Comprehensive Analysis of Chemical Kinetics & Equilibrium
VerifiedAdded on 2023/03/31
|4
|591
|465
Homework Assignment
AI Summary
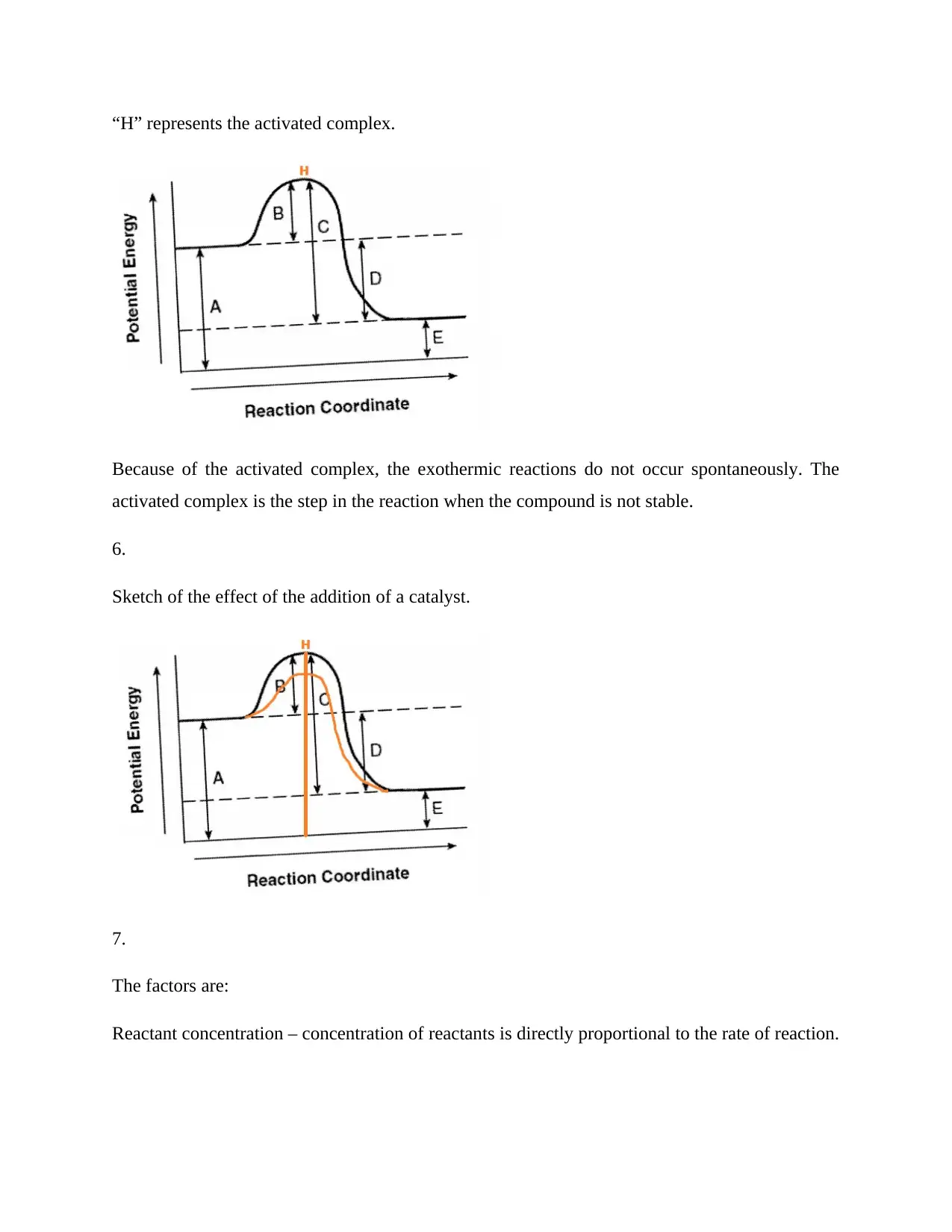
This assignment delves into the fundamental principles of chemical kinetics and equilibrium. It addresses collision theory, activation energy, and molecular orientation as key factors influencing reaction rates. The exothermic nature of a specific reaction is identified, and the role of activation energy in chemical reactions is discussed. The assignment further explores the impact of catalysts on reaction rates, emphasizing how they lower activation energy. Factors affecting reaction rates, such as reactant concentration, physical state, surface area, temperature, and the presence of catalysts, are examined. The concept of equilibrium is explained, along with Le Chatelier's principle, to predict the effects of changes in pressure, reactant concentration, and the addition of catalysts on equilibrium reactions. The point at which equilibrium is reached is determined using a graph. Desklib provides students with access to this solved assignment and a variety of study resources to enhance their understanding.
1 out of 4









![[object Object]](/_next/static/media/star-bottom.7253800d.svg)