4BIOM004W Histology Staining Assessment Practical Report
VerifiedAdded on 2023/04/23
|11
|2350
|261
Practical Assignment
AI Summary
This practical report details a student's assessment of tissue and organ staining techniques, focusing on Hematoxylin and Eosin (H&E) and Masson Trichrome staining methods. The introduction explains the significance of histological assessments in biological studies, followed by the aims to examine tissue components under these staining methods. The report outlines the materials and methods used, including Mallory's Trichome and H&E techniques, and presents the results through microscopic images of various organs like the thymus, parotid gland, and liver. The findings are compared with benchmark images to aid in cell differentiation and tissue description. The discussion section elaborates on the principles of trichrome staining, the properties of hematoxylin and eosin, and the advantages of each technique. The report concludes with a reference list, providing a comprehensive overview of the staining processes and their applications in histology.

UNIVERSITY
Task
Tissue and organ staining assessment practical
Unit
Name
Date
Tutor
Task
Tissue and organ staining assessment practical
Unit
Name
Date
Tutor
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

2
Introduction
Histological assessments are a key aspect in the study of plant and animal tissues through
staining procedures and dissection in order to allow comprehensive examination in a
microscope. Currently, there are varied methods utilized in tissue characteristics and
examining cell macroscopic structures. Histological assessments are essential in forensic
investigation, diagnosis, autopsy and education setups. Histology is utilized extensively
Medicare aiding treatment protocols, (Black .2012).
Histological staining offers a series of technique process which is undertaken during sample
preparation of sample tissues through staining aiding microscope studies, (Anderson, 2011).
The process of staining normally takes five stages which entail, fixation, processing,
embedding, sectioning, and staining process, (Titford, 2009). Great strides have been
undertaken in techniques for histological staining which collectively facilitates studies of cell
and tissues (Shostak, 2013). This has aided in faster approaches used to identify and
categorisie tissues on cells, thus facilitating diagnosis and differentiation process.
Staining is essential in highlighting the essential features of the tissue. Hematoxylin dye is the
basic component used in the staining process (Iyiolo & Avwioro, 2011). Fixation referred to
the use of chemical components in order to preserve the tissue structure. Fixation improves
and enhances tissue and cell preservation, (Young, O'Dowed & Steward, 2010). Dehydration
aims at removing water from the selected tissues facilitating thinning section much simpler
for light microscopic examination (Shostak, 2013). Embedding process facilitates easier
extraction of the underlying cellular structures, (Musumeci, 2014). Fixation can lead to cell
and tissue degradation occurring during prolonged heating. During the sectioning process,
ribbon preparation is made to allow for mounting on the slide for examination purposes.
Aims
The aim of this practical assessment was to conduct tissue assessment using two techniques.
Haematoxylin, Eosin and Masson Trichome staining process. The two approaches are
employed in this study so as to examine tissue. H&E allows basic staining on chromosomes
while Masson trichome allows for usage of hematoxylin staining. Thus the practical
assessment aims at examining the microscopic images of tissue components under these two
components.
Introduction
Histological assessments are a key aspect in the study of plant and animal tissues through
staining procedures and dissection in order to allow comprehensive examination in a
microscope. Currently, there are varied methods utilized in tissue characteristics and
examining cell macroscopic structures. Histological assessments are essential in forensic
investigation, diagnosis, autopsy and education setups. Histology is utilized extensively
Medicare aiding treatment protocols, (Black .2012).
Histological staining offers a series of technique process which is undertaken during sample
preparation of sample tissues through staining aiding microscope studies, (Anderson, 2011).
The process of staining normally takes five stages which entail, fixation, processing,
embedding, sectioning, and staining process, (Titford, 2009). Great strides have been
undertaken in techniques for histological staining which collectively facilitates studies of cell
and tissues (Shostak, 2013). This has aided in faster approaches used to identify and
categorisie tissues on cells, thus facilitating diagnosis and differentiation process.
Staining is essential in highlighting the essential features of the tissue. Hematoxylin dye is the
basic component used in the staining process (Iyiolo & Avwioro, 2011). Fixation referred to
the use of chemical components in order to preserve the tissue structure. Fixation improves
and enhances tissue and cell preservation, (Young, O'Dowed & Steward, 2010). Dehydration
aims at removing water from the selected tissues facilitating thinning section much simpler
for light microscopic examination (Shostak, 2013). Embedding process facilitates easier
extraction of the underlying cellular structures, (Musumeci, 2014). Fixation can lead to cell
and tissue degradation occurring during prolonged heating. During the sectioning process,
ribbon preparation is made to allow for mounting on the slide for examination purposes.
Aims
The aim of this practical assessment was to conduct tissue assessment using two techniques.
Haematoxylin, Eosin and Masson Trichome staining process. The two approaches are
employed in this study so as to examine tissue. H&E allows basic staining on chromosomes
while Masson trichome allows for usage of hematoxylin staining. Thus the practical
assessment aims at examining the microscopic images of tissue components under these two
components.

3
Methods and Materials
The tissue organs used in this assessment entails usage of two methods Mallory's Trichome
method and Hematoxylin and Eosin Method. In Mallory's method, Weigert iron hematoxylin
and iron solution were used. The slides were undertaken through various stages of staining.
The iron solution was filtered and added to an equal volume of hematoxylin solution. The
mixture color observed was violet/black in color. In solution A of Weigert's Iron
hematoxylin, 1 g of hematoxylin with alcohol 100mL was used while in solution B, 30% 4
ml aqueous solution of iron III chlorides was used mixed, 1 ml of concentrated hydrochloric
acid together with 95 ml of water.
Te selected slides were arranged in a stack and immersion began. Weigert's solution was used
for staining the nuclei, water runnel and later further staining was done using ponceau-acid
fuchsine solution. Rinsing was done followed for differentiation using phosphor molybdic
acid. After passing through the alcohol reagents, the slides were mounted on a microscope for
viewing.
In hematoxylin and Eosin technique, the needed reagents entailed Harris solution, acid
alcohol (1% hydrochloric acid in 70% v/v ethanol/IMS) 1% aqueous eosin, absolute ethanol,
xylene, and DPX mountant. The selected slides were dewaxed in arrange of solutions using
xylene, alcohol, and washing in running tap water. The slides were covered in hematoxylin
solution and immersed in running water. Thereafter, the slides were mounted for viewing.
Results
Mallory’s Trichrome
Mallory's trichrome technique used to analyze multi-block organ specimen revealed various
components and tissues of the human body. The figure 1 below illustrates the different organs
under observation. The organs indicated below entail; A; thymus organ B; parotid gland C;
thyroid gland D; tongue E; spleen
Methods and Materials
The tissue organs used in this assessment entails usage of two methods Mallory's Trichome
method and Hematoxylin and Eosin Method. In Mallory's method, Weigert iron hematoxylin
and iron solution were used. The slides were undertaken through various stages of staining.
The iron solution was filtered and added to an equal volume of hematoxylin solution. The
mixture color observed was violet/black in color. In solution A of Weigert's Iron
hematoxylin, 1 g of hematoxylin with alcohol 100mL was used while in solution B, 30% 4
ml aqueous solution of iron III chlorides was used mixed, 1 ml of concentrated hydrochloric
acid together with 95 ml of water.
Te selected slides were arranged in a stack and immersion began. Weigert's solution was used
for staining the nuclei, water runnel and later further staining was done using ponceau-acid
fuchsine solution. Rinsing was done followed for differentiation using phosphor molybdic
acid. After passing through the alcohol reagents, the slides were mounted on a microscope for
viewing.
In hematoxylin and Eosin technique, the needed reagents entailed Harris solution, acid
alcohol (1% hydrochloric acid in 70% v/v ethanol/IMS) 1% aqueous eosin, absolute ethanol,
xylene, and DPX mountant. The selected slides were dewaxed in arrange of solutions using
xylene, alcohol, and washing in running tap water. The slides were covered in hematoxylin
solution and immersed in running water. Thereafter, the slides were mounted for viewing.
Results
Mallory’s Trichrome
Mallory's trichrome technique used to analyze multi-block organ specimen revealed various
components and tissues of the human body. The figure 1 below illustrates the different organs
under observation. The organs indicated below entail; A; thymus organ B; parotid gland C;
thyroid gland D; tongue E; spleen
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
D
A
B
C
E
4
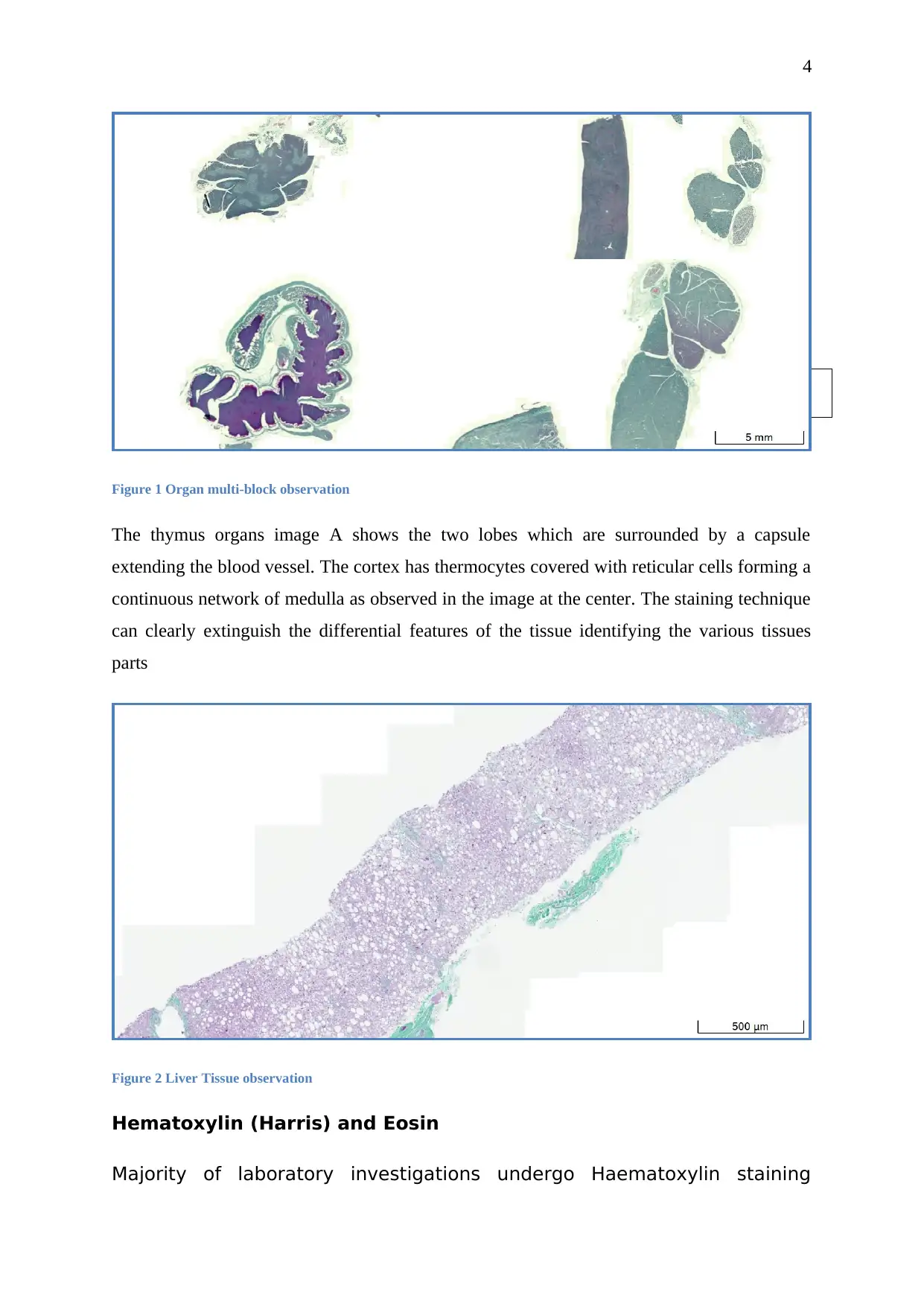
Figure 1 Organ multi-block observation
The thymus organs image A shows the two lobes which are surrounded by a capsule
extending the blood vessel. The cortex has thermocytes covered with reticular cells forming a
continuous network of medulla as observed in the image at the center. The staining technique
can clearly extinguish the differential features of the tissue identifying the various tissues
parts
Figure 2 Liver Tissue observation
Hematoxylin (Harris) and Eosin
Majority of laboratory investigations undergo Haematoxylin staining
A
B
C
E
4
Figure 1 Organ multi-block observation
The thymus organs image A shows the two lobes which are surrounded by a capsule
extending the blood vessel. The cortex has thermocytes covered with reticular cells forming a
continuous network of medulla as observed in the image at the center. The staining technique
can clearly extinguish the differential features of the tissue identifying the various tissues
parts
Figure 2 Liver Tissue observation
Hematoxylin (Harris) and Eosin
Majority of laboratory investigations undergo Haematoxylin staining
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

5
process. Tissues are normally treated with hematoxylin and eosin. The
results above indicated a microscopic examination of the different tissue
organs using this method. The liver tissue histology displays various
sheets of connective tissue which divide it into a thousand small units
referred to as lobules. The connective tissues extend deeply into batched
septae, delineating it into hepatic lobules and the overall unit of the liver.
The connective tissue surrounding the lobules is abundant. The rough
hexagonal plates arrangement can be vividly observed in the tissue
observed. The plates radiate outwards from the central vein (Sapno &
Flores, 2017).
The vertices located in the lobule are equally distributed in portal triads.
The presence of lymphatic system is present however they are vivid to
see. The presence of the lobules can be vividly observed. The precise
boundaries can be difficult to discern but a closer look illustrates the
orienting central veins and the portal tracts which allows smooth
identification process (Carriel et al., 2017).
The prescience of observable hepatocytes is observed as they are
arranged in plates which anatomizes on one another. The formed and
observable polygonal shapes and their sides can locate themselves on
sinusoidal. The hepatocyte nucleus is round having one or two nuclei
while many of the cells have binucleate cells.
Hepatocytes in the liver are actively engaged in protein synthesis and
exportation of lipids, hence conducting ultrastructural assessment shows
rough and smooth endoplasmic reticulum.
process. Tissues are normally treated with hematoxylin and eosin. The
results above indicated a microscopic examination of the different tissue
organs using this method. The liver tissue histology displays various
sheets of connective tissue which divide it into a thousand small units
referred to as lobules. The connective tissues extend deeply into batched
septae, delineating it into hepatic lobules and the overall unit of the liver.
The connective tissue surrounding the lobules is abundant. The rough
hexagonal plates arrangement can be vividly observed in the tissue
observed. The plates radiate outwards from the central vein (Sapno &
Flores, 2017).
The vertices located in the lobule are equally distributed in portal triads.
The presence of lymphatic system is present however they are vivid to
see. The presence of the lobules can be vividly observed. The precise
boundaries can be difficult to discern but a closer look illustrates the
orienting central veins and the portal tracts which allows smooth
identification process (Carriel et al., 2017).
The prescience of observable hepatocytes is observed as they are
arranged in plates which anatomizes on one another. The formed and
observable polygonal shapes and their sides can locate themselves on
sinusoidal. The hepatocyte nucleus is round having one or two nuclei
while many of the cells have binucleate cells.
Hepatocytes in the liver are actively engaged in protein synthesis and
exportation of lipids, hence conducting ultrastructural assessment shows
rough and smooth endoplasmic reticulum.

6
Figure 3 Liver Tissue assessment Image
The liver has a dense covering of connective tissues which capsules and extends in the liver
forming septae. The connective tissue provides support and highway along with afferent
blood vessels, lymphatic vessels, and bile ducts transverse, thus dividing the parenchyma of
the liver yielding lobules.
The lobule offers the structural unit for the liver. The rough hexagonal plates arranged
irregularly forming hepatocytes radiating outwardly. The vertices entail the lobules which are
regularly distributed in the portal triads having bile duct and terminal branch of the hepatic
artery and portal vein. The lobules are not easily observed due to poor delineation from the
connective tissue.
Usage of H& E staining technique allows for the differentiation of the various tissue organs
such as the lobules and tiny central veins. The results obtained from this assessment are
similar to the benchmark images provided (Bejnordi et al., 2016).
Figure 3 Liver Tissue assessment Image
The liver has a dense covering of connective tissues which capsules and extends in the liver
forming septae. The connective tissue provides support and highway along with afferent
blood vessels, lymphatic vessels, and bile ducts transverse, thus dividing the parenchyma of
the liver yielding lobules.
The lobule offers the structural unit for the liver. The rough hexagonal plates arranged
irregularly forming hepatocytes radiating outwardly. The vertices entail the lobules which are
regularly distributed in the portal triads having bile duct and terminal branch of the hepatic
artery and portal vein. The lobules are not easily observed due to poor delineation from the
connective tissue.
Usage of H& E staining technique allows for the differentiation of the various tissue organs
such as the lobules and tiny central veins. The results obtained from this assessment are
similar to the benchmark images provided (Bejnordi et al., 2016).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
D
A
B
C
E
A
C
B
D
E
7
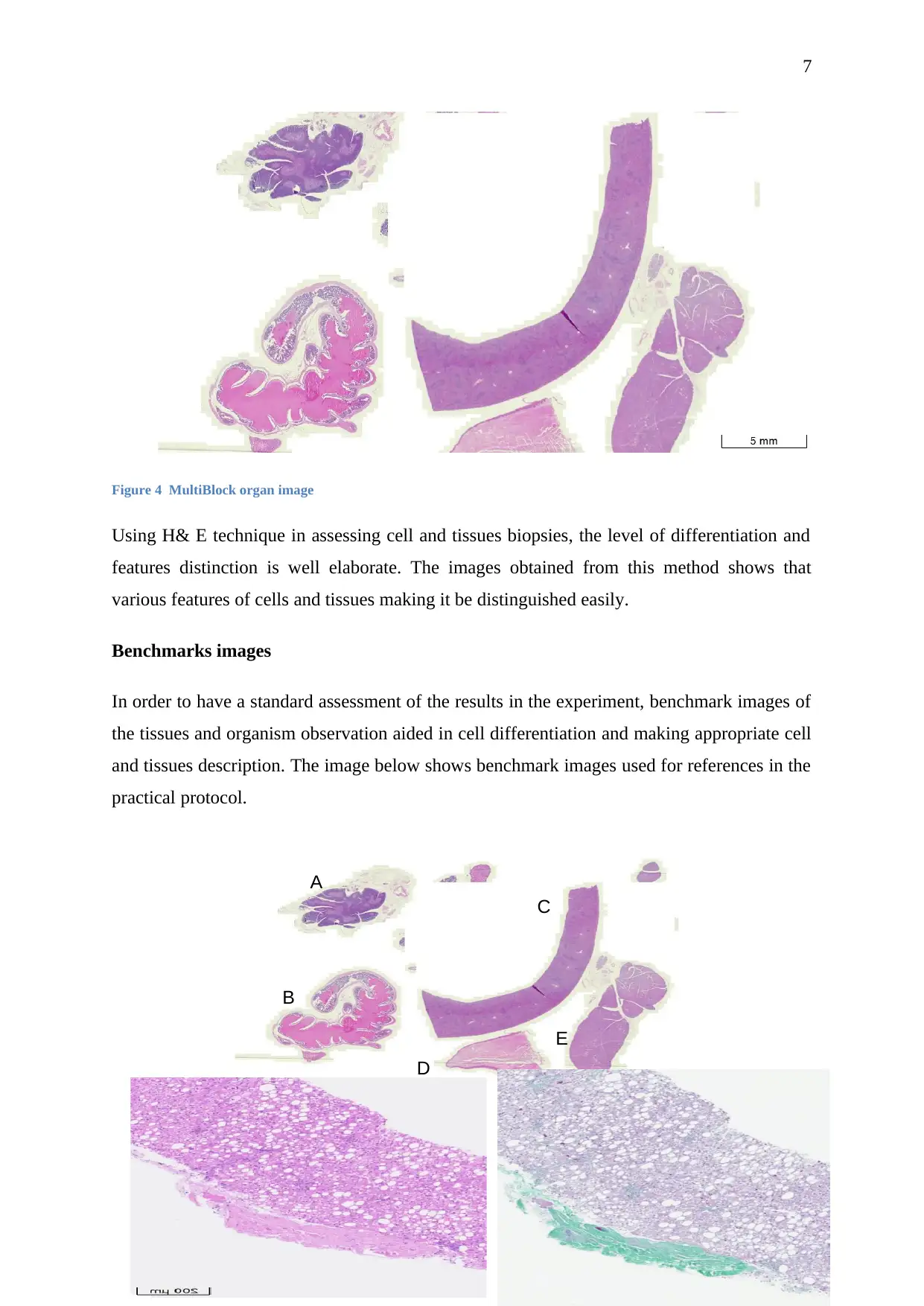
Figure 4 MultiBlock organ image
Using H& E technique in assessing cell and tissues biopsies, the level of differentiation and
features distinction is well elaborate. The images obtained from this method shows that
various features of cells and tissues making it be distinguished easily.
Benchmarks images
In order to have a standard assessment of the results in the experiment, benchmark images of
the tissues and organism observation aided in cell differentiation and making appropriate cell
and tissues description. The image below shows benchmark images used for references in the
practical protocol.
A
B
C
E
A
C
B
D
E
7
Figure 4 MultiBlock organ image
Using H& E technique in assessing cell and tissues biopsies, the level of differentiation and
features distinction is well elaborate. The images obtained from this method shows that
various features of cells and tissues making it be distinguished easily.
Benchmarks images
In order to have a standard assessment of the results in the experiment, benchmark images of
the tissues and organism observation aided in cell differentiation and making appropriate cell
and tissues description. The image below shows benchmark images used for references in the
practical protocol.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

8
Discussion
Trichome staining offers a histological method utilizing two or more dyes. The staining is
aimed at differentiating the tinting tissues through color contrasting. Staining facilitates
contrasting of cell and tissue features making them visible through the microscope. Trichome
staining can offer a difficult challenge when discerning one feature from another. Liver tissue
biopsies have fine fibers of collagen located between the cells and collagen strands can offer
an effective approach in determining s number. Trichome is an effective method in
differentiating collagen from the muscles (Swiderska-Chadaj, Markiewicz & Koktysz 2016
p.244).
Trichome staining uses two more dyes. Staining with polyacids is a crucial step in the
staining process.polydyes has molecular weight; hence they remove dye from the collagen
section. Red dyes in dilute acetic acid are applied to over stain the components. Polyacid
application removes the dye in the collagen. Repeated application of dye displaces the
polyacid which yields contrasting colors of collagen. Staining of erythrocytes allows
application of smaller molecular weight of orange or yellow color being applied with the red
dye (Alturkistani, Tashkandi & Mohammedsaleh, 2016).
Hematoxylin stain used in H&E technique is violet stain having positive polarity. Its action
entails binding of the basophilic substances such as the Ribonucleic acid and DNA which are
both acidic and negative charge ability. The nuclei DNA/RNA have the acidic properties due
to the nucleic acid backbone which are negatively charged. This offers to bind the DNA and
RNA giving them distinctive violet color (Wang et al., 2016 pp 53-56).
Eosin stain is red or pink in color having both acidic and negative charge. It acts by binding
the acidophilic substances. Most of the proteins located in the cytoplasm are basic in nature
due to their positive charge based on their amino acid residues. The resulting slats formed
contain negative charges such as eosin, forming biding to the amino acids staining them to be
Discussion
Trichome staining offers a histological method utilizing two or more dyes. The staining is
aimed at differentiating the tinting tissues through color contrasting. Staining facilitates
contrasting of cell and tissue features making them visible through the microscope. Trichome
staining can offer a difficult challenge when discerning one feature from another. Liver tissue
biopsies have fine fibers of collagen located between the cells and collagen strands can offer
an effective approach in determining s number. Trichome is an effective method in
differentiating collagen from the muscles (Swiderska-Chadaj, Markiewicz & Koktysz 2016
p.244).
Trichome staining uses two more dyes. Staining with polyacids is a crucial step in the
staining process.polydyes has molecular weight; hence they remove dye from the collagen
section. Red dyes in dilute acetic acid are applied to over stain the components. Polyacid
application removes the dye in the collagen. Repeated application of dye displaces the
polyacid which yields contrasting colors of collagen. Staining of erythrocytes allows
application of smaller molecular weight of orange or yellow color being applied with the red
dye (Alturkistani, Tashkandi & Mohammedsaleh, 2016).
Hematoxylin stain used in H&E technique is violet stain having positive polarity. Its action
entails binding of the basophilic substances such as the Ribonucleic acid and DNA which are
both acidic and negative charge ability. The nuclei DNA/RNA have the acidic properties due
to the nucleic acid backbone which are negatively charged. This offers to bind the DNA and
RNA giving them distinctive violet color (Wang et al., 2016 pp 53-56).
Eosin stain is red or pink in color having both acidic and negative charge. It acts by binding
the acidophilic substances. Most of the proteins located in the cytoplasm are basic in nature
due to their positive charge based on their amino acid residues. The resulting slats formed
contain negative charges such as eosin, forming biding to the amino acids staining them to be

9
pink in color.
H&E offers a relatively cheaper method of staining compared to Trichome technique. It
widely used means of pathological assessment. It utilizes two dyes in assessing tissue and
cells under investigation. It is the most commonly used stain as it stains both nucleus and cell
contents such as the cytoplasm. Haemotoxin stain is key in the identification of nucleus
which gives a blue color. Eosin stains on the cell are able to distinguish the various features
of a cell such as a cytoplasm, mitochondrion, and endoplasmic reticulum among other
features thus a suitable method of assessment (Copper et al., 2018 p. 39).
pink in color.
H&E offers a relatively cheaper method of staining compared to Trichome technique. It
widely used means of pathological assessment. It utilizes two dyes in assessing tissue and
cells under investigation. It is the most commonly used stain as it stains both nucleus and cell
contents such as the cytoplasm. Haemotoxin stain is key in the identification of nucleus
which gives a blue color. Eosin stains on the cell are able to distinguish the various features
of a cell such as a cytoplasm, mitochondrion, and endoplasmic reticulum among other
features thus a suitable method of assessment (Copper et al., 2018 p. 39).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

10
Reference
Alturkistani, H.A., Tashkandi, F.M. and Mohammed Saleh, Z.M., 2016. Histological stains: a
literature review and case study. Global journal of health science, 8(3), p.72.
Anderson, J., 2011. An introduction to Routine and special staining. Retrieved on August,
188(3), p.2014.
Bejnordi, B.E., Litjens, G., Timofeeva, N., Otte-Höller, I., Homeyer, A., Karssemeijer, N.
and van der Laak, J.A., 2016. Stain specific standardization of whole-slide histopathological
images. IEEE transactions on medical imaging, 35(2), pp.404-415.
Black J. Microbiology: Principles and exploration. 8th ed. John Wiley Sons. 45(3), p. 68
Carriel, V., Campos, F., Aneiros-Fernández, J. and Kiernan, J.A., 2017. Tissue fixation and
processing for the histological identification of lipids. In Histochemistry of Single Molecules
(pp. 197-206). Humana Press, New York, NY.
Copper, J.E., Budgeon, L.R., Foutz, C.A., van Rossum, D.B., Vanselow, D.J., Hubley, M.J.,
Clark, D.P., Mandrell, D.T. and Cheng, K.C., 2018. Comparative analysis of fixation and
embedding techniques for optimized histological preparation of zebrafish. Comparative
Biochemistry and Physiology Part C: Toxicology & Pharmacology, 208, pp.38-46.
Musumeci, G., 2014. Past, present and future: overview on histology and histopathology. J
Histol Histopathol 1(5). p.5
Shostak, S., 2013. Histology’s nomenclature: Past, present and future. Biol Syst Open Access,
2(122), p.2.
Spano Perez, C.A. and Flores, V., 2017. Staining protocol for the histological study of sea
anemones (Anthozoa: Actiniaria), with recommendation for anesthesia and fixation of
specimens. Submission article platform-Latin American Journal of Aquatic Research, 107(4),
pp.261-273
Swiderska-Chadaj, Z., Markiewicz, T. and Koktysz, R., 2016. Image Processing Method for
Detection and Description of Structures in the Histological Images of Placental Villi. Main
Themes, 22(4), pp.599-601.
Reference
Alturkistani, H.A., Tashkandi, F.M. and Mohammed Saleh, Z.M., 2016. Histological stains: a
literature review and case study. Global journal of health science, 8(3), p.72.
Anderson, J., 2011. An introduction to Routine and special staining. Retrieved on August,
188(3), p.2014.
Bejnordi, B.E., Litjens, G., Timofeeva, N., Otte-Höller, I., Homeyer, A., Karssemeijer, N.
and van der Laak, J.A., 2016. Stain specific standardization of whole-slide histopathological
images. IEEE transactions on medical imaging, 35(2), pp.404-415.
Black J. Microbiology: Principles and exploration. 8th ed. John Wiley Sons. 45(3), p. 68
Carriel, V., Campos, F., Aneiros-Fernández, J. and Kiernan, J.A., 2017. Tissue fixation and
processing for the histological identification of lipids. In Histochemistry of Single Molecules
(pp. 197-206). Humana Press, New York, NY.
Copper, J.E., Budgeon, L.R., Foutz, C.A., van Rossum, D.B., Vanselow, D.J., Hubley, M.J.,
Clark, D.P., Mandrell, D.T. and Cheng, K.C., 2018. Comparative analysis of fixation and
embedding techniques for optimized histological preparation of zebrafish. Comparative
Biochemistry and Physiology Part C: Toxicology & Pharmacology, 208, pp.38-46.
Musumeci, G., 2014. Past, present and future: overview on histology and histopathology. J
Histol Histopathol 1(5). p.5
Shostak, S., 2013. Histology’s nomenclature: Past, present and future. Biol Syst Open Access,
2(122), p.2.
Spano Perez, C.A. and Flores, V., 2017. Staining protocol for the histological study of sea
anemones (Anthozoa: Actiniaria), with recommendation for anesthesia and fixation of
specimens. Submission article platform-Latin American Journal of Aquatic Research, 107(4),
pp.261-273
Swiderska-Chadaj, Z., Markiewicz, T. and Koktysz, R., 2016. Image Processing Method for
Detection and Description of Structures in the Histological Images of Placental Villi. Main
Themes, 22(4), pp.599-601.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

11
Titford, M., 2009. Progress in the development of microscopical techniques for diagnostic
pathology. Journal of Histotechnology, 32(1), pp.9-19.
Wang, H., Yang, L.L., Ji, Y.L., Chen, Y.H., Hu, J., Zhang, C., Zhang, J. and Xu, D.X., 2016.
Different fixative methods influence histological morphology and TUNEL staining in mouse
testes. Reproductive Toxicology, 60, pp.53-61.
Young B, O’Dowd G, Stewart W. Wheater's Basic Pathology: A Text, Atlas and Review of
Histopathology, 10(39), pp.337-356.
Titford, M., 2009. Progress in the development of microscopical techniques for diagnostic
pathology. Journal of Histotechnology, 32(1), pp.9-19.
Wang, H., Yang, L.L., Ji, Y.L., Chen, Y.H., Hu, J., Zhang, C., Zhang, J. and Xu, D.X., 2016.
Different fixative methods influence histological morphology and TUNEL staining in mouse
testes. Reproductive Toxicology, 60, pp.53-61.
Young B, O’Dowd G, Stewart W. Wheater's Basic Pathology: A Text, Atlas and Review of
Histopathology, 10(39), pp.337-356.
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




