Transgenes in Plant Breeding for Chemical Production: A Biology Report
VerifiedAdded on 2022/11/24
|6
|2125
|54
Report
AI Summary
This report delves into the application of transgenes in plant breeding to enhance the production of specific chemicals, such as chemical X. It explores the design of transgenes, including the selection of genes of interest, promoters, and terminator sequences, as well as the construction of transgenic constructs. The report also discusses the vectors used in transgene construction, focusing on the Ti plasmid and its role in Agrobacterium-mediated gene transfer. Furthermore, it covers the process of plant transformation, generation, and selection of transgenic plants, including direct and indirect gene transfer methods, and the use of selectable markers. Finally, the report examines the methods used to confirm the generated transgenic plants, such as PCR and Southern blotting, providing a comprehensive overview of the techniques and processes involved in producing genetically modified plants for increased chemical production. The report references several scientific articles to support the information provided.

1. Transgenes for the increased production of chemical X
Producing crops with desirable features, excellent quality, and high yield is the core goal of
transgenic plant breeding. In addition to helping the agriculture industry, it has been
discovered that the plants can serve as a factory for the manufacturing of pharmaceutical
proteins. The deoxyribonucleic acid (DNA) of transgenic or genetically modified plants has
been altered using genetic engineering methods. A transgenic construct is created while
creating a transgenic plant. This design contains the gene of interested in or want to test, and
it also needs a promoter. The promoter essentially drives the gene by causing it to produce
messenger RNA, which is then converted into protein. The terminator sequence is also
required to construct the transgenic construct (Rani & Usha, 2013).
Standard transgenic constructions should include the interest gene and/or marker gene, all
necessary 5' and 3' regulatory sequences for transgene expression, and restriction sites that
permit extraction of a full length translational transgenic segment for microinjection.
Sequence addition that can boost transgenic expression should be taken into consideration.
For instance, depending on the type of investigation, some gene constructs may incorporate
unique sequences like reporter sequences, silencer, and enhancer. The construct design
should also include a method for detecting your transgene or its byproduct (Rani & Usha,
2013).
Design of the transgene:
While designing a transgene, a transgene or foreign gene from organisms belonging to a
distinct species or different kingdom can be chosen. However, the mutated gene sequence
from the same plant can also be used.
In this case, there are two methods to construct a transgene:
Use the mutated genes of Gene XA, Gene XB, and Gene XC from the same plant.
Further, enhancers along with the petal-specific transcription factors, TFX are also used.
Genes responsible for desired traits (such as plants having long leaves, non-seasonal
leaves growth with the same genome) should be taken from plants belonging to same
species but different plants. Since, the gene of interest for dominant traits such as long
leaves with non-seasonal growth are taken from the plant of same species, its genomic
content is conserved or have same genome.
Producing crops with desirable features, excellent quality, and high yield is the core goal of
transgenic plant breeding. In addition to helping the agriculture industry, it has been
discovered that the plants can serve as a factory for the manufacturing of pharmaceutical
proteins. The deoxyribonucleic acid (DNA) of transgenic or genetically modified plants has
been altered using genetic engineering methods. A transgenic construct is created while
creating a transgenic plant. This design contains the gene of interested in or want to test, and
it also needs a promoter. The promoter essentially drives the gene by causing it to produce
messenger RNA, which is then converted into protein. The terminator sequence is also
required to construct the transgenic construct (Rani & Usha, 2013).
Standard transgenic constructions should include the interest gene and/or marker gene, all
necessary 5' and 3' regulatory sequences for transgene expression, and restriction sites that
permit extraction of a full length translational transgenic segment for microinjection.
Sequence addition that can boost transgenic expression should be taken into consideration.
For instance, depending on the type of investigation, some gene constructs may incorporate
unique sequences like reporter sequences, silencer, and enhancer. The construct design
should also include a method for detecting your transgene or its byproduct (Rani & Usha,
2013).
Design of the transgene:
While designing a transgene, a transgene or foreign gene from organisms belonging to a
distinct species or different kingdom can be chosen. However, the mutated gene sequence
from the same plant can also be used.
In this case, there are two methods to construct a transgene:
Use the mutated genes of Gene XA, Gene XB, and Gene XC from the same plant.
Further, enhancers along with the petal-specific transcription factors, TFX are also used.
Genes responsible for desired traits (such as plants having long leaves, non-seasonal
leaves growth with the same genome) should be taken from plants belonging to same
species but different plants. Since, the gene of interest for dominant traits such as long
leaves with non-seasonal growth are taken from the plant of same species, its genomic
content is conserved or have same genome.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Figure 1: Transgene construct containing promoter sequence, transgene, terminator sequence,
and antibiotic resistance genes (Making a Transgenic Plant, 2018).
2. Description of vector involved construction of transgenes
A vector serves as a means of delivering the desired gene to the target cell for multiplication
and expression. An origin of replication, a multicloning site or recombination site, and a
selectable marker make up a common vector. The origin of replication is an AT-rich
sequence on the vector that binds to a protein complex to start the replication of the vector,
unwinds the vector, and then replicates it with the assistance of polymerases (Low et al.,
2018).
An area known as a multicloning site has several distinct sequences that, when cut by a
particular restriction enzyme, allow the insertion of the desired gene. Site-specific
recombination between two plasmids is possible thanks to the recombination site. The
selectable markers are genetic markers that carry out the gene construct section's description
of how they work, confirming that the vector was successfully inserted into the
Agrobacterium species. The tumor inducing (Ti) plasmid-based vectors and plant viral-based
vectors are frequently employed in plant transformation (Low et al., 2018).
Ti-Plasmid
The Ti plasmid is the vector that is most frequently used to create transgenic plants.
Depending on the Ti plasmid classes, the estimated size of the Ti plasmid ranges from 200 to
800 kilobase pairs (kbp). The transfer DNA (T-DNA) area, virulence region, and opine
catabolism region are the three primary portions of the Ti plasmid. Approximately, 24 kbp of
T-DNA are transported into the plant genome (Hernandez-Garcia et al., 2014).
The right and left border are repetition sequences that encircle this area on either end. For the
transfer of DNA that leads to cancer, the correct boundary is a crucial component. However,
the virulence region is in charge of encoding the vir genes, that facilitates the transmission of
the T-DNA. The T-DNA sequence also specifies the manufacture of auxin and cytokinin, two
and antibiotic resistance genes (Making a Transgenic Plant, 2018).
2. Description of vector involved construction of transgenes
A vector serves as a means of delivering the desired gene to the target cell for multiplication
and expression. An origin of replication, a multicloning site or recombination site, and a
selectable marker make up a common vector. The origin of replication is an AT-rich
sequence on the vector that binds to a protein complex to start the replication of the vector,
unwinds the vector, and then replicates it with the assistance of polymerases (Low et al.,
2018).
An area known as a multicloning site has several distinct sequences that, when cut by a
particular restriction enzyme, allow the insertion of the desired gene. Site-specific
recombination between two plasmids is possible thanks to the recombination site. The
selectable markers are genetic markers that carry out the gene construct section's description
of how they work, confirming that the vector was successfully inserted into the
Agrobacterium species. The tumor inducing (Ti) plasmid-based vectors and plant viral-based
vectors are frequently employed in plant transformation (Low et al., 2018).
Ti-Plasmid
The Ti plasmid is the vector that is most frequently used to create transgenic plants.
Depending on the Ti plasmid classes, the estimated size of the Ti plasmid ranges from 200 to
800 kilobase pairs (kbp). The transfer DNA (T-DNA) area, virulence region, and opine
catabolism region are the three primary portions of the Ti plasmid. Approximately, 24 kbp of
T-DNA are transported into the plant genome (Hernandez-Garcia et al., 2014).
The right and left border are repetition sequences that encircle this area on either end. For the
transfer of DNA that leads to cancer, the correct boundary is a crucial component. However,
the virulence region is in charge of encoding the vir genes, that facilitates the transmission of
the T-DNA. The T-DNA sequence also specifies the manufacture of auxin and cytokinin, two
phytohormones. The three T-DNA oncogenes (auxin, cytokinin, and opine biosynthesis gene)
are the primary factors in plant tumorigenesis, which results in the crown gall disease.
Synthesized growth hormones are to blame for the unchecked plant cell proliferation, which
makes matters worse by promoting the development of crown galls. The primary carbon
source that A. tumefaciens uses that is not produced naturally by plant metabolism is opiates.
By genetically altering the host cells, A. tumefaciens will produce its biosynthetic machinery
for the manufacture of nutrients. The genes encoding the proteins responsible for opines
catabolism are encoded by the opines catabolism region. The Ti plasmid can be kept stable in
the bacteria because of origin of DNA replication. The Ti plasmid is often disarmed for plant
transformation, with the tumor-inducing genes gets deleted. Further, the deleted genes of
interest are replaced with the reporter genes by genes of interest (Van Montagu & Zambryski,
2013).
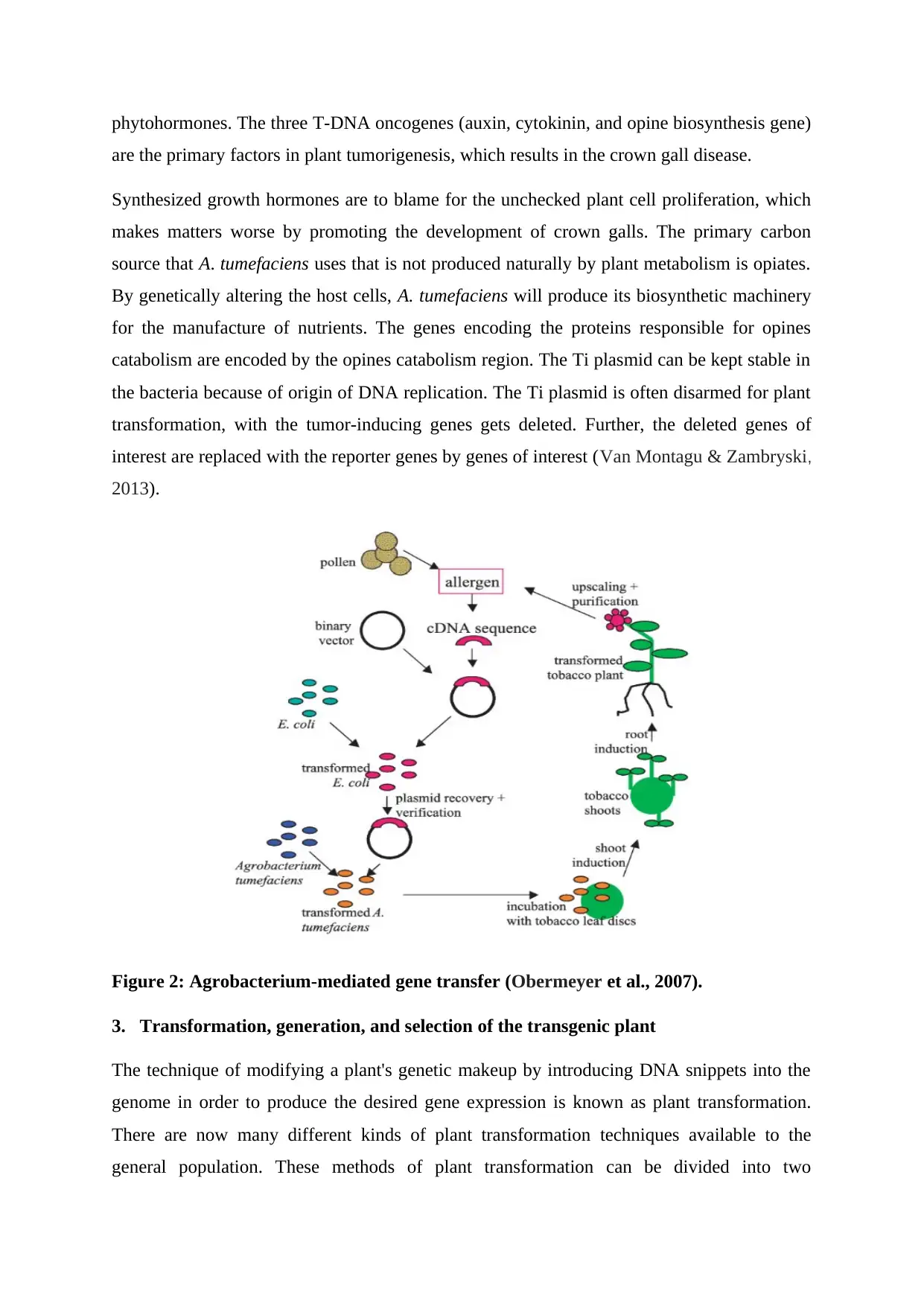
Figure 2: Agrobacterium-mediated gene transfer (Obermeyer et al., 2007).
3. Transformation, generation, and selection of the transgenic plant
The technique of modifying a plant's genetic makeup by introducing DNA snippets into the
genome in order to produce the desired gene expression is known as plant transformation.
There are now many different kinds of plant transformation techniques available to the
general population. These methods of plant transformation can be divided into two
are the primary factors in plant tumorigenesis, which results in the crown gall disease.
Synthesized growth hormones are to blame for the unchecked plant cell proliferation, which
makes matters worse by promoting the development of crown galls. The primary carbon
source that A. tumefaciens uses that is not produced naturally by plant metabolism is opiates.
By genetically altering the host cells, A. tumefaciens will produce its biosynthetic machinery
for the manufacture of nutrients. The genes encoding the proteins responsible for opines
catabolism are encoded by the opines catabolism region. The Ti plasmid can be kept stable in
the bacteria because of origin of DNA replication. The Ti plasmid is often disarmed for plant
transformation, with the tumor-inducing genes gets deleted. Further, the deleted genes of
interest are replaced with the reporter genes by genes of interest (Van Montagu & Zambryski,
2013).
Figure 2: Agrobacterium-mediated gene transfer (Obermeyer et al., 2007).
3. Transformation, generation, and selection of the transgenic plant
The technique of modifying a plant's genetic makeup by introducing DNA snippets into the
genome in order to produce the desired gene expression is known as plant transformation.
There are now many different kinds of plant transformation techniques available to the
general population. These methods of plant transformation can be divided into two
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

categories: indirect gene transfer and direct gene transfer. Direct gene transfer methods
introduce foreign DNA directly into the plant genome by physical or chemical interactions,
whereas indirect gene transfer methods use biological vectors to introduce exogenous DNA
into the plant genome. This technique is also known as vector-mediated gene transfer (Low et
al., 2018).
Transient or stable incorporation of transgenes in plant cells is possible. Transgenes are often
inserted into the nucleus of plant tissue to start the stable transformation process. Some
transgenes effectively integrating into the cell's genome results in stable transformation. The
following generation can inherit and express the transgene because these transgenes
subsequently integrate into the genome and are reproduced simultaneously. Transient
transformant, on the other hand, only expressed the transgene momentarily and did not
integrate the transgene into the plant genome (Low et al., 2018).
Currently, the Agrobacterium-mediated approach can produce both temporary and sustained
transformation. The T-DNA region is incorporated into the plant genome via the
Agrobacterium-mediated technique, creating a stable transformant, as opposed to the non-
integrated T-DNA plasmid, which only expresses the transgene momentarily (Xiong et al.,
2013).
Based on the transgenic constructions, selectable marker, and reporter gene used, an
appropriate approach must be applied for analysis and validation of transgene integration.
Herbicides or antibiotics are added to the growing medium to screen transgenic plant cells
inserted with antibiotic resistance genes, separating them from non-transformed plant cells.
This strategy calls for a lot of expensive antibiotics and herbicides, which is made worse by
the possibility of horizontal gene transfer to other bacteria. As an alternate screening strategy
for transgenic plants, more accurate techniques such as reporter gene expression screening
and polymerase chain reaction (PCR) are applied (Low et al., 2018).
4. Confirmation of generated transgenic plants
Among all the molecular techniques used to confirm the transgene, the polymerase chain
reaction (PCR) approach is one of the most accurate and straightforward. Primers are
typically employed in PCR that are specific to the gene of interest and the location of plasmid
constructs used to create transgenic plants. Positive multiplication of the DNA fragment with
the anticipated band suggests the potential presence of a transgene, and DNA sequencing is
used to confirm this DNA fragment. In comparison to the conventional Southern blot
introduce foreign DNA directly into the plant genome by physical or chemical interactions,
whereas indirect gene transfer methods use biological vectors to introduce exogenous DNA
into the plant genome. This technique is also known as vector-mediated gene transfer (Low et
al., 2018).
Transient or stable incorporation of transgenes in plant cells is possible. Transgenes are often
inserted into the nucleus of plant tissue to start the stable transformation process. Some
transgenes effectively integrating into the cell's genome results in stable transformation. The
following generation can inherit and express the transgene because these transgenes
subsequently integrate into the genome and are reproduced simultaneously. Transient
transformant, on the other hand, only expressed the transgene momentarily and did not
integrate the transgene into the plant genome (Low et al., 2018).
Currently, the Agrobacterium-mediated approach can produce both temporary and sustained
transformation. The T-DNA region is incorporated into the plant genome via the
Agrobacterium-mediated technique, creating a stable transformant, as opposed to the non-
integrated T-DNA plasmid, which only expresses the transgene momentarily (Xiong et al.,
2013).
Based on the transgenic constructions, selectable marker, and reporter gene used, an
appropriate approach must be applied for analysis and validation of transgene integration.
Herbicides or antibiotics are added to the growing medium to screen transgenic plant cells
inserted with antibiotic resistance genes, separating them from non-transformed plant cells.
This strategy calls for a lot of expensive antibiotics and herbicides, which is made worse by
the possibility of horizontal gene transfer to other bacteria. As an alternate screening strategy
for transgenic plants, more accurate techniques such as reporter gene expression screening
and polymerase chain reaction (PCR) are applied (Low et al., 2018).
4. Confirmation of generated transgenic plants
Among all the molecular techniques used to confirm the transgene, the polymerase chain
reaction (PCR) approach is one of the most accurate and straightforward. Primers are
typically employed in PCR that are specific to the gene of interest and the location of plasmid
constructs used to create transgenic plants. Positive multiplication of the DNA fragment with
the anticipated band suggests the potential presence of a transgene, and DNA sequencing is
used to confirm this DNA fragment. In comparison to the conventional Southern blot
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

analysis, real-time PCR offers quick, sensitive, and high-throughput molecular analysis,
particularly in the domain of zygosity detection and transgene copy number in transgenic
plants. Moreover, real-time PCR is practical because it enables real-time quantitative, semi-
quantitative, or qualitative (RT-qPCR) monitoring of the target DNA (Bubner & Baldwin,
2004).
Another method for the detection of the transgene is southern blotting. A molecular technique
called southern blotting is used to identify specific DNA sequences in DNA samples. The
detection of transgenic integrity and transgene rearrangement, as well as the amount of
transgenes introduced into the host genome, are often accomplished by southern blotting.
Endonuclease restriction enzymes are used to cleave the DNA into fragments, which are then
separated by size during electrophoresis and put onto a nitrocellulose or nylon membrane.
Incubation of membranes containing bound DNA in a solution containing labelled probes is
followed by autoradiography or chromogenic detection of the pattern of hybridization. The
number of bands detected is inversely correlated with the transgene copy number (Bubner &
Baldwin, 2004).
References
Making a transgenic plant. (2018, February 19). Science Learning Hub. Retrieved August 7,
2022, from https://www.sciencelearn.org.nz/image_maps/62-making-a-transgenic-
plant#:%7E:text=When%20making%20a%20transgenic%20plant%2C%20you
%20build%20what,messenger%20RNA%2C%20and%20that%20gets%20turned
%20into%20protein.
Rani, S. J., & Usha, R. (2013). Transgenic plants: Types, benefits, public concerns and
future. Journal of Pharmacy Research, 6(8), 879-883.
Hernandez-Garcia, C. M., & Finer, J. J. (2014). Identification and validation of promoters
and cis-acting regulatory elements. Plant Science, 217, 109-119.
Low, L. Y., Yang, S. K., Andrew Kok, D. X., Ong-Abdullah, J., Tan, N. P., & Lai, K. S.
(2018). Transgenic plants: Gene constructs, vector and transformation method. New visions
in plant science, 41-61.
Van Montagu, M., & Zambryski, P. (2013). Agrobacterium and Ti plasmids. In Brenner's
encyclopedia of genetics, vol. 1 (pp. 55-57). Elsevier Science.
particularly in the domain of zygosity detection and transgene copy number in transgenic
plants. Moreover, real-time PCR is practical because it enables real-time quantitative, semi-
quantitative, or qualitative (RT-qPCR) monitoring of the target DNA (Bubner & Baldwin,
2004).
Another method for the detection of the transgene is southern blotting. A molecular technique
called southern blotting is used to identify specific DNA sequences in DNA samples. The
detection of transgenic integrity and transgene rearrangement, as well as the amount of
transgenes introduced into the host genome, are often accomplished by southern blotting.
Endonuclease restriction enzymes are used to cleave the DNA into fragments, which are then
separated by size during electrophoresis and put onto a nitrocellulose or nylon membrane.
Incubation of membranes containing bound DNA in a solution containing labelled probes is
followed by autoradiography or chromogenic detection of the pattern of hybridization. The
number of bands detected is inversely correlated with the transgene copy number (Bubner &
Baldwin, 2004).
References
Making a transgenic plant. (2018, February 19). Science Learning Hub. Retrieved August 7,
2022, from https://www.sciencelearn.org.nz/image_maps/62-making-a-transgenic-
plant#:%7E:text=When%20making%20a%20transgenic%20plant%2C%20you
%20build%20what,messenger%20RNA%2C%20and%20that%20gets%20turned
%20into%20protein.
Rani, S. J., & Usha, R. (2013). Transgenic plants: Types, benefits, public concerns and
future. Journal of Pharmacy Research, 6(8), 879-883.
Hernandez-Garcia, C. M., & Finer, J. J. (2014). Identification and validation of promoters
and cis-acting regulatory elements. Plant Science, 217, 109-119.
Low, L. Y., Yang, S. K., Andrew Kok, D. X., Ong-Abdullah, J., Tan, N. P., & Lai, K. S.
(2018). Transgenic plants: Gene constructs, vector and transformation method. New visions
in plant science, 41-61.
Van Montagu, M., & Zambryski, P. (2013). Agrobacterium and Ti plasmids. In Brenner's
encyclopedia of genetics, vol. 1 (pp. 55-57). Elsevier Science.

Xiong, Y., Jung, J., Zeng, Q., Gallo, M., & Altpeter, F. (2013). Comparison of procedures for
DNA coating of micro-carriers in the transient and stable biolistic transformation of
sugarcane. Plant Cell, Tissue and Organ Culture (PCTOC), 112(1), 95-99.
Bubner, B., & Baldwin, I. T. (2004). Use of real-time PCR for determining copy number and
zygosity in transgenic plants. Plant cell reports, 23(5), 263-271.
Gheysen, G., Montagu, M. V., & Zambryski, P. (1987). Integration of Agrobacterium
tumefaciens transfer DNA (T-DNA) involves rearrangements of target plant DNA
sequences. Proceedings of the National Academy of Sciences, 84(17), 6169-6173.
Bubner, B., & Baldwin, I. T. (2004). Use of real-time PCR for determining copy number and
zygosity in transgenic plants. Plant cell reports, 23(5), 263-271.
Obermeyer, G., Gehwolf, R., Sebesta, W., Hamilton, N., Gadermaier, G., Ferreira, F., ... &
Bentrup, F. W. (2004). Over-expression and production of plant allergens by molecular
farming strategies. Methods, 32(3), 235-240.
DNA coating of micro-carriers in the transient and stable biolistic transformation of
sugarcane. Plant Cell, Tissue and Organ Culture (PCTOC), 112(1), 95-99.
Bubner, B., & Baldwin, I. T. (2004). Use of real-time PCR for determining copy number and
zygosity in transgenic plants. Plant cell reports, 23(5), 263-271.
Gheysen, G., Montagu, M. V., & Zambryski, P. (1987). Integration of Agrobacterium
tumefaciens transfer DNA (T-DNA) involves rearrangements of target plant DNA
sequences. Proceedings of the National Academy of Sciences, 84(17), 6169-6173.
Bubner, B., & Baldwin, I. T. (2004). Use of real-time PCR for determining copy number and
zygosity in transgenic plants. Plant cell reports, 23(5), 263-271.
Obermeyer, G., Gehwolf, R., Sebesta, W., Hamilton, N., Gadermaier, G., Ferreira, F., ... &
Bentrup, F. W. (2004). Over-expression and production of plant allergens by molecular
farming strategies. Methods, 32(3), 235-240.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 6
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
