Chemistry Report: Analysis of Titration Graphs and pH
VerifiedAdded on 2022/12/29
|4
|428
|38
Report
AI Summary
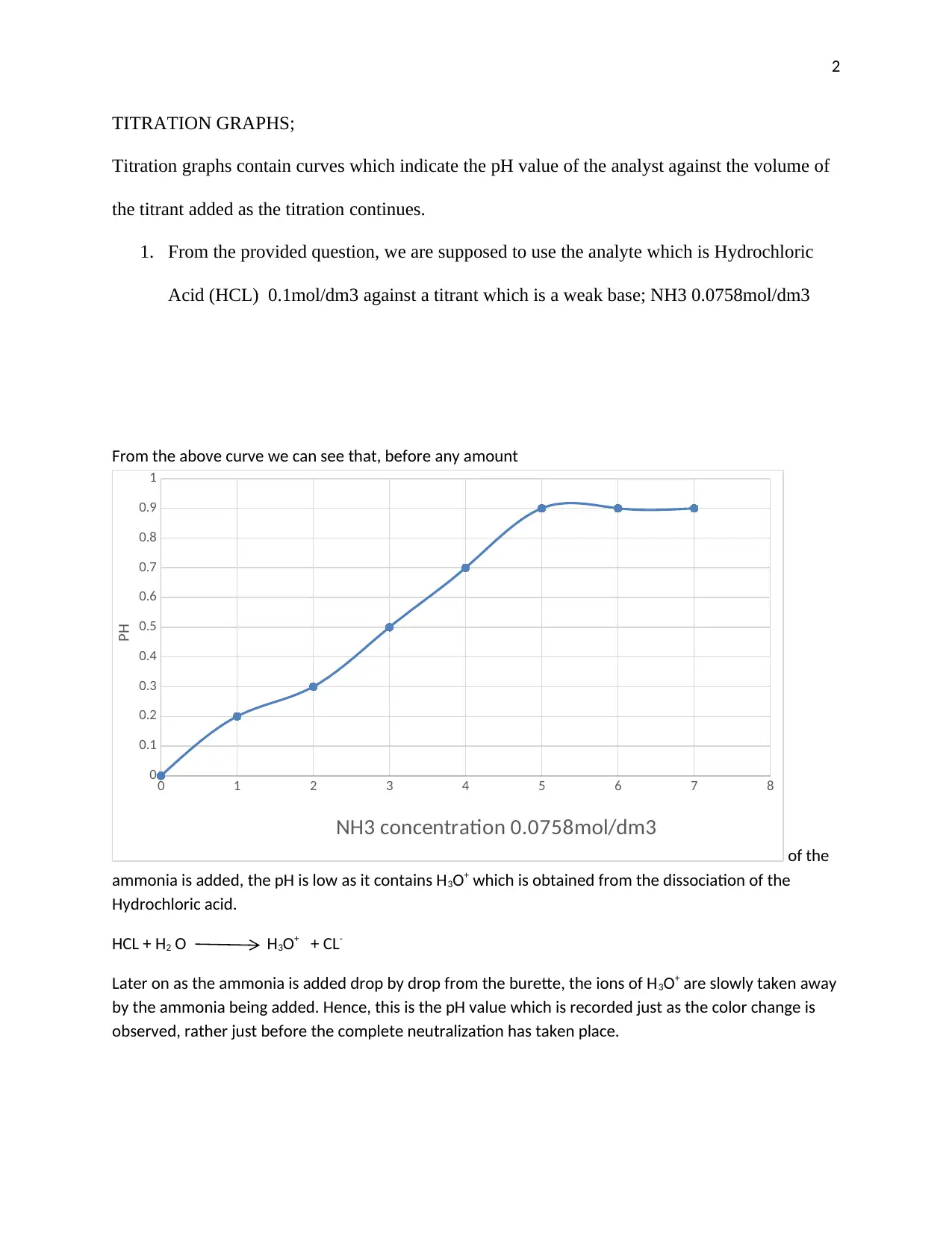
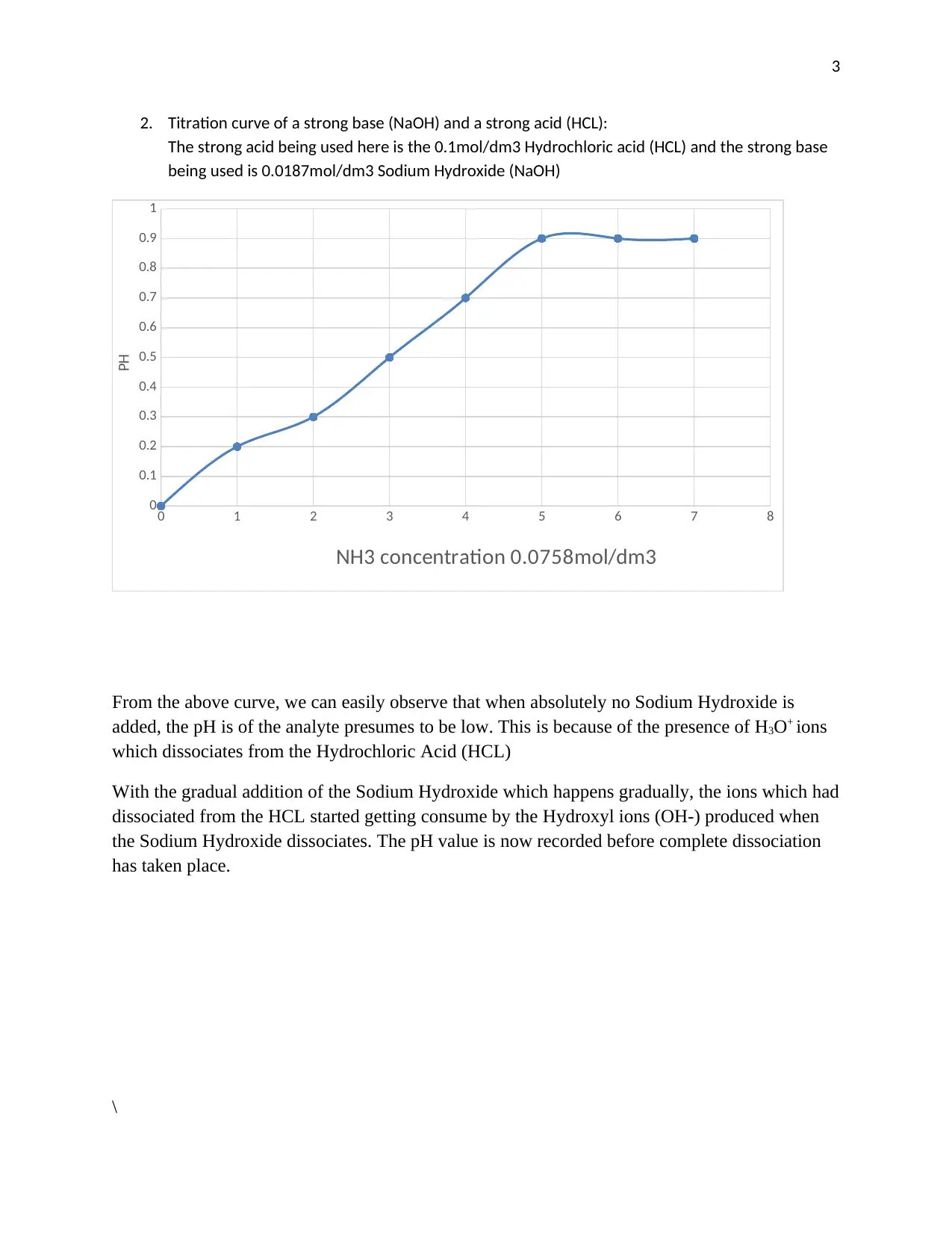
This report provides an analysis of titration graphs, focusing on the reactions between acids and bases. It examines the titration of hydrochloric acid (HCl) with ammonia (NH3), a weak base, and the titration of a strong acid (HCl) with a strong base (NaOH). The report explains how the pH changes during titration, providing insights into the behavior of H3O+ ions and the dissociation of acids and bases. The analysis includes the use of titration graphs to visualize the changes in pH as the titrant is added. References are provided for further reading on the topic, including the infrared titration of aqueous NaOH by aqueous HCl and computer-based projects for a chemistry curriculum.
1 out of 4




![[object Object]](/_next/static/media/star-bottom.7253800d.svg)