the hydrogel origination
from cross linked polymer which has a network formation. In this formation, there is cross-
linking of polymer components and other key components which are not cross-linked
polymer (Hacker & Mikos, 2011).
Configuration classification
This mode of classification often depends on the physical structure and the chemical
composition underlying the noncrystalline structure, semi crystalline, and crystalline
structures.
Cross-linking type of classification
Hydrogels tend to comprise of various categories based on the chemical and physical
5
molecular control such as tailored density properties, such as biodegradation of chemical
compounds l response, biological factors, and mechanical strengthened and stimuli response.
Hydrogel classification
The hydrogels have been classified into various stages linked to various natural original
forms of synthetics (Zhao et al 2013). Classification can be undertaken using polymer
composition. Preparation methods tend to lead to critical formation of critical key classess
related to the hdrogel which include polymer classes of the hydrogel. They are exemplified
by the following types;
Homopolymeric hydrogels commonly referred to as network polymers and are obtained from
single species which entail the monomers and are the basic structure which has structural unit
polymer network. Homopolymers have been shown to have crosslinking skeletal structure
design to the monomer and polymer nature. The cross-linked skeletal structure tends to have
homopolymers which have cross-linked skeletal structures depending on the polymerization
technique undertaken on the polymer (Maeda et al., 2008).
Copolymeric hydrogels originated from two different monomers. They have at least a single
component of hydrophilic arrangement undertaken at random alternating or configured along
the polymer network.
Multi polymer interpenetrating polymer hydrogel is a critical class of the hydrogel origination
from cross linked polymer which has a network formation. In this formation, there is cross-
linking of polymer components and other key components which are not cross-linked
polymer (Hacker & Mikos, 2011).
Configuration classification
This mode of classification often depends on the physical structure and the chemical
composition underlying the noncrystalline structure, semi crystalline, and crystalline
structures.
Cross-linking type of classification
Hydrogels tend to comprise of various categories based on the chemical and physical
5
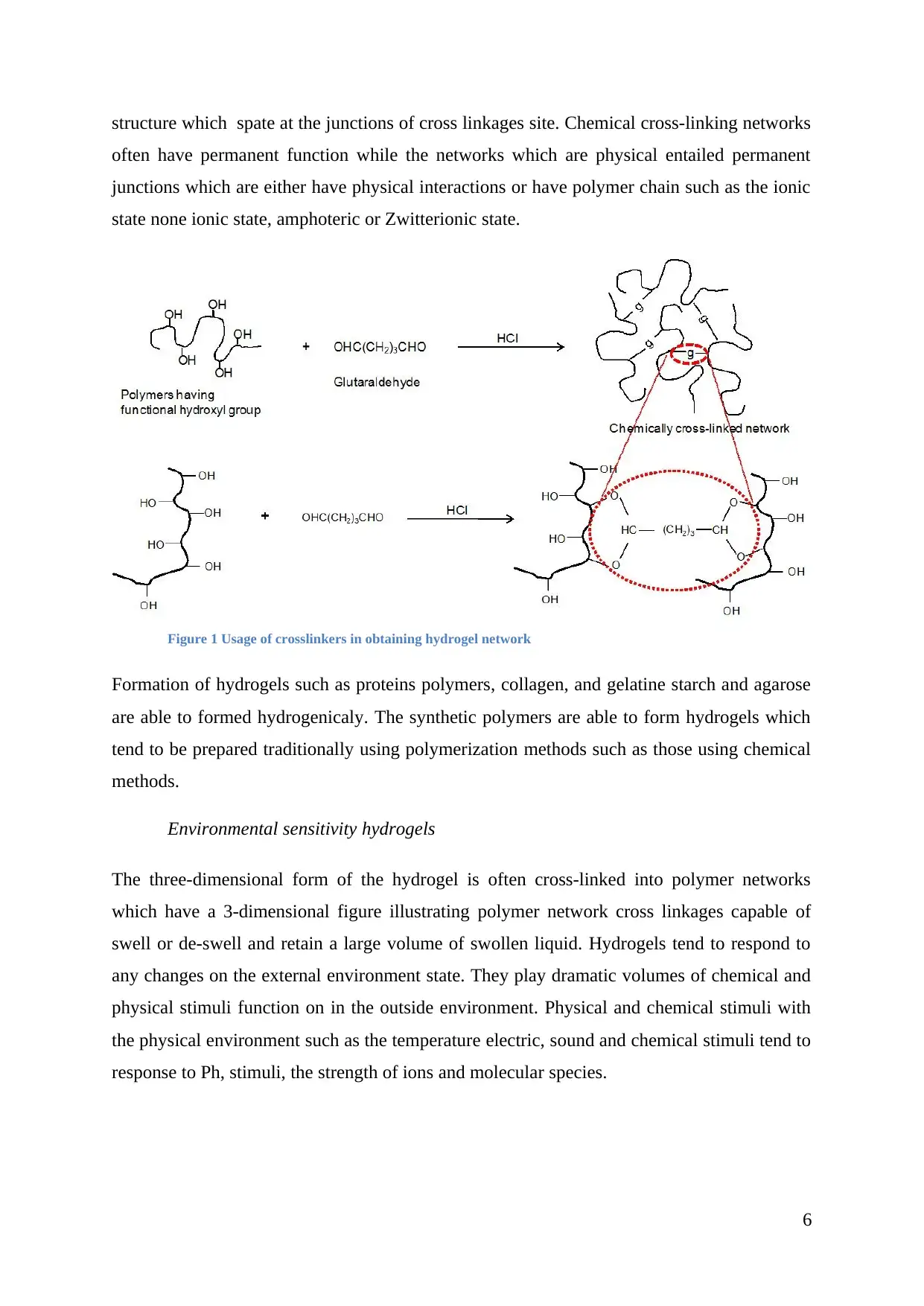
structure which spate at the junctions of cross linkages site. Chemical cross-linking networks
often have permanent function while the networks which are physical entailed permanent
junctions which are either have physical interactions or have polymer chain such as the ionic
state none ionic state, amphoteric or Zwitterionic state.
Figure 1 Usage of crosslinkers in obtaining hydrogel network
Formation of hydrogels such as proteins polymers, collagen, and gelatine starch and agarose
are able to formed hydrogenicaly. The synthetic polymers are able to form hydrogels which
tend to be prepared traditionally using polymerization methods such as those using chemical
methods.
Environmental sensitivity hydrogels
The three-dimensional form of the hydrogel is often cross-linked into polymer networks
which have a 3-dimensional figure illustrating polymer network cross linkages capable of
swell or de-swell and retain a large volume of swollen liquid. Hydrogels tend to respond to
any changes on the external environment state. They play dramatic volumes of chemical and
physical stimuli function on in the outside environment. Physical and chemical stimuli with
the physical environment such as the temperature electric, sound and chemical stimuli tend to
response to Ph, stimuli, the strength of ions and molecular species.
6
often have permanent function while the networks which are physical entailed permanent
junctions which are either have physical interactions or have polymer chain such as the ionic
state none ionic state, amphoteric or Zwitterionic state.
Figure 1 Usage of crosslinkers in obtaining hydrogel network
Formation of hydrogels such as proteins polymers, collagen, and gelatine starch and agarose
are able to formed hydrogenicaly. The synthetic polymers are able to form hydrogels which
tend to be prepared traditionally using polymerization methods such as those using chemical
methods.
Environmental sensitivity hydrogels
The three-dimensional form of the hydrogel is often cross-linked into polymer networks
which have a 3-dimensional figure illustrating polymer network cross linkages capable of
swell or de-swell and retain a large volume of swollen liquid. Hydrogels tend to respond to
any changes on the external environment state. They play dramatic volumes of chemical and
physical stimuli function on in the outside environment. Physical and chemical stimuli with
the physical environment such as the temperature electric, sound and chemical stimuli tend to
response to Ph, stimuli, the strength of ions and molecular species.
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
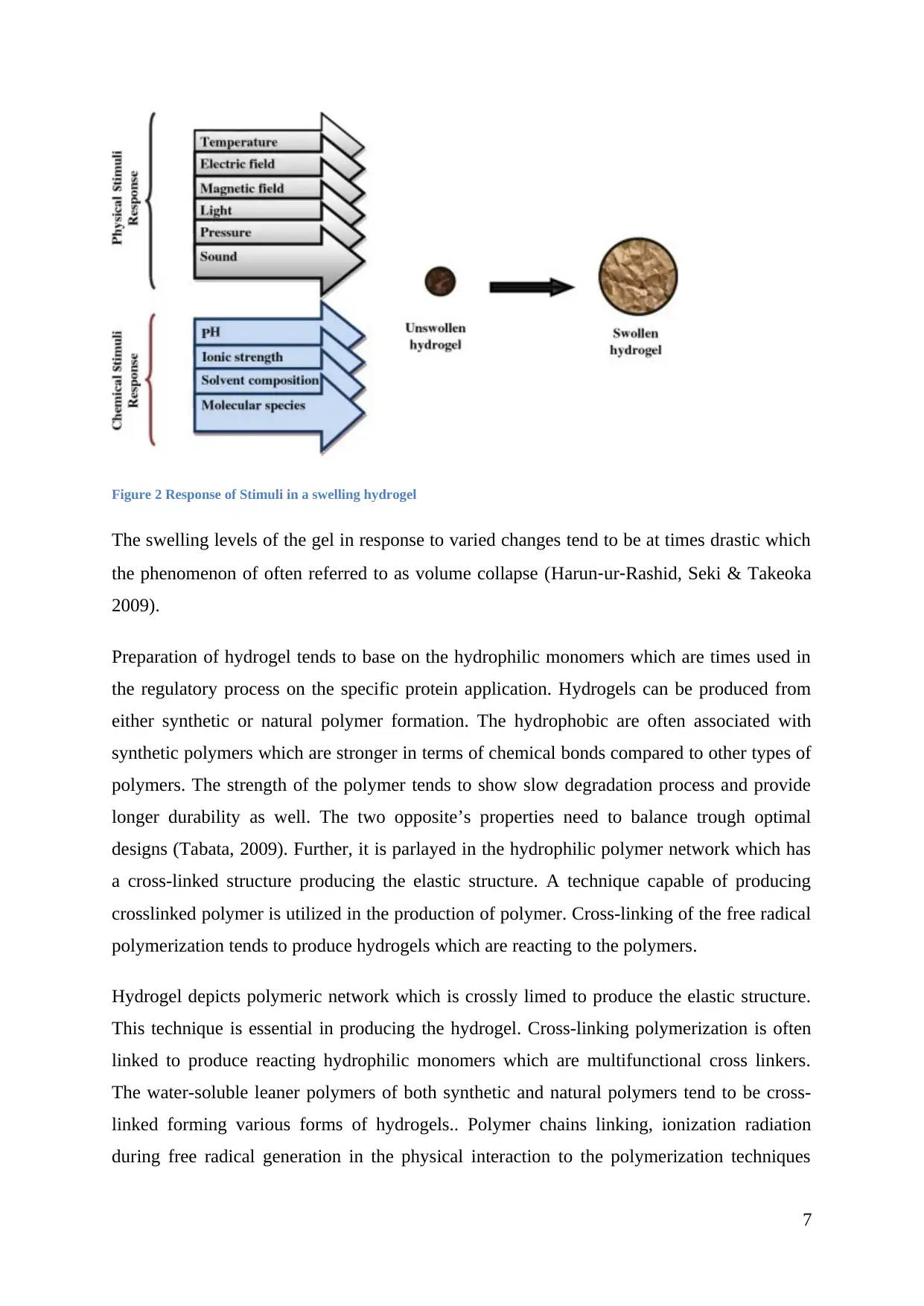
Figure 2 Response of Stimuli in a swelling hydrogel
The swelling levels of the gel in response to varied changes tend to be at times drastic which
the phenomenon of often referred to as volume collapse (Harun‐ur‐Rashid, Seki & Takeoka
2009).
Preparation of hydrogel tends to base on the hydrophilic monomers which are times used in
the regulatory process on the specific protein application. Hydrogels can be produced from
either synthetic or natural polymer formation. The hydrophobic are often associated with
synthetic polymers which are stronger in terms of chemical bonds compared to other types of
polymers. The strength of the polymer tends to show slow degradation process and provide
longer durability as well. The two opposite’s properties need to balance trough optimal
designs (Tabata, 2009). Further, it is parlayed in the hydrophilic polymer network which has
a cross-linked structure producing the elastic structure. A technique capable of producing
crosslinked polymer is utilized in the production of polymer. Cross-linking of the free radical
polymerization tends to produce hydrogels which are reacting to the polymers.
Hydrogel depicts polymeric network which is crossly limed to produce the elastic structure.
This technique is essential in producing the hydrogel. Cross-linking polymerization is often
linked to produce reacting hydrophilic monomers which are multifunctional cross linkers.
The water-soluble leaner polymers of both synthetic and natural polymers tend to be cross-
linked forming various forms of hydrogels.. Polymer chains linking, ionization radiation
during free radical generation in the physical interaction to the polymerization techniques
7
The swelling levels of the gel in response to varied changes tend to be at times drastic which
the phenomenon of often referred to as volume collapse (Harun‐ur‐Rashid, Seki & Takeoka
2009).
Preparation of hydrogel tends to base on the hydrophilic monomers which are times used in
the regulatory process on the specific protein application. Hydrogels can be produced from
either synthetic or natural polymer formation. The hydrophobic are often associated with
synthetic polymers which are stronger in terms of chemical bonds compared to other types of
polymers. The strength of the polymer tends to show slow degradation process and provide
longer durability as well. The two opposite’s properties need to balance trough optimal
designs (Tabata, 2009). Further, it is parlayed in the hydrophilic polymer network which has
a cross-linked structure producing the elastic structure. A technique capable of producing
crosslinked polymer is utilized in the production of polymer. Cross-linking of the free radical
polymerization tends to produce hydrogels which are reacting to the polymers.
Hydrogel depicts polymeric network which is crossly limed to produce the elastic structure.
This technique is essential in producing the hydrogel. Cross-linking polymerization is often
linked to produce reacting hydrophilic monomers which are multifunctional cross linkers.
The water-soluble leaner polymers of both synthetic and natural polymers tend to be cross-
linked forming various forms of hydrogels.. Polymer chains linking, ionization radiation
during free radical generation in the physical interaction to the polymerization techniques
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
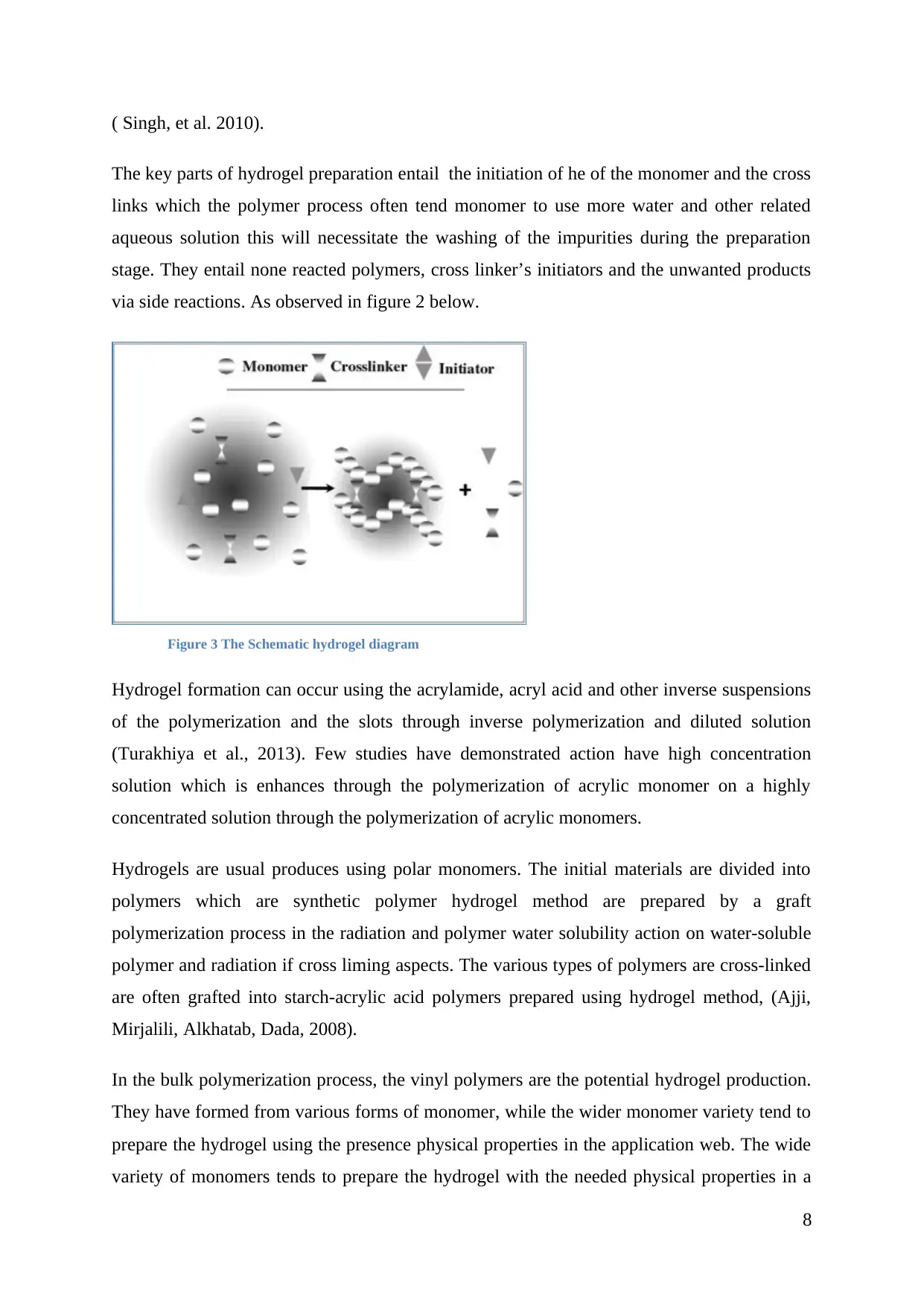
( Singh, et al. 2010).
The key parts of hydrogel preparation entail the initiation of he of the monomer and the cross
links which the polymer process often tend monomer to use more water and other related
aqueous solution this will necessitate the washing of the impurities during the preparation
stage. They entail none reacted polymers, cross linker’s initiators and the unwanted products
via side reactions. As observed in figure 2 below.
Figure 3 The Schematic hydrogel diagram
Hydrogel formation can occur using the acrylamide, acryl acid and other inverse suspensions
of the polymerization and the slots through inverse polymerization and diluted solution
(Turakhiya et al., 2013). Few studies have demonstrated action have high concentration
solution which is enhances through the polymerization of acrylic monomer on a highly
concentrated solution through the polymerization of acrylic monomers.
Hydrogels are usual produces using polar monomers. The initial materials are divided into
polymers which are synthetic polymer hydrogel method are prepared by a graft
polymerization process in the radiation and polymer water solubility action on water-soluble
polymer and radiation if cross liming aspects. The various types of polymers are cross-linked
are often grafted into starch-acrylic acid polymers prepared using hydrogel method, (Ajji,
Mirjalili, Alkhatab, Dada, 2008).
In the bulk polymerization process, the vinyl polymers are the potential hydrogel production.
They have formed from various forms of monomer, while the wider monomer variety tend to
prepare the hydrogel using the presence physical properties in the application web. The wide
variety of monomers tends to prepare the hydrogel with the needed physical properties in a
8
The key parts of hydrogel preparation entail the initiation of he of the monomer and the cross
links which the polymer process often tend monomer to use more water and other related
aqueous solution this will necessitate the washing of the impurities during the preparation
stage. They entail none reacted polymers, cross linker’s initiators and the unwanted products
via side reactions. As observed in figure 2 below.
Figure 3 The Schematic hydrogel diagram
Hydrogel formation can occur using the acrylamide, acryl acid and other inverse suspensions
of the polymerization and the slots through inverse polymerization and diluted solution
(Turakhiya et al., 2013). Few studies have demonstrated action have high concentration
solution which is enhances through the polymerization of acrylic monomer on a highly
concentrated solution through the polymerization of acrylic monomers.
Hydrogels are usual produces using polar monomers. The initial materials are divided into
polymers which are synthetic polymer hydrogel method are prepared by a graft
polymerization process in the radiation and polymer water solubility action on water-soluble
polymer and radiation if cross liming aspects. The various types of polymers are cross-linked
are often grafted into starch-acrylic acid polymers prepared using hydrogel method, (Ajji,
Mirjalili, Alkhatab, Dada, 2008).
In the bulk polymerization process, the vinyl polymers are the potential hydrogel production.
They have formed from various forms of monomer, while the wider monomer variety tend to
prepare the hydrogel using the presence physical properties in the application web. The wide
variety of monomers tends to prepare the hydrogel with the needed physical properties in a
8
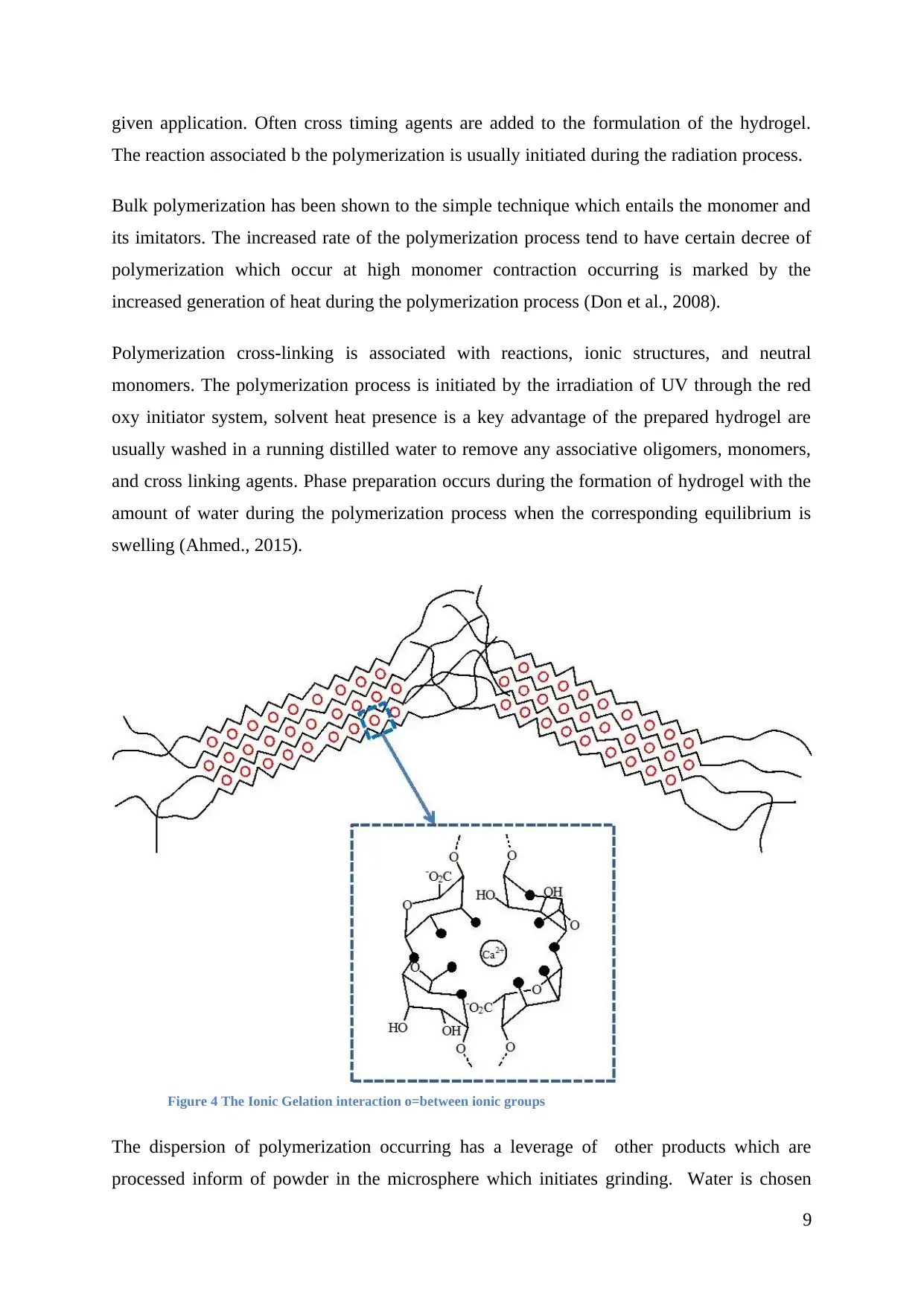
given application. Often cross timing agents are added to the formulation of the hydrogel.
The reaction associated b the polymerization is usually initiated during the radiation process.
Bulk polymerization has been shown to the simple technique which entails the monomer and
its imitators. The increased rate of the polymerization process tend to have certain decree of
polymerization which occur at high monomer contraction occurring is marked by the
increased generation of heat during the polymerization process (Don et al., 2008).
Polymerization cross-linking is associated with reactions, ionic structures, and neutral
monomers. The polymerization process is initiated by the irradiation of UV through the red
oxy initiator system, solvent heat presence is a key advantage of the prepared hydrogel are
usually washed in a running distilled water to remove any associative oligomers, monomers,
and cross linking agents. Phase preparation occurs during the formation of hydrogel with the
amount of water during the polymerization process when the corresponding equilibrium is
swelling (Ahmed., 2015).
Figure 4 The Ionic Gelation interaction o=between ionic groups
The dispersion of polymerization occurring has a leverage of other products which are
processed inform of powder in the microsphere which initiates grinding. Water is chosen
9
The reaction associated b the polymerization is usually initiated during the radiation process.
Bulk polymerization has been shown to the simple technique which entails the monomer and
its imitators. The increased rate of the polymerization process tend to have certain decree of
polymerization which occur at high monomer contraction occurring is marked by the
increased generation of heat during the polymerization process (Don et al., 2008).
Polymerization cross-linking is associated with reactions, ionic structures, and neutral
monomers. The polymerization process is initiated by the irradiation of UV through the red
oxy initiator system, solvent heat presence is a key advantage of the prepared hydrogel are
usually washed in a running distilled water to remove any associative oligomers, monomers,
and cross linking agents. Phase preparation occurs during the formation of hydrogel with the
amount of water during the polymerization process when the corresponding equilibrium is
swelling (Ahmed., 2015).
Figure 4 The Ionic Gelation interaction o=between ionic groups
The dispersion of polymerization occurring has a leverage of other products which are
processed inform of powder in the microsphere which initiates grinding. Water is chosen
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

instead of oil. This leads to dispersion of the monomer and the initiator which undergoes
dispersion in the hydrocarbon phase leading to a compound formation. The agitation of the
monomer tends to form agitation and dispersant mainly covering in the resin particle
formation and shape is (McLeod et al., 2016). This fundamental issue entails heterophase
polymerization occurring dispersion of the thermodynamically unstable requires continues
agitation process and addition low hydrophilic and hydrophobic balance effect in the body.
The hydrogel often has a weak structure which has bulk polymerization and has a weak
structure. This improves the mechanical support and properties if hydrogel they are grafted
on the surface into stronger support. This process entails the generation of free radicals which
tend to have stronger support and surface which the polymer monomer is directed into which
lead to covalently bonding in order to support.
The occurring polymerization through radiation tends to cause high ionization process.
Which entail gamma rays and electron usage (Torisu et al., 2017). The radiation process of
the prepared hydrogel tends to form unsaturated compounds’ he irradiation of this aqueous
forms tends to radical formation in the polymer chains. Water molecules radiolysis result in
the formation of the hydroxyl radicals which tend to attack the polymer chains and leading to
macromolecules. The macroradicals combination on various chains releases covalent bonds
which lead to formation of covalent bonds.
The key properties for the formation of hydrogel tend to have high absorbency, lower soluble
content, lower prices, high durability, high biodegradability, pH neutrality, and photo
stability. Most often haemophilic hydrogel tends to full fill all these criteria, they need to at
least have fulfilment on few features. The design implications of the hydrogel tend to be
accomplished through the monomer polymerization process and the modification to the
existing polymers. The original source is often divided into key classes which entail artificial
and natural methods. The natural method pathway is divided into two groups of
polysaccharide and polypeptides. The natural occurring based hydrogels are produced from
the addition of synthetic patch found in the natural occurring substrates (Ma, Li & Bao,
2015).
The greatest volume of hydrogel tends to comprise of artificial petrochemical which is
obtained from the acrylic monomers. The acid from the acrylics tend to form a produced from
the acrylic monomers. The acrylic acid and the relaxant acryl amide are used often in the
10
dispersion in the hydrocarbon phase leading to a compound formation. The agitation of the
monomer tends to form agitation and dispersant mainly covering in the resin particle
formation and shape is (McLeod et al., 2016). This fundamental issue entails heterophase
polymerization occurring dispersion of the thermodynamically unstable requires continues
agitation process and addition low hydrophilic and hydrophobic balance effect in the body.
The hydrogel often has a weak structure which has bulk polymerization and has a weak
structure. This improves the mechanical support and properties if hydrogel they are grafted
on the surface into stronger support. This process entails the generation of free radicals which
tend to have stronger support and surface which the polymer monomer is directed into which
lead to covalently bonding in order to support.
The occurring polymerization through radiation tends to cause high ionization process.
Which entail gamma rays and electron usage (Torisu et al., 2017). The radiation process of
the prepared hydrogel tends to form unsaturated compounds’ he irradiation of this aqueous
forms tends to radical formation in the polymer chains. Water molecules radiolysis result in
the formation of the hydroxyl radicals which tend to attack the polymer chains and leading to
macromolecules. The macroradicals combination on various chains releases covalent bonds
which lead to formation of covalent bonds.
The key properties for the formation of hydrogel tend to have high absorbency, lower soluble
content, lower prices, high durability, high biodegradability, pH neutrality, and photo
stability. Most often haemophilic hydrogel tends to full fill all these criteria, they need to at
least have fulfilment on few features. The design implications of the hydrogel tend to be
accomplished through the monomer polymerization process and the modification to the
existing polymers. The original source is often divided into key classes which entail artificial
and natural methods. The natural method pathway is divided into two groups of
polysaccharide and polypeptides. The natural occurring based hydrogels are produced from
the addition of synthetic patch found in the natural occurring substrates (Ma, Li & Bao,
2015).
The greatest volume of hydrogel tends to comprise of artificial petrochemical which is
obtained from the acrylic monomers. The acid from the acrylics tend to form a produced from
the acrylic monomers. The acrylic acid and the relaxant acryl amide are used often in the
10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

production of the hydrogel. The two key networks for simultaneous polymerization entail
cross-linking to the polyvinyl crosslinker and water-soluble polymers. The most efficient
way of producing synthetic hydrogels tend to sue free radical which is multifunctional vinyl
polymers which have double carbon bonds and active centre which propagate the production
of longer chains.
Characterization process
In vitro tests
Injectable formulation
Surgical interventions of solid tumors have been used as the most preferred form of treatment
in cancer, which occurrence of the tumor is imminent. The need for controlled delivery of the
tumour site with therapeutic agents was assessed using injectable chitosan gel – DZCGs
having doxorubicin loaded in Aein nanoparticles. The synthesis was performed and the size
of DOX-SC SNPs was determined. In vitro drug tests release on this profile revealed that the
tumor was more controlled using the DZ CGs compared to DOX SC ZNPs-Doxorubicin. The
comparison was assessed using great importance on breast cancer cell lines and DZ-CGs
composite is more effective in suppressing cancer cells (Arunkumar, Indulekha,
Vijayalakshmi & Srivstava, 2017).
Tramadol release from poloxamer thermogels
Poloxamer based gels have been sued on tramadol use. The thermosensitive gel formation
entailed gelling behavior, drug content and drug release. The assessment used immersion
cells to investigate on tramadol drug. The findings of this in vitro drug testing revealed that
the use of cell immersion leads to lower drug release compared to the absence of the drug.
The key factor which influences the drug release was significantly associated with a
membrane pore size in vitro assessment protocol process (Bisharat Perinelli & Palmieri,
2017).
In vivo tests
Vulvovaginal candidiasis using amphotericin tests
Assessment of vulvovaginal candidiasis a vaginal mucosal infection has been established.
11
cross-linking to the polyvinyl crosslinker and water-soluble polymers. The most efficient
way of producing synthetic hydrogels tend to sue free radical which is multifunctional vinyl
polymers which have double carbon bonds and active centre which propagate the production
of longer chains.
Characterization process
In vitro tests
Injectable formulation
Surgical interventions of solid tumors have been used as the most preferred form of treatment
in cancer, which occurrence of the tumor is imminent. The need for controlled delivery of the
tumour site with therapeutic agents was assessed using injectable chitosan gel – DZCGs
having doxorubicin loaded in Aein nanoparticles. The synthesis was performed and the size
of DOX-SC SNPs was determined. In vitro drug tests release on this profile revealed that the
tumor was more controlled using the DZ CGs compared to DOX SC ZNPs-Doxorubicin. The
comparison was assessed using great importance on breast cancer cell lines and DZ-CGs
composite is more effective in suppressing cancer cells (Arunkumar, Indulekha,
Vijayalakshmi & Srivstava, 2017).
Tramadol release from poloxamer thermogels
Poloxamer based gels have been sued on tramadol use. The thermosensitive gel formation
entailed gelling behavior, drug content and drug release. The assessment used immersion
cells to investigate on tramadol drug. The findings of this in vitro drug testing revealed that
the use of cell immersion leads to lower drug release compared to the absence of the drug.
The key factor which influences the drug release was significantly associated with a
membrane pore size in vitro assessment protocol process (Bisharat Perinelli & Palmieri,
2017).
In vivo tests
Vulvovaginal candidiasis using amphotericin tests
Assessment of vulvovaginal candidiasis a vaginal mucosal infection has been established.
11

Amphotericin drug was developed using high-pressure homogenization and poloxamer
p407/p188 hydrogel. The participle size of amphotericin as hydrogenized in 247 nm. In vivo
assessment of this drug towards the management of vulva, vagina candidiasis showed better
efficacy compared to commercial amphotericin effervescent tablets (Tianyuan et al., 2018).
Reversible PDLLA-PEG-PDLLA copolymer thermogels
Injectable thermoreversible and thermo gelling agent PDLLA-PEG-PDLLA copolymers was
developed to assess the thermal gelling effect of in vivo test. In vivo experiment on rat
illustrated that the hydrogel had the capacity to retain its integrity and entail hydrolysis effect.
There was observed a significant reduction on postoperative adhesion associated with
PDLLA-PEG-PDLLA treated with hydrogel, (Shi et al., 2016).
A fundamental way of quantifying the presence of hydrogel is through dispersal at the
polymer system using water in a cylindrical vial and visual observation using the insoluble
formation. The viscosity visual monitor colludes to achieve tuning to the universal upside
down which offers quick measures of the bulky viscosity.
Characterization methods
Solubility
In this method, the hydrogel component tends to estimated by insoluble part using the dried
sample and immersed in deionized water for an estimate of 16 hours. The sample further is
dilated in the concentration at 1% to ensure that there is full dispersion of the hydrogel
material (Sung, et al., 2015). The formula for calculating the gel fraction entailed the formula,
Gel Fraction(hydrogel%)=(WdWi)*100 E1
Where the Wi is the first weight of dry sample while Wd indicates the dry sample obtained
from water extraction.
Swelling method
A more common form of standardization been used is the Japanese Industrial Standards
k8150 method which is essential in the hydrogels swelling.. The dry hydrogel is deeper into
deionized water for an estimate of 48 hours in room temperature using a role mix (Wahid et
al., 2017). After the swelling process, the filtration of hydrogel is initiated using stainless
12
p407/p188 hydrogel. The participle size of amphotericin as hydrogenized in 247 nm. In vivo
assessment of this drug towards the management of vulva, vagina candidiasis showed better
efficacy compared to commercial amphotericin effervescent tablets (Tianyuan et al., 2018).
Reversible PDLLA-PEG-PDLLA copolymer thermogels
Injectable thermoreversible and thermo gelling agent PDLLA-PEG-PDLLA copolymers was
developed to assess the thermal gelling effect of in vivo test. In vivo experiment on rat
illustrated that the hydrogel had the capacity to retain its integrity and entail hydrolysis effect.
There was observed a significant reduction on postoperative adhesion associated with
PDLLA-PEG-PDLLA treated with hydrogel, (Shi et al., 2016).
A fundamental way of quantifying the presence of hydrogel is through dispersal at the
polymer system using water in a cylindrical vial and visual observation using the insoluble
formation. The viscosity visual monitor colludes to achieve tuning to the universal upside
down which offers quick measures of the bulky viscosity.
Characterization methods
Solubility
In this method, the hydrogel component tends to estimated by insoluble part using the dried
sample and immersed in deionized water for an estimate of 16 hours. The sample further is
dilated in the concentration at 1% to ensure that there is full dispersion of the hydrogel
material (Sung, et al., 2015). The formula for calculating the gel fraction entailed the formula,
Gel Fraction(hydrogel%)=(WdWi)*100 E1
Where the Wi is the first weight of dry sample while Wd indicates the dry sample obtained
from water extraction.
Swelling method
A more common form of standardization been used is the Japanese Industrial Standards
k8150 method which is essential in the hydrogels swelling.. The dry hydrogel is deeper into
deionized water for an estimate of 48 hours in room temperature using a role mix (Wahid et
al., 2017). After the swelling process, the filtration of hydrogel is initiated using stainless
12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 20
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.