Ublituximab: A Novel Disease Target Therapy for MS - Desklib Report
VerifiedAdded on 2023/04/21
|6
|1612
|424
Report
AI Summary
This report explores Ublituximab, a glycoengineered anti-CD20 monoclonal antibody, as a potential therapy for relapsing multiple sclerosis (MS). It highlights that B cells are active during MS relapse and Ublituximab targets the CD20 antigen on mature B cells, depleting their volume. Initially aimed at treating chronic lymphocytic leukemia, Ublituximab has shown efficacy in managing relapsing forms of MS in phase 2 trials, reducing brain and spine lesions. The therapy targets the CD20 protein in immune B cells involved in MS development, with infusions administered over several weeks. While minor infusion-related side effects have been observed, no serious adverse events were reported. The company plans a marketing strategy with a $6 price target, aiming for significant sales. Ublituximab offers a new mechanism of action, validated against competitors, and demonstrates potential advantages in inhibiting BCR signaling. The report emphasizes the need for randomized trials across diverse ethnic origins to further assess its efficacy and safety, particularly in comparison to existing therapies like ibrutinib. Further research is needed to optimize GMP protocols and assess toxicity levels to expand the use of Ublituximab in managing chronic leukemia.

Name: TG Therapeutics
Date 13/02/2018
Address
35264220
Georgia, USA
Ublituximab is a glycoengineered anti-CD 20 monoclonal antibodies used as a potential treatment for
relapsing. Studies have shown that the B cells are active during the relapse phase in which the cells of the
immune system are found in the acute lesions located in the spinal cord which have a role in MS flares,
(Burger et al., 2014).
Ublituximab offers target receptors of the CD 20 on the antigen located in the mature B cells depleting the
volume in the blood vessels. Its original management is aimed at treating disorders such as chronic
lymphocytic leukemia. Currently, Ublituximab is being observed in uncovering autoimmune response.
Results obtained from phase 2 trial shows the drug Ublituximab continues to be effective efficacy in
managing relapsing forms of sclerosis, (Barr et al., 2015). Managing using 450 mg Ublituximab offered
using intravenous means induced depletion of B cells. Treatment of brain lesions and spine of code
patients were reduced significantly, (Morabito et al., 2015).
Ublituximab targets the region of CD 20 protein present in the immune B cells which are involved in MS
development. This therapeutically study has been undertaken to assess safety and evaluate how different
Ublituximab, a monoclonal antibody for a novel
Disease Target As A Therapy For Effectively
Lowering Relapses, Depleting B-Cells In MS
Patients
Opportunity
What we have:
Date 13/02/2018
Address
35264220
Georgia, USA
Ublituximab is a glycoengineered anti-CD 20 monoclonal antibodies used as a potential treatment for
relapsing. Studies have shown that the B cells are active during the relapse phase in which the cells of the
immune system are found in the acute lesions located in the spinal cord which have a role in MS flares,
(Burger et al., 2014).
Ublituximab offers target receptors of the CD 20 on the antigen located in the mature B cells depleting the
volume in the blood vessels. Its original management is aimed at treating disorders such as chronic
lymphocytic leukemia. Currently, Ublituximab is being observed in uncovering autoimmune response.
Results obtained from phase 2 trial shows the drug Ublituximab continues to be effective efficacy in
managing relapsing forms of sclerosis, (Barr et al., 2015). Managing using 450 mg Ublituximab offered
using intravenous means induced depletion of B cells. Treatment of brain lesions and spine of code
patients were reduced significantly, (Morabito et al., 2015).
Ublituximab targets the region of CD 20 protein present in the immune B cells which are involved in MS
development. This therapeutically study has been undertaken to assess safety and evaluate how different
Ublituximab, a monoclonal antibody for a novel
Disease Target As A Therapy For Effectively
Lowering Relapses, Depleting B-Cells In MS
Patients
Opportunity
What we have:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

dosages of drug management alters body functions. The infusion applied in this study were done in three
periods on 1st, 15th, and 24th week.
Intellectual property rights
IPR process for monotherapy has been undertaken with patent rights documented according to world
intellectual property organization rights, (Braga, 2018).
Trials have shown that ublituximab, has a remarkable reduction in multiple sclerosis of the patient's brain
and spine lesions. The therapy works through the honing of CD 20 protein which is present in immune B
cells involved in MS development. Side effects have been observed on ublituximab as reported in few
cases however there were minor and safe side effects. The management of adverse effects was related to
infusion while no serious events were reported.
In vitro / in vivo data showed on the initial doses administered as an administration of 150 mg infusion on
day 1, 450 mg on day 15 and 450 mg at week 24 and 600 mg. Ublituximab has no major adverse reactions
noted, (Byrd et al., 2014).
The company is initiating marketing strategy of the therapy drug with a buy rating of $6 price target. The
target price is meant to generate more forecast sales for drug therapy. The price is lower as expected to
market catalysts.
The buying rating of $6 as the target price is aimed at ensuring that there is continuous cash flow. The
cash flow of the therapy is aimed to be smooth after 2 years of its launch. The therapy is a monoclonal
antibody which induce antibody-dependent cell-mediated cytotoxicity.
Current treatment options for chronic lymphocytic leukemia include:
- Fludarabine treatment, Cytoxan and rituximab, high dos of prediction and rituximab, Ibrutinib and
alemtuzumab with rituximab.
The market and competitors
periods on 1st, 15th, and 24th week.
Intellectual property rights
IPR process for monotherapy has been undertaken with patent rights documented according to world
intellectual property organization rights, (Braga, 2018).
Trials have shown that ublituximab, has a remarkable reduction in multiple sclerosis of the patient's brain
and spine lesions. The therapy works through the honing of CD 20 protein which is present in immune B
cells involved in MS development. Side effects have been observed on ublituximab as reported in few
cases however there were minor and safe side effects. The management of adverse effects was related to
infusion while no serious events were reported.
In vitro / in vivo data showed on the initial doses administered as an administration of 150 mg infusion on
day 1, 450 mg on day 15 and 450 mg at week 24 and 600 mg. Ublituximab has no major adverse reactions
noted, (Byrd et al., 2014).
The company is initiating marketing strategy of the therapy drug with a buy rating of $6 price target. The
target price is meant to generate more forecast sales for drug therapy. The price is lower as expected to
market catalysts.
The buying rating of $6 as the target price is aimed at ensuring that there is continuous cash flow. The
cash flow of the therapy is aimed to be smooth after 2 years of its launch. The therapy is a monoclonal
antibody which induce antibody-dependent cell-mediated cytotoxicity.
Current treatment options for chronic lymphocytic leukemia include:
- Fludarabine treatment, Cytoxan and rituximab, high dos of prediction and rituximab, Ibrutinib and
alemtuzumab with rituximab.
The market and competitors
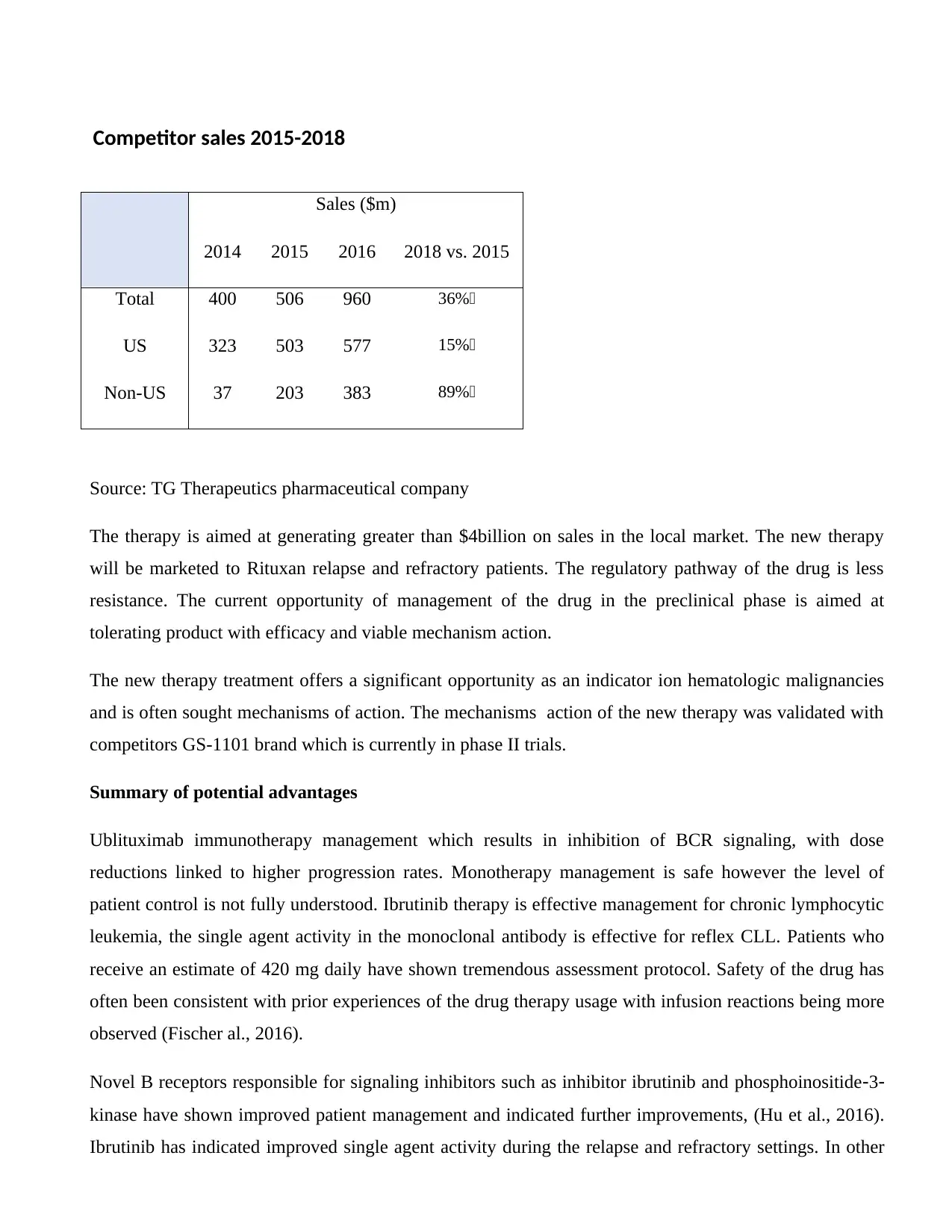
Sales ($m)
2014 2015 2016 2018 vs. 2015
Total 400 506 960 36%
US 323 503 577 15%
Non-US 37 203 383 89%
Source: TG Therapeutics pharmaceutical company
The therapy is aimed at generating greater than $4billion on sales in the local market. The new therapy
will be marketed to Rituxan relapse and refractory patients. The regulatory pathway of the drug is less
resistance. The current opportunity of management of the drug in the preclinical phase is aimed at
tolerating product with efficacy and viable mechanism action.
The new therapy treatment offers a significant opportunity as an indicator ion hematologic malignancies
and is often sought mechanisms of action. The mechanisms action of the new therapy was validated with
competitors GS-1101 brand which is currently in phase II trials.
Summary of potential advantages
Ublituximab immunotherapy management which results in inhibition of BCR signaling, with dose
reductions linked to higher progression rates. Monotherapy management is safe however the level of
patient control is not fully understood. Ibrutinib therapy is effective management for chronic lymphocytic
leukemia, the single agent activity in the monoclonal antibody is effective for reflex CLL. Patients who
receive an estimate of 420 mg daily have shown tremendous assessment protocol. Safety of the drug has
often been consistent with prior experiences of the drug therapy usage with infusion reactions being more
observed (Fischer al., 2016).
Novel B receptors responsible for signaling inhibitors such as inhibitor ibrutinib and phosphoinositide‐3‐
kinase have shown improved patient management and indicated further improvements, (Hu et al., 2016).
Ibrutinib has indicated improved single agent activity during the relapse and refractory settings. In other
Competitor sales 2015-2018
2014 2015 2016 2018 vs. 2015
Total 400 506 960 36%
US 323 503 577 15%
Non-US 37 203 383 89%
Source: TG Therapeutics pharmaceutical company
The therapy is aimed at generating greater than $4billion on sales in the local market. The new therapy
will be marketed to Rituxan relapse and refractory patients. The regulatory pathway of the drug is less
resistance. The current opportunity of management of the drug in the preclinical phase is aimed at
tolerating product with efficacy and viable mechanism action.
The new therapy treatment offers a significant opportunity as an indicator ion hematologic malignancies
and is often sought mechanisms of action. The mechanisms action of the new therapy was validated with
competitors GS-1101 brand which is currently in phase II trials.
Summary of potential advantages
Ublituximab immunotherapy management which results in inhibition of BCR signaling, with dose
reductions linked to higher progression rates. Monotherapy management is safe however the level of
patient control is not fully understood. Ibrutinib therapy is effective management for chronic lymphocytic
leukemia, the single agent activity in the monoclonal antibody is effective for reflex CLL. Patients who
receive an estimate of 420 mg daily have shown tremendous assessment protocol. Safety of the drug has
often been consistent with prior experiences of the drug therapy usage with infusion reactions being more
observed (Fischer al., 2016).
Novel B receptors responsible for signaling inhibitors such as inhibitor ibrutinib and phosphoinositide‐3‐
kinase have shown improved patient management and indicated further improvements, (Hu et al., 2016).
Ibrutinib has indicated improved single agent activity during the relapse and refractory settings. In other
Competitor sales 2015-2018
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

studies, ibrutinib has improved the overall survival compared to other drug therapy management options,
(Burger et al., 2015).
The company needs to randomized trials and expand across the various human ethnic origins. In other
studies, racial differences have been noted on mechanisms of known therapy management. This
monotherapy being a type 1 anti CD20 binds to a unique epitome can exhibit low fructose fragment,
(Robak & Robak, 2011). There is a need for more studies to assess whether the ibrutinib could result in
quicker responses and greater response depth for patients with chronic leukemia approaches could be
sought in seeking for more expansive trials for the management of chronic leukemia. This will entail
looking for lead candidates for the trial, optimizing the GMP protocols, assessing and generating toxicity
and safety levels and conducting the trials.
What we need
(Burger et al., 2015).
The company needs to randomized trials and expand across the various human ethnic origins. In other
studies, racial differences have been noted on mechanisms of known therapy management. This
monotherapy being a type 1 anti CD20 binds to a unique epitome can exhibit low fructose fragment,
(Robak & Robak, 2011). There is a need for more studies to assess whether the ibrutinib could result in
quicker responses and greater response depth for patients with chronic leukemia approaches could be
sought in seeking for more expansive trials for the management of chronic leukemia. This will entail
looking for lead candidates for the trial, optimizing the GMP protocols, assessing and generating toxicity
and safety levels and conducting the trials.
What we need
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References
Barr, P.M., Brown, J.R., Hillmen, P., O'Brien, S., Barrientos, J.C., Reddy, N.M., Coutre, S., Mulligan,
S.P., Jaeger, U., Furman, R.R. and Cymbalista, F., 2017. Impact of ibrutinib dose adherence on
therapeutic efficacy in patients with previously treated CLL/SLL. Blood, pp.blood-2016.
Braga, C.A.P., 2018. Innovation, Trade, and Intellectual Property Rights: Implications for Trade
Negotiations. Megaregionalism 2.0: Trade And Innovation Within Global Networks, 67, p.343.
Burger, J.A., Keating, M.J., Wierda, W.G., Hartmann, E., Hoellenriegel, J., Rosin, N.Y., de Weerdt, I.,
Jeyakumar, G., Ferrajoli, A., Cardenas-Turanzas, M. and Lerner, S., 2014. Safety and activity of ibrutinib
plus rituximab for patients with high-risk chronic lymphocytic leukemia: a single-arm, phase 2 study. The
Lancet Oncology, 15(10), pp.1090-1099.
Burger, J.A., Tedeschi, A., Barr, P.M., Robak, T., Owen, C., Ghia, P., Bairey, O., Hillmen, P., Bartlett,
N.L., Li, J. and Simpson, D., 2015. Ibrutinib as initial therapy for patients with chronic lymphocytic
leukemia. New England Journal of Medicine, 373(25), pp.2425-2437.
Byrd, J.C., Hillmen, P., O’Brien, S., Barrientos, J.C., Reddy, N.M., Coutre, S., Tam, C.S., Mulligan, S.P.,
Jäger, U., Barr, P.M. and Furman, R.R., 2017. Long-term efficacy and safety with ibrutinib (IBR) in
previously treated chronic lymphocytic leukemia (CLL): up to four years follow-up of the RESONATE
study. J Clin Oncol, 35(suppl).
Fischer, K., Bahlo, J., Fink, A.M., Goede, V., Herling, C.D., Cramer, P., Langerbeins, P., Von Tresckow,
J., Engelke, A., Maurer, C. and Kovacs, G., 2016. Long-term remissions after FCR chemoimmunotherapy
in previously untreated patients with CLL: updated results of the CLL8 trial. Blood, 127(2), pp.208-215.
Hu, H., Juvekar, A., Lyssiotis, C.A., Lien, E.C., Albeck, J.G., Oh, D., Varma, G., Hung, Y.P., Ullas, S.,
Lauring, J. and Seth, P., 2016. Phosphoinositide 3-kinase regulates glycolysis through mobilization of
aldolase from the actin cytoskeleton. Cell, 164(3), pp.433-446.
Morabito, F., Recchia, A.G., Vigna, E., De Stefano, L., Bossio, S., Morabito, L., Pellicanò, M., Palumbo,
A., Storino, F., Caruso, N. and Gentile, M., 2015. Promising therapies for the treatment of chronic
lymphocytic leukemia. Expert opinion on investigational drugs, 24(6), pp.795-807.
Robak, T. and Robak, E., 2011. New anti-CD20 monoclonal antibodies for the treatment of B-cell
lymphoid malignancies. BioDrugs, 25(1), pp.13-25.
Barr, P.M., Brown, J.R., Hillmen, P., O'Brien, S., Barrientos, J.C., Reddy, N.M., Coutre, S., Mulligan,
S.P., Jaeger, U., Furman, R.R. and Cymbalista, F., 2017. Impact of ibrutinib dose adherence on
therapeutic efficacy in patients with previously treated CLL/SLL. Blood, pp.blood-2016.
Braga, C.A.P., 2018. Innovation, Trade, and Intellectual Property Rights: Implications for Trade
Negotiations. Megaregionalism 2.0: Trade And Innovation Within Global Networks, 67, p.343.
Burger, J.A., Keating, M.J., Wierda, W.G., Hartmann, E., Hoellenriegel, J., Rosin, N.Y., de Weerdt, I.,
Jeyakumar, G., Ferrajoli, A., Cardenas-Turanzas, M. and Lerner, S., 2014. Safety and activity of ibrutinib
plus rituximab for patients with high-risk chronic lymphocytic leukemia: a single-arm, phase 2 study. The
Lancet Oncology, 15(10), pp.1090-1099.
Burger, J.A., Tedeschi, A., Barr, P.M., Robak, T., Owen, C., Ghia, P., Bairey, O., Hillmen, P., Bartlett,
N.L., Li, J. and Simpson, D., 2015. Ibrutinib as initial therapy for patients with chronic lymphocytic
leukemia. New England Journal of Medicine, 373(25), pp.2425-2437.
Byrd, J.C., Hillmen, P., O’Brien, S., Barrientos, J.C., Reddy, N.M., Coutre, S., Tam, C.S., Mulligan, S.P.,
Jäger, U., Barr, P.M. and Furman, R.R., 2017. Long-term efficacy and safety with ibrutinib (IBR) in
previously treated chronic lymphocytic leukemia (CLL): up to four years follow-up of the RESONATE
study. J Clin Oncol, 35(suppl).
Fischer, K., Bahlo, J., Fink, A.M., Goede, V., Herling, C.D., Cramer, P., Langerbeins, P., Von Tresckow,
J., Engelke, A., Maurer, C. and Kovacs, G., 2016. Long-term remissions after FCR chemoimmunotherapy
in previously untreated patients with CLL: updated results of the CLL8 trial. Blood, 127(2), pp.208-215.
Hu, H., Juvekar, A., Lyssiotis, C.A., Lien, E.C., Albeck, J.G., Oh, D., Varma, G., Hung, Y.P., Ullas, S.,
Lauring, J. and Seth, P., 2016. Phosphoinositide 3-kinase regulates glycolysis through mobilization of
aldolase from the actin cytoskeleton. Cell, 164(3), pp.433-446.
Morabito, F., Recchia, A.G., Vigna, E., De Stefano, L., Bossio, S., Morabito, L., Pellicanò, M., Palumbo,
A., Storino, F., Caruso, N. and Gentile, M., 2015. Promising therapies for the treatment of chronic
lymphocytic leukemia. Expert opinion on investigational drugs, 24(6), pp.795-807.
Robak, T. and Robak, E., 2011. New anti-CD20 monoclonal antibodies for the treatment of B-cell
lymphoid malignancies. BioDrugs, 25(1), pp.13-25.

⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 6
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
