Antimicrobial Peptides: A Promising Therapy for Tuberculosis (TB)
VerifiedAdded on 2023/05/30
|12
|3552
|141
Report
AI Summary
This report provides an overview of antimicrobial peptides (AMPs) as a potential therapeutic strategy against Tuberculosis (TB), caused by Mycobacterium tuberculosis. It highlights the issues with current TB therapies, including long treatment durations, adverse events, and the rise of drug resistance. The report details the unique structure of the bacterial cell wall and the mechanisms of action of AMPs, including membrane disruption, metabolic inhibition, and immunomodulation. It discusses both human-derived and synthetic AMPs, their sources, and their modes of action against mycobacteria. The conclusion emphasizes the potential of AMPs to overcome drug resistance and the need for low-cost synthesis methods and novel drug delivery systems to address proteolytic cleavage. The report references several studies supporting the use of AMPs in TB treatment.

Antimicrobial Peptides
1
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Tuberculosis:
Tuberculosis (TB) is caused by disease causing pathogen called Mycobacterium tuberculosis.
Tuberculosis generally affects lungs; however, it can also affect other parts of the body. Most
common symptoms of TB include chronic cough with blood-containing sputum, fever, night
sweats, and weight loss. TB usually spreads through air when active TB patient cough, spit,
speak, or sneeze (Venketaraman et al., 2015).
Issues with current therapy for TB:
Duration and complexity of treatment; and adverse events associated with TB treatment leads
to nonadherence to treatment. It results in the suboptimal response and emergence of
resistance. Increased incidence of multidrug-resistance and drug-resistant TB are the serious
problems associated with TB. Prophylactic treatment of latent TB with drugs like isoniazid is
associated with nonadherence to the treatment. Efforts to shorten treatment duration with
alternative drugs resulted in the severe adverse events (Mandal et al., 2014).
Bacterial unique structure:
Even though antimicrobial peptides (AMPs) have low level of amino acid sequence, these are
associated with similar structural scaffolds. Hence, AMPs have potential antimicrobial action.
It is difficult for the antimicrobial agents to cross the microbial cell-wall scaffold because it is
composed of complex grid of macromolecules like peptidoglycan, arabinogalactan, and
mycolic acids (MAgP complex) (Arranz-Trullén et al., 2017).
AMPs:
AMPs are small, cationic and amphipathic peptides which make part of the innate immune
system; hence, considered as host defence peptides (HDPs). Expression of endogenous AMPs
is an effective host defence strategy of living organisms. Characteristics of AMPs like
multifunctional model of action, natural origin and effectiveness at low concentration made
them potential candidates for anti-tubercular treatment (Arranz-Trullén et al., 2017).
Mechanism of action:
AMPs exhibit its action through three different mechanisms like membrane disruption,
metabolic inhibitor and immunomodulator. AMPs exhibit its action on the bacterial
membranes. AMPs possess positive charge and these positive charges get attracted towards
the negative charges of bacterial membrane. Immune response develops following infection
2
Tuberculosis (TB) is caused by disease causing pathogen called Mycobacterium tuberculosis.
Tuberculosis generally affects lungs; however, it can also affect other parts of the body. Most
common symptoms of TB include chronic cough with blood-containing sputum, fever, night
sweats, and weight loss. TB usually spreads through air when active TB patient cough, spit,
speak, or sneeze (Venketaraman et al., 2015).
Issues with current therapy for TB:
Duration and complexity of treatment; and adverse events associated with TB treatment leads
to nonadherence to treatment. It results in the suboptimal response and emergence of
resistance. Increased incidence of multidrug-resistance and drug-resistant TB are the serious
problems associated with TB. Prophylactic treatment of latent TB with drugs like isoniazid is
associated with nonadherence to the treatment. Efforts to shorten treatment duration with
alternative drugs resulted in the severe adverse events (Mandal et al., 2014).
Bacterial unique structure:
Even though antimicrobial peptides (AMPs) have low level of amino acid sequence, these are
associated with similar structural scaffolds. Hence, AMPs have potential antimicrobial action.
It is difficult for the antimicrobial agents to cross the microbial cell-wall scaffold because it is
composed of complex grid of macromolecules like peptidoglycan, arabinogalactan, and
mycolic acids (MAgP complex) (Arranz-Trullén et al., 2017).
AMPs:
AMPs are small, cationic and amphipathic peptides which make part of the innate immune
system; hence, considered as host defence peptides (HDPs). Expression of endogenous AMPs
is an effective host defence strategy of living organisms. Characteristics of AMPs like
multifunctional model of action, natural origin and effectiveness at low concentration made
them potential candidates for anti-tubercular treatment (Arranz-Trullén et al., 2017).
Mechanism of action:
AMPs exhibit its action through three different mechanisms like membrane disruption,
metabolic inhibitor and immunomodulator. AMPs exhibit its action on the bacterial
membranes. AMPs possess positive charge and these positive charges get attracted towards
the negative charges of bacterial membrane. Immune response develops following infection
2
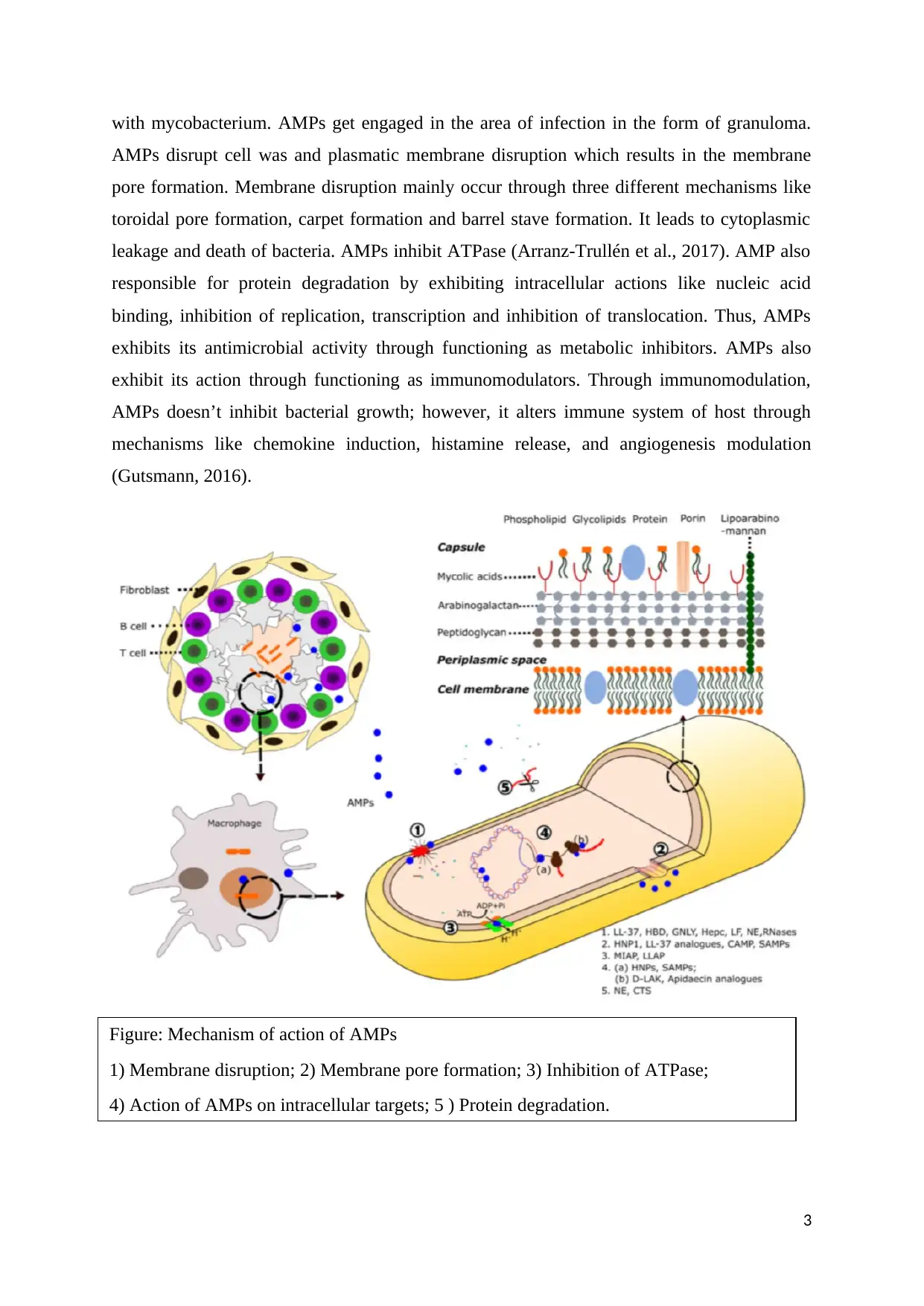
with mycobacterium. AMPs get engaged in the area of infection in the form of granuloma.
AMPs disrupt cell was and plasmatic membrane disruption which results in the membrane
pore formation. Membrane disruption mainly occur through three different mechanisms like
toroidal pore formation, carpet formation and barrel stave formation. It leads to cytoplasmic
leakage and death of bacteria. AMPs inhibit ATPase (Arranz-Trullén et al., 2017). AMP also
responsible for protein degradation by exhibiting intracellular actions like nucleic acid
binding, inhibition of replication, transcription and inhibition of translocation. Thus, AMPs
exhibits its antimicrobial activity through functioning as metabolic inhibitors. AMPs also
exhibit its action through functioning as immunomodulators. Through immunomodulation,
AMPs doesn’t inhibit bacterial growth; however, it alters immune system of host through
mechanisms like chemokine induction, histamine release, and angiogenesis modulation
(Gutsmann, 2016).
3
Figure: Mechanism of action of AMPs
1) Membrane disruption; 2) Membrane pore formation; 3) Inhibition of ATPase;
4) Action of AMPs on intracellular targets; 5 ) Protein degradation.
AMPs disrupt cell was and plasmatic membrane disruption which results in the membrane
pore formation. Membrane disruption mainly occur through three different mechanisms like
toroidal pore formation, carpet formation and barrel stave formation. It leads to cytoplasmic
leakage and death of bacteria. AMPs inhibit ATPase (Arranz-Trullén et al., 2017). AMP also
responsible for protein degradation by exhibiting intracellular actions like nucleic acid
binding, inhibition of replication, transcription and inhibition of translocation. Thus, AMPs
exhibits its antimicrobial activity through functioning as metabolic inhibitors. AMPs also
exhibit its action through functioning as immunomodulators. Through immunomodulation,
AMPs doesn’t inhibit bacterial growth; however, it alters immune system of host through
mechanisms like chemokine induction, histamine release, and angiogenesis modulation
(Gutsmann, 2016).
3
Figure: Mechanism of action of AMPs
1) Membrane disruption; 2) Membrane pore formation; 3) Inhibition of ATPase;
4) Action of AMPs on intracellular targets; 5 ) Protein degradation.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Human derived AMPs:
Human derived AMPs are mainly responsible for the immune host defense against
mycobacteria. Human AMPs include cathelicidin, defensins, hepcidins, lactoferrin,
azurocidin, elastases, antimicrobial RNases, eosinophil peroxidase, cathepsins, granulysin,
calgranulin/calprotectin, ubiquitinated peptides and lipocalin2 (Arranz-Trullén et al., 2017).
Synthetic AMPs:
Synthetic AMPs are considered as the next generation antibiotics and these are useful to
combat drug-resistant strains. Most widely used strategy for synthetic AMPs is to engineer
stabilized amphipathic α-helix with selected antimicrobial prone amino acids. Synthetic AMP
include 1-C134mer, A18G5, A24C1ac, A29C5FA, A38A1guan, CAMP/PL-D, CP26, d-LAK
120, d-LL37, E2 and E6, HHC-10, hLFcin1-11/ hLFcin17-30, Innate defense regulators like
(IDR)1002, -HH2, and IDR-1018, LLAP, LLKKK18, MU1140, MIAP, Pin2 variants,
RN3(1-45) RN6(1-45) RN7(1-45), SAMPs-Dma and X(LLKK) 2X: II-D, II-Orn, IIDab, and
IIDa (Arranz-Trullén et al., 2017).
Human AMPs:
Name Source Mode of action Activity
Cathelicidin
(hCAP18/LL-37)
(Torres-Juarez et al.,
2015; Yu et al., 2013;
Rekha et al., 2015)
Neutrophils,
Monocytes,
Epithelial cells, Mast
cells, Macrophages,
Dendritic cells,
Natural killer cells.
Monocytes, Epithelial
cells, Mast cells,
Macrophages, Dendritic
cells, Natural killer cells,
Mycobacterial cell wall
lysis, Immunomodulation,
Pro-inflammatory action,
Autophagy activation,
Chemotaxis, Neutrophil
extracellular traps (NETs)
promotion, Bind with
Mycobacterium
tuberculosis within the
In vitro,
In vivo
4
Human derived AMPs are mainly responsible for the immune host defense against
mycobacteria. Human AMPs include cathelicidin, defensins, hepcidins, lactoferrin,
azurocidin, elastases, antimicrobial RNases, eosinophil peroxidase, cathepsins, granulysin,
calgranulin/calprotectin, ubiquitinated peptides and lipocalin2 (Arranz-Trullén et al., 2017).
Synthetic AMPs:
Synthetic AMPs are considered as the next generation antibiotics and these are useful to
combat drug-resistant strains. Most widely used strategy for synthetic AMPs is to engineer
stabilized amphipathic α-helix with selected antimicrobial prone amino acids. Synthetic AMP
include 1-C134mer, A18G5, A24C1ac, A29C5FA, A38A1guan, CAMP/PL-D, CP26, d-LAK
120, d-LL37, E2 and E6, HHC-10, hLFcin1-11/ hLFcin17-30, Innate defense regulators like
(IDR)1002, -HH2, and IDR-1018, LLAP, LLKKK18, MU1140, MIAP, Pin2 variants,
RN3(1-45) RN6(1-45) RN7(1-45), SAMPs-Dma and X(LLKK) 2X: II-D, II-Orn, IIDab, and
IIDa (Arranz-Trullén et al., 2017).
Human AMPs:
Name Source Mode of action Activity
Cathelicidin
(hCAP18/LL-37)
(Torres-Juarez et al.,
2015; Yu et al., 2013;
Rekha et al., 2015)
Neutrophils,
Monocytes,
Epithelial cells, Mast
cells, Macrophages,
Dendritic cells,
Natural killer cells.
Monocytes, Epithelial
cells, Mast cells,
Macrophages, Dendritic
cells, Natural killer cells,
Mycobacterial cell wall
lysis, Immunomodulation,
Pro-inflammatory action,
Autophagy activation,
Chemotaxis, Neutrophil
extracellular traps (NETs)
promotion, Bind with
Mycobacterium
tuberculosis within the
In vitro,
In vivo
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
macrophage phagosome.
Defensins
(Sharma et al., 2000;
Sharma et al., 2001;
Rivas-Santiago et al.,
2011)
Eosinophils,
Macrophages,
Epithelial cells,
Dendritic cells,
Neutrophils
Mycobacterial cell
membrane lysis (HBD),
Membrane pore formation
(HNPs), Mycobacterial
growth inhibition,
Dendritic and macrophage
cells chemotaxis
(HBD/HNPs),
Inflammation regulation
(HBD), zHNP1),
Intracellular DNA target
(HNPs).
In vitro,
In vivo,
ex vivo
Hepcidin
(Gutsmann, 2016;
Yamaji, 2004)
Hepatocytes,
Macrophages,
Dendritic cells, Lung
epithelial cells,
Lymphocytes.
Mycobacterial cell wall
lysis, Inhibition of
mycobacterial infection,
Iron homeostasis
regulation, Pro-
inflammatory activity.
In vitro,
In vivo
Lactoferrin
(Hwang et al., 2007)
Epithelial cells,
Neutrophils,
Polymorphonuclear
(PMN) leukocytes
Bacterial cell permeation,
Iron kidnapping, Anti-
inflammatory activity.
In vitro,
In vivo
Azurocidin
(Jena et al., 2012)
PMN leukocytes,
Neutrophils
Mycobacterial cell wall
lysis, Promotion of
phagolysosomal fusion
In vitro
Elastases
(Wong and Jacobs, 2013)
Neutrophil
azurophilic granules,
bone marrow cells,
Macrophages
Bacterial cell membrane
lysis, Serine protease
activity, Cell chemotaxis
induction,
Immunomodulation, NETs
formation, Macrophage
extracellular traps (METs)
In vitro,
In vivo
5
Defensins
(Sharma et al., 2000;
Sharma et al., 2001;
Rivas-Santiago et al.,
2011)
Eosinophils,
Macrophages,
Epithelial cells,
Dendritic cells,
Neutrophils
Mycobacterial cell
membrane lysis (HBD),
Membrane pore formation
(HNPs), Mycobacterial
growth inhibition,
Dendritic and macrophage
cells chemotaxis
(HBD/HNPs),
Inflammation regulation
(HBD), zHNP1),
Intracellular DNA target
(HNPs).
In vitro,
In vivo,
ex vivo
Hepcidin
(Gutsmann, 2016;
Yamaji, 2004)
Hepatocytes,
Macrophages,
Dendritic cells, Lung
epithelial cells,
Lymphocytes.
Mycobacterial cell wall
lysis, Inhibition of
mycobacterial infection,
Iron homeostasis
regulation, Pro-
inflammatory activity.
In vitro,
In vivo
Lactoferrin
(Hwang et al., 2007)
Epithelial cells,
Neutrophils,
Polymorphonuclear
(PMN) leukocytes
Bacterial cell permeation,
Iron kidnapping, Anti-
inflammatory activity.
In vitro,
In vivo
Azurocidin
(Jena et al., 2012)
PMN leukocytes,
Neutrophils
Mycobacterial cell wall
lysis, Promotion of
phagolysosomal fusion
In vitro
Elastases
(Wong and Jacobs, 2013)
Neutrophil
azurophilic granules,
bone marrow cells,
Macrophages
Bacterial cell membrane
lysis, Serine protease
activity, Cell chemotaxis
induction,
Immunomodulation, NETs
formation, Macrophage
extracellular traps (METs)
In vitro,
In vivo
5
formation.
Antimicrobial RNases
(Becknell et al., 2015)
Eosinophils
(RNase3/ECP),
Neutrophils and
monocytes, Epithelial
cells and leukocytes
Mycobacterial cell
agglutination,
Mycobacteria cell wall and
membrane lysis.
In
vitroI,
In vivo,
Clinical
Eosinophil peroxidase
(Pulido et al., 2013)
Eosinophils Bacterial cell wall lysis. In vitro
Cathepsins
(Walter et al., 2015)
Neutrophils,
Monocytes
Mediation of apoptosis
pathway,
Immunomodualtion.
In vitro,
In vivo
Granulysin
(Stenger et al., 1998)
Lymphocytes Mycobacterial cell lysis. In vitro
Calgranulin/calprotectin
(Dhiman et al., 2014)
Neutrophils,
Monocytes,
Keratinocytes,
Leukocytes
Phagolysosomal fusion,
Pro-inflammatory action.
In vitro,
In vivo
Ubiquitinated peptides
(Gutsmann, 2016)
Macrophages Mycobacterial cell lysis. In vitro
Lipocalin2
((Gutsmann, 2016))
Neutrophils Mycobacterial growth
inhibition,
Immunoregulation.
In vitro,
In vivo
Synthetic AMPs:
Name Source Mode of action Activity
1-C134mer
(Kapoor et al.,
2011)
De novo design by oligo N-
substituted glycines (peptoid)
and alkylation
Pore formation In vitro
A18G5,
A24C1ac,
A29C5FA, and
A38A1guan
Derived from the insect
proline-rich peptide
Apidaecin. Steps involved are
alkylation, tetramethyl
guanidinilation, and
Bacterial membrane
permeation and inhibition
of protein synthesis
In vitro
6
Antimicrobial RNases
(Becknell et al., 2015)
Eosinophils
(RNase3/ECP),
Neutrophils and
monocytes, Epithelial
cells and leukocytes
Mycobacterial cell
agglutination,
Mycobacteria cell wall and
membrane lysis.
In
vitroI,
In vivo,
Clinical
Eosinophil peroxidase
(Pulido et al., 2013)
Eosinophils Bacterial cell wall lysis. In vitro
Cathepsins
(Walter et al., 2015)
Neutrophils,
Monocytes
Mediation of apoptosis
pathway,
Immunomodualtion.
In vitro,
In vivo
Granulysin
(Stenger et al., 1998)
Lymphocytes Mycobacterial cell lysis. In vitro
Calgranulin/calprotectin
(Dhiman et al., 2014)
Neutrophils,
Monocytes,
Keratinocytes,
Leukocytes
Phagolysosomal fusion,
Pro-inflammatory action.
In vitro,
In vivo
Ubiquitinated peptides
(Gutsmann, 2016)
Macrophages Mycobacterial cell lysis. In vitro
Lipocalin2
((Gutsmann, 2016))
Neutrophils Mycobacterial growth
inhibition,
Immunoregulation.
In vitro,
In vivo
Synthetic AMPs:
Name Source Mode of action Activity
1-C134mer
(Kapoor et al.,
2011)
De novo design by oligo N-
substituted glycines (peptoid)
and alkylation
Pore formation In vitro
A18G5,
A24C1ac,
A29C5FA, and
A38A1guan
Derived from the insect
proline-rich peptide
Apidaecin. Steps involved are
alkylation, tetramethyl
guanidinilation, and
Bacterial membrane
permeation and inhibition
of protein synthesis
In vitro
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
(Hoffmann and
Czihal, 2009)
polyethylene glycol
conjugation.
CAMP/PL-D
(Ramón-García et
al., 2013)
Short cationic peptides (10
AA) rich in W and R selected
from peptide libraries
Pore formation. In vitro
CP26
(Rivas-Santiago
et al., 2013)
Derived from cecropin A:
mellitin
Bacterial cell wall
disruption.
In vitro
d-LAK 120
(Lan et al., 2014)
Synthetic α-helical peptides Pore formation and
inhibition of protein
synthesis.
In vitro,
Ex vivo
d-LL37
(Jiang et al.,
2011)
Derived from LL-37 Pore formation and
immunomodulatory
activity.
In vitro
E2 and E6
(Rivas-Santiago
et al., 2013)
Derived from bactenecin
(bovine cathelicidin) Bac8c (8
AA)
Bacterial cell wall
disruption.
In vitro
HHC-10
(Llamas-
González et al.,
2013)
Derived from bactenecin Bacteria membrane lysis. In vitro,
In vivo
hLFcin1-11/
hLFcin17-30
(Silva et al.,
2014)
Derived from lactoferricin
(All-R and All-K
substitutions)
Bacterial cell wall and
membrane lysis.
In vivo
Innate defense
regulators [innate
defense regulator
(IDR)1002, -
HH2, IDR-1018]
(Rivas-Santiago
et al., 2013)
Derived from macrophage
chemotactic protein-1 (MCP-
1)
Immunomodulatory and
anti-inflammatory
activity.
In vitro,
In vivo
LLAP Derived from LL-37 Inhibition of ATPase. In vitro
7
Czihal, 2009)
polyethylene glycol
conjugation.
CAMP/PL-D
(Ramón-García et
al., 2013)
Short cationic peptides (10
AA) rich in W and R selected
from peptide libraries
Pore formation. In vitro
CP26
(Rivas-Santiago
et al., 2013)
Derived from cecropin A:
mellitin
Bacterial cell wall
disruption.
In vitro
d-LAK 120
(Lan et al., 2014)
Synthetic α-helical peptides Pore formation and
inhibition of protein
synthesis.
In vitro,
Ex vivo
d-LL37
(Jiang et al.,
2011)
Derived from LL-37 Pore formation and
immunomodulatory
activity.
In vitro
E2 and E6
(Rivas-Santiago
et al., 2013)
Derived from bactenecin
(bovine cathelicidin) Bac8c (8
AA)
Bacterial cell wall
disruption.
In vitro
HHC-10
(Llamas-
González et al.,
2013)
Derived from bactenecin Bacteria membrane lysis. In vitro,
In vivo
hLFcin1-11/
hLFcin17-30
(Silva et al.,
2014)
Derived from lactoferricin
(All-R and All-K
substitutions)
Bacterial cell wall and
membrane lysis.
In vivo
Innate defense
regulators [innate
defense regulator
(IDR)1002, -
HH2, IDR-1018]
(Rivas-Santiago
et al., 2013)
Derived from macrophage
chemotactic protein-1 (MCP-
1)
Immunomodulatory and
anti-inflammatory
activity.
In vitro,
In vivo
LLAP Derived from LL-37 Inhibition of ATPase. In vitro
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
(Chingaté et al.,
2015)
LLKKK18
(Silva et al.,
2016)
Derived from LL-37 through
Hyaluronic acid nanogel
conjugation.
Pore formation and
immunomodulatory
activity.
In vivo
MU1140
(Ghobrial et al.,
2010)
Derived from Streptococcus
mutans lantibiotics
Inhibition of cell wall
synthesis.
In vivo,
In vivo
MIAP
(Santos et al.,
2012)
Derived from Magainin-I Inhibition of ATPase. In vitro
Pin2 variants
(Rodríguez et al.,
2014)
Derived from short helical
peptides like pandinin2
Membrane disruption. In vitro
RN3(1-45)
RN6(1-45)
RN7(1-45)
(Pulido et al.,
2013)
Derived from human RNases
N-terminus
Bacterial cell wall
disruption and cell
agglutination and
intracellular macrophage
killing.
In vitro,
ex-vivo
Synthetic AMPs
(SAMPs-Dma)
(Sharma et al.,
2015)
De novo design through
Dimethylamination and
imidazolation.
Cell penetration and
DNA binding.
In vitro
X(LLKK) 2X: II-
D, II-Orn, IIDab,
and IIDap
(Khara et al.,
2014).
Short stabilized α-helix
amphipatic peptides
Pore formation. In vitro
Conclusion:
In recent past, number of AMPs are discovered to combat resistance in TB. These AMPs
exhibit its action through direct killing of bacteria and immunomodulation; hence, there is
more potential to evade resistance problem. In future, AMPs with low-cost synthesis method
8
2015)
LLKKK18
(Silva et al.,
2016)
Derived from LL-37 through
Hyaluronic acid nanogel
conjugation.
Pore formation and
immunomodulatory
activity.
In vivo
MU1140
(Ghobrial et al.,
2010)
Derived from Streptococcus
mutans lantibiotics
Inhibition of cell wall
synthesis.
In vivo,
In vivo
MIAP
(Santos et al.,
2012)
Derived from Magainin-I Inhibition of ATPase. In vitro
Pin2 variants
(Rodríguez et al.,
2014)
Derived from short helical
peptides like pandinin2
Membrane disruption. In vitro
RN3(1-45)
RN6(1-45)
RN7(1-45)
(Pulido et al.,
2013)
Derived from human RNases
N-terminus
Bacterial cell wall
disruption and cell
agglutination and
intracellular macrophage
killing.
In vitro,
ex-vivo
Synthetic AMPs
(SAMPs-Dma)
(Sharma et al.,
2015)
De novo design through
Dimethylamination and
imidazolation.
Cell penetration and
DNA binding.
In vitro
X(LLKK) 2X: II-
D, II-Orn, IIDab,
and IIDap
(Khara et al.,
2014).
Short stabilized α-helix
amphipatic peptides
Pore formation. In vitro
Conclusion:
In recent past, number of AMPs are discovered to combat resistance in TB. These AMPs
exhibit its action through direct killing of bacteria and immunomodulation; hence, there is
more potential to evade resistance problem. In future, AMPs with low-cost synthesis method
8

should be prepared because its cost of synthesis is more. AMPs are susceptible to proteolytic
cleavage after systemic administration; hence, these should be produced using novel drug
delivery systems. It is essential to produce sufficient evidence to address the issues related to
peptide-based therapy for TB.
9
cleavage after systemic administration; hence, these should be produced using novel drug
delivery systems. It is essential to produce sufficient evidence to address the issues related to
peptide-based therapy for TB.
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

References:
Arranz-Trullén, J., Lu, L., Pulido, D., Bhakta, S. and Boix, E. (2017) Host Antimicrobial
Peptides: The Promise of New Treatment Strategies against Tuberculosis. Frontiers in
Immunology, 8, 1499. doi: 10.3389/fimmu.2017.01499.
Becknell, B., Eichler, T.E., Beceiro, S., Li, B., Easterling, R.S. and Carpenter, A.R. (2015)
Ribonucleases 6 and 7 have antimicrobial function in the human and murine urinary tract.
Kidney International, 87, pp. 151–61. doi:10.1038/ki.2014.268.
Chingaté, S., Delgado, G., Salazar, L.M. and Soto, C.Y. (2015) The ATPase activity of the
mycobacterial plasma membrane is inhibited by the LL37-analogous peptide LLAP.
Peptides, 71, pp. 222–8. doi:10.1016/j.peptides.2015.07.021.
Dhiman, R., Venkatasubramanian, S., Paidipally, P., Barnes, P.F., Tvinnereim, A. and
Vankayalapati, R. (2014) Interleukin 22 inhibits intracellular growth of Mycobacterium
tuberculosis by enhancing calgranulin an expression. Journal of Infectious Diseases, 209, pp.
578–87. doi:10.1093/infdis/jit495.
Gutsmann, T. (2016) Interaction between antimicrobial peptides and mycobacteria.
Biochimica et Biophysica Acta, 1858, pp. 1034–43. doi:10.1016/j.bbamem. 2016.01.031.
Ghobrial, O., Derendorf, H. and Hillman, J.D. (2010) Pharmacokinetic and
pharmacodynamic evaluation of the lantibiotic MU1140. Journal of Pharmaceutical
Sciences, 99, pp. 2521–8. doi:10.1002/jps.22015.
Hoffmann and Czihal. (2009) Antibiotic peptides Patent. WO2009013262 A1.
Hwang, S. A., Wilk, K.M., Bangale, Y.A., Kruzel, M.L. and Actor, J.K. (2007) Lactoferrin
modulation of IL-12 and IL-10 response from activated murine leukocytes. Medical
Microbiology and Immunology, 196, pp. 171–80. doi:10.1007/s00430-007-0041-6.
Jena, P., Mohanty, S., Mohanty, T., Kallert, S., Morgelin, M. and Lindstrøm, T. (2012)
Azurophil granule proteins constitute the major mycobactericidal proteins in human
neutrophils and enhance the killing of mycobacteria in macrophages. PLoS One, 7:e50345.
doi:10.1371/journal.pone.0050345.
Jiang, Z., Higgins, M.P., Whitehurst, J., Kisich, K.O., Voskuil, M.I. and Hodges, R.S. (2011)
Antituberculosis activity of α-helical antimicrobial peptides: de novo designed L- and D-
enantiomers versus L- and D-LL-37. Protein & Peptide Letters, 18, pp. 241–52.
doi:10.2174/092986611794578288.
Kapoor, R., Eimerman, P.R., Hardy, J.W., Cirillo, J.D., Contag, C.H. and Barron, A.E. (2011)
Efficacy of antimicrobial peptoids against Mycobacterium tuberculosis. Antimicrobial Agents
and Chemotherapy, 55, pp. 3058–62. doi:10.1128/AAC.01667-10.
Khara, J.S., Wang, Y., Ke, X. Y., Liu, S., Newton, S.M. and Langford, P.R. (2014).
Antimycobacterial activities of synthetic cationic α-helical peptides and their synergism with
rifampicin. Biomaterials, 35, pp. 2032–8. doi:10.1016/ j.biomaterials.2013.11.035.
Lan, Y., Lam, J.T., Siu, G.K.H., Yam, W.C., Mason, A.J. and Lam, J.K.W. (2014) Cationic
amphipathic D-enantiomeric antimicrobial peptides with in vitro and ex vivo activity against
10
Arranz-Trullén, J., Lu, L., Pulido, D., Bhakta, S. and Boix, E. (2017) Host Antimicrobial
Peptides: The Promise of New Treatment Strategies against Tuberculosis. Frontiers in
Immunology, 8, 1499. doi: 10.3389/fimmu.2017.01499.
Becknell, B., Eichler, T.E., Beceiro, S., Li, B., Easterling, R.S. and Carpenter, A.R. (2015)
Ribonucleases 6 and 7 have antimicrobial function in the human and murine urinary tract.
Kidney International, 87, pp. 151–61. doi:10.1038/ki.2014.268.
Chingaté, S., Delgado, G., Salazar, L.M. and Soto, C.Y. (2015) The ATPase activity of the
mycobacterial plasma membrane is inhibited by the LL37-analogous peptide LLAP.
Peptides, 71, pp. 222–8. doi:10.1016/j.peptides.2015.07.021.
Dhiman, R., Venkatasubramanian, S., Paidipally, P., Barnes, P.F., Tvinnereim, A. and
Vankayalapati, R. (2014) Interleukin 22 inhibits intracellular growth of Mycobacterium
tuberculosis by enhancing calgranulin an expression. Journal of Infectious Diseases, 209, pp.
578–87. doi:10.1093/infdis/jit495.
Gutsmann, T. (2016) Interaction between antimicrobial peptides and mycobacteria.
Biochimica et Biophysica Acta, 1858, pp. 1034–43. doi:10.1016/j.bbamem. 2016.01.031.
Ghobrial, O., Derendorf, H. and Hillman, J.D. (2010) Pharmacokinetic and
pharmacodynamic evaluation of the lantibiotic MU1140. Journal of Pharmaceutical
Sciences, 99, pp. 2521–8. doi:10.1002/jps.22015.
Hoffmann and Czihal. (2009) Antibiotic peptides Patent. WO2009013262 A1.
Hwang, S. A., Wilk, K.M., Bangale, Y.A., Kruzel, M.L. and Actor, J.K. (2007) Lactoferrin
modulation of IL-12 and IL-10 response from activated murine leukocytes. Medical
Microbiology and Immunology, 196, pp. 171–80. doi:10.1007/s00430-007-0041-6.
Jena, P., Mohanty, S., Mohanty, T., Kallert, S., Morgelin, M. and Lindstrøm, T. (2012)
Azurophil granule proteins constitute the major mycobactericidal proteins in human
neutrophils and enhance the killing of mycobacteria in macrophages. PLoS One, 7:e50345.
doi:10.1371/journal.pone.0050345.
Jiang, Z., Higgins, M.P., Whitehurst, J., Kisich, K.O., Voskuil, M.I. and Hodges, R.S. (2011)
Antituberculosis activity of α-helical antimicrobial peptides: de novo designed L- and D-
enantiomers versus L- and D-LL-37. Protein & Peptide Letters, 18, pp. 241–52.
doi:10.2174/092986611794578288.
Kapoor, R., Eimerman, P.R., Hardy, J.W., Cirillo, J.D., Contag, C.H. and Barron, A.E. (2011)
Efficacy of antimicrobial peptoids against Mycobacterium tuberculosis. Antimicrobial Agents
and Chemotherapy, 55, pp. 3058–62. doi:10.1128/AAC.01667-10.
Khara, J.S., Wang, Y., Ke, X. Y., Liu, S., Newton, S.M. and Langford, P.R. (2014).
Antimycobacterial activities of synthetic cationic α-helical peptides and their synergism with
rifampicin. Biomaterials, 35, pp. 2032–8. doi:10.1016/ j.biomaterials.2013.11.035.
Lan, Y., Lam, J.T., Siu, G.K.H., Yam, W.C., Mason, A.J. and Lam, J.K.W. (2014) Cationic
amphipathic D-enantiomeric antimicrobial peptides with in vitro and ex vivo activity against
10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

drug-resistant Mycobacterium tuberculosis. Tuberculosis, 94, pp. 678–89.
doi:10.1016/j.tube.2014.08.001.
Llamas-González, Y.Y., Pedroza-Roldán, C., Cortés-Serna, M.B., MárquezAguirre, A.L.,
Gálvez-Gastélum, F.J. and Flores-Valdez, M.A. (2013) The synthetic cathelicidin HHC-10
inhibits Mycobacterium bovis BCG in vitro and in C57BL/6 mice. Microbial Drug
Resistance, 19, pp. 124–9. doi:10.1089/mdr.2012.0149.
Mandal, S.M., Roy, A., Ghosh, A.K., Hazra, T.K., Basak, A. and Franco, O.L. (2014)
Challenges and future prospects of antibiotic therapy: from peptides to phages utilization.
Frontiers in Pharmacology, 13, 5. p. 105. doi: 10.3389/fphar.2014.00105.
Pulido, D., Torrent, M., Andreu, D., Nogues, M.V. and Boix, E. (2013) Two human host
defense ribonucleases against mycobacteria, the eosinophil cationic protein (RNase 3) and
RNase 7. Antimicrobial Agents and Chemotherapy, 57, pp. 3797–805.
doi:10.1128/AAC.00428-13.
Ramón-García, S., Mikut, R., Ng, C., Ruden, S., Volkmer, R. and Reischl, M. (2013)
Targeting Mycobacterium tuberculosis and other microbial pathogens using improved
synthetic antibacterial peptides. Antimicrobial Agents and Chemotherapy, 57, pp. 2295–303.
doi:10.1128/AAC.00175-13.
Rekha, R.S., Rao Muvva, S.S.V.J., Wan, M., Raqib, R., Bergman, P. and Brighenti, S. (2015)
Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of
Mycobacterium tuberculosis in human macrophages. Autophagy, 11, pp. 1688–99.
doi:10.1080/15548627.2015.1075110.
Rivas-Santiago, B., Rivas Santiago, C.E., Castañeda-Delgado, J.E., LeónContreras, J.C.,
Hancock, R.E.W. and Hernandez-Pando, R. (2013) Activity of LL-37, CRAMP and
antimicrobial peptide-derived compounds E2, E6 and CP26 against Mycobacterium
tuberculosis. International Journal of Antimicrobial Agents, 41, pp. 143–8.
doi:10.1016/j.ijantimicag.2012.09.015.
Rodríguez, A., Villegas, E., Montoya-Rosales, A., Rivas-Santiago, B. and Corzo, G. (2014).
Characterization of antibacterial and hemolytic activity of synthetic pandinin 2 variants and
their inhibition against Mycobacterium tuberculosis. PLoS One, 9, e101742.
doi:10.1371/journal.pone.0101742.
Rivas-Santiago, B., Castañeda-Delgado, J.E., Rivas Santiago, C.E., Waldbrook, M.,
González-Curiel, I. and León-Contreras, J.C. (2013) Ability of innate defence regulator
peptides IDR-1002, IDR-HH2 and IDR-1018 to protect against Mycobacterium tuberculosis
infections in animal models. PLoS One, 8, e59119. doi:10.1371/journal.pone.0059119.
Rivas-Santiago, C.E., Rivas-Santiago, B., León, D.A., Castañeda-Delgado, J. and Hernández
Pando, R. (2011) Induction of β-defensins by l-isoleucine as novel immunotherapy in
experimental murine tuberculosis. Clinical & Experimental Immunology, 164, pp. 80–9.
doi:10.1111/j.1365-2249.2010.04313.x.
Santos, P., Gordillo, A., Osses, L., Salazar, L.M. and Soto, C.Y. (2012) Effect of
antimicrobial peptides on ATPase activity and proton pumping in plasma membrane vesicles
obtained from mycobacteria. Peptides, 36, pp. 121–8. doi:10.1016/ j.peptides.2012.04.018.
11
doi:10.1016/j.tube.2014.08.001.
Llamas-González, Y.Y., Pedroza-Roldán, C., Cortés-Serna, M.B., MárquezAguirre, A.L.,
Gálvez-Gastélum, F.J. and Flores-Valdez, M.A. (2013) The synthetic cathelicidin HHC-10
inhibits Mycobacterium bovis BCG in vitro and in C57BL/6 mice. Microbial Drug
Resistance, 19, pp. 124–9. doi:10.1089/mdr.2012.0149.
Mandal, S.M., Roy, A., Ghosh, A.K., Hazra, T.K., Basak, A. and Franco, O.L. (2014)
Challenges and future prospects of antibiotic therapy: from peptides to phages utilization.
Frontiers in Pharmacology, 13, 5. p. 105. doi: 10.3389/fphar.2014.00105.
Pulido, D., Torrent, M., Andreu, D., Nogues, M.V. and Boix, E. (2013) Two human host
defense ribonucleases against mycobacteria, the eosinophil cationic protein (RNase 3) and
RNase 7. Antimicrobial Agents and Chemotherapy, 57, pp. 3797–805.
doi:10.1128/AAC.00428-13.
Ramón-García, S., Mikut, R., Ng, C., Ruden, S., Volkmer, R. and Reischl, M. (2013)
Targeting Mycobacterium tuberculosis and other microbial pathogens using improved
synthetic antibacterial peptides. Antimicrobial Agents and Chemotherapy, 57, pp. 2295–303.
doi:10.1128/AAC.00175-13.
Rekha, R.S., Rao Muvva, S.S.V.J., Wan, M., Raqib, R., Bergman, P. and Brighenti, S. (2015)
Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of
Mycobacterium tuberculosis in human macrophages. Autophagy, 11, pp. 1688–99.
doi:10.1080/15548627.2015.1075110.
Rivas-Santiago, B., Rivas Santiago, C.E., Castañeda-Delgado, J.E., LeónContreras, J.C.,
Hancock, R.E.W. and Hernandez-Pando, R. (2013) Activity of LL-37, CRAMP and
antimicrobial peptide-derived compounds E2, E6 and CP26 against Mycobacterium
tuberculosis. International Journal of Antimicrobial Agents, 41, pp. 143–8.
doi:10.1016/j.ijantimicag.2012.09.015.
Rodríguez, A., Villegas, E., Montoya-Rosales, A., Rivas-Santiago, B. and Corzo, G. (2014).
Characterization of antibacterial and hemolytic activity of synthetic pandinin 2 variants and
their inhibition against Mycobacterium tuberculosis. PLoS One, 9, e101742.
doi:10.1371/journal.pone.0101742.
Rivas-Santiago, B., Castañeda-Delgado, J.E., Rivas Santiago, C.E., Waldbrook, M.,
González-Curiel, I. and León-Contreras, J.C. (2013) Ability of innate defence regulator
peptides IDR-1002, IDR-HH2 and IDR-1018 to protect against Mycobacterium tuberculosis
infections in animal models. PLoS One, 8, e59119. doi:10.1371/journal.pone.0059119.
Rivas-Santiago, C.E., Rivas-Santiago, B., León, D.A., Castañeda-Delgado, J. and Hernández
Pando, R. (2011) Induction of β-defensins by l-isoleucine as novel immunotherapy in
experimental murine tuberculosis. Clinical & Experimental Immunology, 164, pp. 80–9.
doi:10.1111/j.1365-2249.2010.04313.x.
Santos, P., Gordillo, A., Osses, L., Salazar, L.M. and Soto, C.Y. (2012) Effect of
antimicrobial peptides on ATPase activity and proton pumping in plasma membrane vesicles
obtained from mycobacteria. Peptides, 36, pp. 121–8. doi:10.1016/ j.peptides.2012.04.018.
11

Sharma, S., Verma, I. and Khuller, G.K. (2000) Antibacterial activity of human neutrophil
peptide-1 against Mycobacterium tuberculosis H37Rv: in vitro and ex vivo study. European
Respiratory Journal, 16, pp. 112–7. doi:10.1034/j.1399-3003.2000.16a20.x 50.
Sharma, S., Verma, I. and Khuller, G.K. (2001) Therapeutic potential of human neutrophil
peptide 1 against experimental tuberculosis. Antimicrobial Agents and Chemotherapy, 45, pp.
639–40. doi:10.1128/AAC.45.2.639-640.2001.
Sharma, A., Pohane, A.A., Bansal, S., Bajaj, A., Jain, V. and Srivastava, A. (2015) Cell
penetrating synthetic antimicrobial peptides (SAMPs) exhibiting potent and selective killing
of Mycobacterium by targeting its DNA. Chemistry, 21, pp. 3540–5.
doi:10.1002/chem.201404650.
Silva, T., Magalhães, B., Maia, S., Gomes, P., Nazmi, K. and Bolscher, J.G.M. (2014) Killing
of Mycobacterium avium by lactoferricin peptides: improved activity of arginine- and d-
amino-acid-containing molecules. Antimicrobial Agents and Chemotherapy, 58, pp. 3461–7.
doi:10.1128/AAC.02728-13.
Silva, J.P., Gonçalves, C., Costa, C., Sousa, J., Silva-Gomes, R. and Castro, A.G. (2016).
Delivery of LLKKK18 loaded into self-assembling hyaluronic acid nanogel for tuberculosis
treatment. Journal of Controlled Release, 235, pp. 112–24. doi:10.1016/
j.jconrel.2016.05.064.
Stenger, S., Hanson, D.A., Teitelbaum, R., Dewan, P., Niazi, K.R. and Froelich, C.J. (1998)
An antimicrobial activity of cytolytic T cells mediated by granulysin. Science, 282, pp. 121–
5. doi:10.1126/science.282.5386.121.
Torres-Juarez, F., Cardenas-Vargas, A., Montoya-Rosales, A., González-Curiel, I., Garcia-
Hernandez, M.H. and Enciso-Moreno, J.A. (2015) LL-37 immunomodulatory activity during
Mycobacterium tuberculosis infection in macrophages. Infection and Immunity, 83, pp. 4495–
503. doi:10.1128/IAI.00936-15 58.
Venketaraman, V., Kaushal, D. and Saviola, B. (2015) Mycobacterium tuberculosis. Journal
of Immunology Research, 857598. doi: 10.1155/2015/857598.
Walter, K., Steinwede, K., Aly, S., Reinheckel, T., Bohling, J. and Maus, U.A. (2015)
Cathepsin G in experimental tuberculosis: relevance for antibacterial protection and potential
for immunotherapy. Journal of Immunology, 195, pp. 3325–33.
doi:10.4049/jimmunol.1501012.
Wong, K.W. and Jacobs, W.R. (2013) Mycobacterium tuberculosis exploits human interferon
γ to stimulate macrophage extracellular trap formation and necrosis. Journal of Infectious
Diseases, 208, pp. 109–19. doi:10.1093/infdis/jit097.
Yamaji, S (2004) Inhibition of iron transport across human intestinal epithelial cells by
hepcidin. Blood, 104, pp. 2178–80. doi:10.1182/blood-2004-03-0829.
Yu, X., Li, C., Hong, W., Pan, W. and Xie, J. (2013) Autophagy during Mycobacterium
tuberculosis infection and implications for future tuberculosis medications. Cell Signal, 25,
pp. 1272–8. doi:10.1016/j.cellsig.2013.02.011.
12
peptide-1 against Mycobacterium tuberculosis H37Rv: in vitro and ex vivo study. European
Respiratory Journal, 16, pp. 112–7. doi:10.1034/j.1399-3003.2000.16a20.x 50.
Sharma, S., Verma, I. and Khuller, G.K. (2001) Therapeutic potential of human neutrophil
peptide 1 against experimental tuberculosis. Antimicrobial Agents and Chemotherapy, 45, pp.
639–40. doi:10.1128/AAC.45.2.639-640.2001.
Sharma, A., Pohane, A.A., Bansal, S., Bajaj, A., Jain, V. and Srivastava, A. (2015) Cell
penetrating synthetic antimicrobial peptides (SAMPs) exhibiting potent and selective killing
of Mycobacterium by targeting its DNA. Chemistry, 21, pp. 3540–5.
doi:10.1002/chem.201404650.
Silva, T., Magalhães, B., Maia, S., Gomes, P., Nazmi, K. and Bolscher, J.G.M. (2014) Killing
of Mycobacterium avium by lactoferricin peptides: improved activity of arginine- and d-
amino-acid-containing molecules. Antimicrobial Agents and Chemotherapy, 58, pp. 3461–7.
doi:10.1128/AAC.02728-13.
Silva, J.P., Gonçalves, C., Costa, C., Sousa, J., Silva-Gomes, R. and Castro, A.G. (2016).
Delivery of LLKKK18 loaded into self-assembling hyaluronic acid nanogel for tuberculosis
treatment. Journal of Controlled Release, 235, pp. 112–24. doi:10.1016/
j.jconrel.2016.05.064.
Stenger, S., Hanson, D.A., Teitelbaum, R., Dewan, P., Niazi, K.R. and Froelich, C.J. (1998)
An antimicrobial activity of cytolytic T cells mediated by granulysin. Science, 282, pp. 121–
5. doi:10.1126/science.282.5386.121.
Torres-Juarez, F., Cardenas-Vargas, A., Montoya-Rosales, A., González-Curiel, I., Garcia-
Hernandez, M.H. and Enciso-Moreno, J.A. (2015) LL-37 immunomodulatory activity during
Mycobacterium tuberculosis infection in macrophages. Infection and Immunity, 83, pp. 4495–
503. doi:10.1128/IAI.00936-15 58.
Venketaraman, V., Kaushal, D. and Saviola, B. (2015) Mycobacterium tuberculosis. Journal
of Immunology Research, 857598. doi: 10.1155/2015/857598.
Walter, K., Steinwede, K., Aly, S., Reinheckel, T., Bohling, J. and Maus, U.A. (2015)
Cathepsin G in experimental tuberculosis: relevance for antibacterial protection and potential
for immunotherapy. Journal of Immunology, 195, pp. 3325–33.
doi:10.4049/jimmunol.1501012.
Wong, K.W. and Jacobs, W.R. (2013) Mycobacterium tuberculosis exploits human interferon
γ to stimulate macrophage extracellular trap formation and necrosis. Journal of Infectious
Diseases, 208, pp. 109–19. doi:10.1093/infdis/jit097.
Yamaji, S (2004) Inhibition of iron transport across human intestinal epithelial cells by
hepcidin. Blood, 104, pp. 2178–80. doi:10.1182/blood-2004-03-0829.
Yu, X., Li, C., Hong, W., Pan, W. and Xie, J. (2013) Autophagy during Mycobacterium
tuberculosis infection and implications for future tuberculosis medications. Cell Signal, 25,
pp. 1272–8. doi:10.1016/j.cellsig.2013.02.011.
12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 12
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.