UPEC Infections: Virulence Factors and Antimicrobial Strategies
VerifiedAdded on 2022/08/17
|23
|22834
|10
Report
AI Summary
This review article provides a comprehensive overview of Uropathogenic Escherichia coli (UPEC) infections, a major cause of urinary tract infections (UTIs) worldwide. It details the various virulence factors employed by UPEC strains, including structural components like fimbriae and pili, and secreted toxins, which contribute to their ability to cause disease. The article also explores the multifaceted host responses within the bladder epithelium, such as urine flow and antimicrobial secretions, that act to counteract bacterial infections. It further examines current treatment strategies, including antibiotics, and the emergence of antibiotic-resistant UPEC strains. Additionally, it discusses potential vaccine strategies, natural antimicrobial compounds, and innovative anti-adhesive and prophylactic approaches aimed at preventing UTIs. The intricate interplay between UPEC virulence and host defenses is highlighted, underscoring the complexity of these infections and the ongoing research efforts to develop effective treatment and prevention methods.

REVIEW
published: 15 August 2017
doi: 10.3389/fmicb.2017.01566
Frontiers in Microbiology | www.frontiersin.org 1 August 2017 | Volume 8 | Article 1566
Edited by:
John W. A. Rossen,
University MedicalCenter Groningen,
Netherlands
Reviewed by:
Ariadnna Cruz-Córdova,
HospitalInfantilde México Federico
Gómez, Mexico
Mirjam Kooistra-Smid,
CERTE, Netherlands
*Correspondence:
Massimo E. Maffei
massimo.maffei@unito.it
Specialty section:
This article was submitted to
Infectious Diseases,
a section of the journal
Frontiers in Microbiology
Received: 15 May 2017
Accepted: 02 August 2017
Published: 15 August 2017
Citation:
TerlizziME, Gribaudo G and MaffeiME
(2017) UroPathogenic Escherichia coli
(UPEC) Infections: Virulence Factors,
Bladder Responses, Antibiotic, and
Non-antibiotic Antimicrobial
Strategies. Front. Microbiol. 8:1566.
doi: 10.3389/fmicb.2017.01566
UroPathogenic Escherichia coli
(UPEC) Infections: Virulence Factors,
Bladder Responses, Antibiotic, and
Non-antibiotic Antimicrobial
Strategies
Maria E. Terlizzi, Giorgio Gribaudo and Massimo E. Maffei*
Department of Life Sciences and Systems Biology, University of Turin, Torino, Italy
Urinary tract infections (UTIs) are one of the most common pathological conditions i
community and hospitalsettings. It has been estimated that about 150 million people
worldwide develop UTI each year, with high socialcosts in terms of hospitalizations and
medical expenses. Among the common uropathogens associated to UTIs developme
UroPathogenic Escherichia coli(UPEC) is the primary cause.UPEC strains possess
a plethora of both structural(as fimbriae,pili, curli, flagella)and secreted (toxins,
iron-acquisition systems)virulence factors thatcontribute to theircapacity to cause
disease, although the ability to adhere to host epithelial cells in the urinary tract rep
the mostimportantdeterminantof pathogenicity.On the opposite side,the bladder
epithelium shows a multifaceted array ofhost defenses including the urine flow and
the secretion ofantimicrobialsubstances,which representusefultools to counteract
bacterialinfections.The fascinating and intricate dynamics between these players
determine a complex interaction system that needs to be revealed. This review willfocus
on the most relevant components ofUPEC arsenalof pathogenicity together with the
major host responses to infection, the current approved treatment and the emergen
of resistant UPEC strains, the vaccine strategies, the naturalantimicrobialcompounds
along with innovative anti-adhesive and prophylactic approaches to prevent UTIs.
Keywords: urinary tract infections, uropathogenic Escherichia coli, bladder, antibiotics, non-antibiotic remedies
URINARY TRACT INFECTIONS (UTIs)
Urinary tractinfections(UTIs) are widespread and affecta large proportion ofthe human
population.About 150 million people worldwide develop UTI each year,with high socialcosts
(Flores-Mireles et al.,2015).It is estimated that 40% of women develop at least one UTI durin
their lifetime (Micali et al., 2014) and that 11% of women over 18 years have an episode
year (Foxman and Brown, 2003; Foxman, 2014). With roughly eleven-million cases repo
sole U.S. each year, the costs are estimated $5 billion annually (Figure 1) (Foxman, 201
The UTI refers to the presence of a certain number of bacteria in the urine (generally5/ml)
and symptomatic UTIs are classified in order of severity as urosepsis syndrome, pyelone
upper UTI, with infection in the kidney) and cystitis (or lower UTI, with bacteria into the b
Foxman, 2014; Smelov et al., 2016). Clinically, UTIs classification comprises either uncom
published: 15 August 2017
doi: 10.3389/fmicb.2017.01566
Frontiers in Microbiology | www.frontiersin.org 1 August 2017 | Volume 8 | Article 1566
Edited by:
John W. A. Rossen,
University MedicalCenter Groningen,
Netherlands
Reviewed by:
Ariadnna Cruz-Córdova,
HospitalInfantilde México Federico
Gómez, Mexico
Mirjam Kooistra-Smid,
CERTE, Netherlands
*Correspondence:
Massimo E. Maffei
massimo.maffei@unito.it
Specialty section:
This article was submitted to
Infectious Diseases,
a section of the journal
Frontiers in Microbiology
Received: 15 May 2017
Accepted: 02 August 2017
Published: 15 August 2017
Citation:
TerlizziME, Gribaudo G and MaffeiME
(2017) UroPathogenic Escherichia coli
(UPEC) Infections: Virulence Factors,
Bladder Responses, Antibiotic, and
Non-antibiotic Antimicrobial
Strategies. Front. Microbiol. 8:1566.
doi: 10.3389/fmicb.2017.01566
UroPathogenic Escherichia coli
(UPEC) Infections: Virulence Factors,
Bladder Responses, Antibiotic, and
Non-antibiotic Antimicrobial
Strategies
Maria E. Terlizzi, Giorgio Gribaudo and Massimo E. Maffei*
Department of Life Sciences and Systems Biology, University of Turin, Torino, Italy
Urinary tract infections (UTIs) are one of the most common pathological conditions i
community and hospitalsettings. It has been estimated that about 150 million people
worldwide develop UTI each year, with high socialcosts in terms of hospitalizations and
medical expenses. Among the common uropathogens associated to UTIs developme
UroPathogenic Escherichia coli(UPEC) is the primary cause.UPEC strains possess
a plethora of both structural(as fimbriae,pili, curli, flagella)and secreted (toxins,
iron-acquisition systems)virulence factors thatcontribute to theircapacity to cause
disease, although the ability to adhere to host epithelial cells in the urinary tract rep
the mostimportantdeterminantof pathogenicity.On the opposite side,the bladder
epithelium shows a multifaceted array ofhost defenses including the urine flow and
the secretion ofantimicrobialsubstances,which representusefultools to counteract
bacterialinfections.The fascinating and intricate dynamics between these players
determine a complex interaction system that needs to be revealed. This review willfocus
on the most relevant components ofUPEC arsenalof pathogenicity together with the
major host responses to infection, the current approved treatment and the emergen
of resistant UPEC strains, the vaccine strategies, the naturalantimicrobialcompounds
along with innovative anti-adhesive and prophylactic approaches to prevent UTIs.
Keywords: urinary tract infections, uropathogenic Escherichia coli, bladder, antibiotics, non-antibiotic remedies
URINARY TRACT INFECTIONS (UTIs)
Urinary tractinfections(UTIs) are widespread and affecta large proportion ofthe human
population.About 150 million people worldwide develop UTI each year,with high socialcosts
(Flores-Mireles et al.,2015).It is estimated that 40% of women develop at least one UTI durin
their lifetime (Micali et al., 2014) and that 11% of women over 18 years have an episode
year (Foxman and Brown, 2003; Foxman, 2014). With roughly eleven-million cases repo
sole U.S. each year, the costs are estimated $5 billion annually (Figure 1) (Foxman, 201
The UTI refers to the presence of a certain number of bacteria in the urine (generally5/ml)
and symptomatic UTIs are classified in order of severity as urosepsis syndrome, pyelone
upper UTI, with infection in the kidney) and cystitis (or lower UTI, with bacteria into the b
Foxman, 2014; Smelov et al., 2016). Clinically, UTIs classification comprises either uncom
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Terlizziet al. Uropathogenic Escherichia coliInfections
or complicated cases, depending on the presence of structural or
neurologicalurinary tract abnormalities (Zacché and Giarenis,
2016). The ORENUC system classifies the risk factors according
to the phenotype (Johansen etal., 2011):O, no known risk
factors;R, risk of recurrentUTIs withouta more severe
outcome; E, extraurogenital risk factors; N, relevant nephropathic
diseases;U, urologicresolvable(transient)risk factors;C,
permanentexternalurinary catheter and unresolved urologic
risk factors(seealso Smelovet al., 2016for a modified
classification).The susceptibility to develop an UTI phenotype
is related to several factors,as dysfunctions of the urinary tract
and/orgenetic mechanismsinvolved in the innate immune
responsecontrolto infections(Kovesand Wullt,2016).In
particular,the innateimmunesystem mayrespond either
to UPEC patterns(pathogen-associated molecularpatterns;
PAMPs) or to moleculesderived from damaged ordying
cells(danger/damage-associated molecular patterns;DAMPs).
Pattern recognition receptors (PRRs) recognized these patterns
in specialized immune cells, epithelia, and other tissues (Purves
and Hughes,2016).Assembling in the cytosolof multimeric
protein complexes (inflammasomes) occurs after sensing PAMPs
or DAMPs structures that can be formed in both upper and lower
urinary tract(Guo etal.,2015).They trigger innate immune
responsesthrough mechanismsdepending ornot from the
production of proinflammatory cytokines (Purves and Hughes,
2016).
The bacterialcystitis (also called acute cystitis)can occur
in both women and men and some people develop recurrent
infectionsof the urinary tract(Fiore and Fox,2014).Three
or more urinary tractinfections within 12 months define the
recurring UTI,as well as two or more recurrenceswithin
6 months.The samebacterialspeciesthat caused previous
infection istypically responsible forrelapses.Approximately
20–30% ofadult women with an initialUTI will experience a
recurrence within 3–4 months;whereas,in children,about one
third experiencing a UTI before the age of one,will experience
a recurrence within 3 years,and 18% ofthem willhave a
recurrence within a few months (Nuutinen and Uhari,2001).
However,these figures are understated;in fact,about50% of
UTI does not come to medicalattention.Recurrent UTIs can
be introduced from different sources and the same or different
UTI-causing strainsin the gutare able to (re)inoculate the
bladder. Alternatively, bacteria residing in the bladder epithelium
are able to re-emerge periodically and cause UTIrecurrence
(Silverman etal., 2013).In patients suffering from recurrent
UTIs, maintenance is ensured by antibiotic prophylaxis; however,
in some cases UTI needs to be treated by surgery (Tolg and Bagli,
2012).During pregnancy,recurrent UTIs may be frequent and
can cause severe adverse outcomes for the mother and the baby,
including preterm birth. The interventions in this setting can be
pharmacologic (antibiotics) or non-pharmacological (alternative
remedies; Schneeberger et al., 2012). In pre-menopausal women,
sexualactivitiesthreeor more timesa week,the use of
spermicides, new or multiple sexual partners and having suffered
from UTI beforeage15 arethe main risk factorsin UTI
development and recurrence.In menopausalwomen,systemic
hormonaltherapy isnot an effective prevention and usually
asymptomatic bacteriuria during this period does notrequire
treatment (Milart et al.,2013).In women after menopause,the
risk increases mainly by low estrogen levels after-effects,which
are often associated to vaginalatrophy (Arnold etal.,2016).
In women over the age of61–65 years,halfhave suffered of
genital-urinary symptoms while 29% had episodes ofurinary
incontinence,all symptomsassociated with bacteriuria (Raz,
2001).
UROPATHOGENIC ESCHERICHIA COLI
AND ITS VIRULENCE
UPEC is the main cause of community-acquired UTIs (about 8
90%; Foxman, 2014; Flores-Mireles et al., 2015). Four main U
phylogroups (A, B1, B2, and D) have been identified on the ba
of the occurrence of genomic Pathogenicity Islands (PAI) and
expression of virulence factors, such as adhesins, toxins, surf
polysaccharides,flagella,and iron-acquisition systems(Bien
et al., 2012). Usually, many of these virulence factors are req
for UPEC to cause UTI (Hannan et al., 2012). However, beside
UPEC, UTI can be caused by Klebsiella pneumoniae (about
7%), Proteus mirabilis (about 5%), and Pseudomonas aerugin
Enterococcus faecalis,Enterobacter cloacae,Streptococcus bovis,
and the fungus Candida albicans (for the remaining percentag
Parish and Holliday, 2012; Palou et al., 2013; Hof, 2017). Duri
UTIs, UPEC pathogenesis includes: (a) UPEC colonization of th
periurethral and vaginal areas with colonization of the urethra
(b) ascending into the bladder lumen and growth as plantkton
cells in urine;(c) adherence to the surface and interaction with
the bladder epithelium defense system (see below);(d) biofilm
formation;(e) invasion and replication by forming bladder
IntracellularBacterialCommunities(IBCs) wherequiescent
intracellular reservoirs (QIRs) form and reside in the underlyin
urothelium; (f) kidney colonization and host tissue damage w
increased risk for bacteremia/septicemia.
Replication of bacteria in the IBC can easily reach as many
105 bacteria per cell;furthermore,bacteria in the IBC undergo
morphologicalchanges,flux outof the infected cell,and go
onto infect neighboring cells (Dhakal et al., 2008; Flores-Mire
et al., 2015; Spaulding and Hultgren, 2016). The flushing of u
removes most of the invading bacteria,along with UPEC-filled
exfoliated bladder epithelium cells (BECs; Kaper et al., 2004).
UPEC colonizethe bladderusing a variety ofvirulence
factors thattherefore play criticalroles in UTI pathogenesis.
These include surface structuralcomponents,such as
lipopolysaccharide(LPS), polysaccharidecapsule,flagella,
outer-membrane vesicles,pili, curli,non-pilus adhesins,outer-
membrane proteins (OMPs), as well as secreted toxins, secre
systems,and TonB-dependent iron-uptake receptors,including
siderophore receptors (Figure 2).All of these components are
attractive candidatesfor the developmentof new drugsand
vaccines (Klemm et al.,2010;Werneburg et al.,2015;O’Brien
et al., 2016).
LPS are molecules with amphipathic properties consisting o
fatty acids lined to an oligosaccharide core,which in turn is
bound to a long polysaccharide chain commonly called O anti
Frontiers in Microbiology | www.frontiersin.org 2 August 2017 | Volume 8 | Article 1566
or complicated cases, depending on the presence of structural or
neurologicalurinary tract abnormalities (Zacché and Giarenis,
2016). The ORENUC system classifies the risk factors according
to the phenotype (Johansen etal., 2011):O, no known risk
factors;R, risk of recurrentUTIs withouta more severe
outcome; E, extraurogenital risk factors; N, relevant nephropathic
diseases;U, urologicresolvable(transient)risk factors;C,
permanentexternalurinary catheter and unresolved urologic
risk factors(seealso Smelovet al., 2016for a modified
classification).The susceptibility to develop an UTI phenotype
is related to several factors,as dysfunctions of the urinary tract
and/orgenetic mechanismsinvolved in the innate immune
responsecontrolto infections(Kovesand Wullt,2016).In
particular,the innateimmunesystem mayrespond either
to UPEC patterns(pathogen-associated molecularpatterns;
PAMPs) or to moleculesderived from damaged ordying
cells(danger/damage-associated molecular patterns;DAMPs).
Pattern recognition receptors (PRRs) recognized these patterns
in specialized immune cells, epithelia, and other tissues (Purves
and Hughes,2016).Assembling in the cytosolof multimeric
protein complexes (inflammasomes) occurs after sensing PAMPs
or DAMPs structures that can be formed in both upper and lower
urinary tract(Guo etal.,2015).They trigger innate immune
responsesthrough mechanismsdepending ornot from the
production of proinflammatory cytokines (Purves and Hughes,
2016).
The bacterialcystitis (also called acute cystitis)can occur
in both women and men and some people develop recurrent
infectionsof the urinary tract(Fiore and Fox,2014).Three
or more urinary tractinfections within 12 months define the
recurring UTI,as well as two or more recurrenceswithin
6 months.The samebacterialspeciesthat caused previous
infection istypically responsible forrelapses.Approximately
20–30% ofadult women with an initialUTI will experience a
recurrence within 3–4 months;whereas,in children,about one
third experiencing a UTI before the age of one,will experience
a recurrence within 3 years,and 18% ofthem willhave a
recurrence within a few months (Nuutinen and Uhari,2001).
However,these figures are understated;in fact,about50% of
UTI does not come to medicalattention.Recurrent UTIs can
be introduced from different sources and the same or different
UTI-causing strainsin the gutare able to (re)inoculate the
bladder. Alternatively, bacteria residing in the bladder epithelium
are able to re-emerge periodically and cause UTIrecurrence
(Silverman etal., 2013).In patients suffering from recurrent
UTIs, maintenance is ensured by antibiotic prophylaxis; however,
in some cases UTI needs to be treated by surgery (Tolg and Bagli,
2012).During pregnancy,recurrent UTIs may be frequent and
can cause severe adverse outcomes for the mother and the baby,
including preterm birth. The interventions in this setting can be
pharmacologic (antibiotics) or non-pharmacological (alternative
remedies; Schneeberger et al., 2012). In pre-menopausal women,
sexualactivitiesthreeor more timesa week,the use of
spermicides, new or multiple sexual partners and having suffered
from UTI beforeage15 arethe main risk factorsin UTI
development and recurrence.In menopausalwomen,systemic
hormonaltherapy isnot an effective prevention and usually
asymptomatic bacteriuria during this period does notrequire
treatment (Milart et al.,2013).In women after menopause,the
risk increases mainly by low estrogen levels after-effects,which
are often associated to vaginalatrophy (Arnold etal.,2016).
In women over the age of61–65 years,halfhave suffered of
genital-urinary symptoms while 29% had episodes ofurinary
incontinence,all symptomsassociated with bacteriuria (Raz,
2001).
UROPATHOGENIC ESCHERICHIA COLI
AND ITS VIRULENCE
UPEC is the main cause of community-acquired UTIs (about 8
90%; Foxman, 2014; Flores-Mireles et al., 2015). Four main U
phylogroups (A, B1, B2, and D) have been identified on the ba
of the occurrence of genomic Pathogenicity Islands (PAI) and
expression of virulence factors, such as adhesins, toxins, surf
polysaccharides,flagella,and iron-acquisition systems(Bien
et al., 2012). Usually, many of these virulence factors are req
for UPEC to cause UTI (Hannan et al., 2012). However, beside
UPEC, UTI can be caused by Klebsiella pneumoniae (about
7%), Proteus mirabilis (about 5%), and Pseudomonas aerugin
Enterococcus faecalis,Enterobacter cloacae,Streptococcus bovis,
and the fungus Candida albicans (for the remaining percentag
Parish and Holliday, 2012; Palou et al., 2013; Hof, 2017). Duri
UTIs, UPEC pathogenesis includes: (a) UPEC colonization of th
periurethral and vaginal areas with colonization of the urethra
(b) ascending into the bladder lumen and growth as plantkton
cells in urine;(c) adherence to the surface and interaction with
the bladder epithelium defense system (see below);(d) biofilm
formation;(e) invasion and replication by forming bladder
IntracellularBacterialCommunities(IBCs) wherequiescent
intracellular reservoirs (QIRs) form and reside in the underlyin
urothelium; (f) kidney colonization and host tissue damage w
increased risk for bacteremia/septicemia.
Replication of bacteria in the IBC can easily reach as many
105 bacteria per cell;furthermore,bacteria in the IBC undergo
morphologicalchanges,flux outof the infected cell,and go
onto infect neighboring cells (Dhakal et al., 2008; Flores-Mire
et al., 2015; Spaulding and Hultgren, 2016). The flushing of u
removes most of the invading bacteria,along with UPEC-filled
exfoliated bladder epithelium cells (BECs; Kaper et al., 2004).
UPEC colonizethe bladderusing a variety ofvirulence
factors thattherefore play criticalroles in UTI pathogenesis.
These include surface structuralcomponents,such as
lipopolysaccharide(LPS), polysaccharidecapsule,flagella,
outer-membrane vesicles,pili, curli,non-pilus adhesins,outer-
membrane proteins (OMPs), as well as secreted toxins, secre
systems,and TonB-dependent iron-uptake receptors,including
siderophore receptors (Figure 2).All of these components are
attractive candidatesfor the developmentof new drugsand
vaccines (Klemm et al.,2010;Werneburg et al.,2015;O’Brien
et al., 2016).
LPS are molecules with amphipathic properties consisting o
fatty acids lined to an oligosaccharide core,which in turn is
bound to a long polysaccharide chain commonly called O anti
Frontiers in Microbiology | www.frontiersin.org 2 August 2017 | Volume 8 | Article 1566
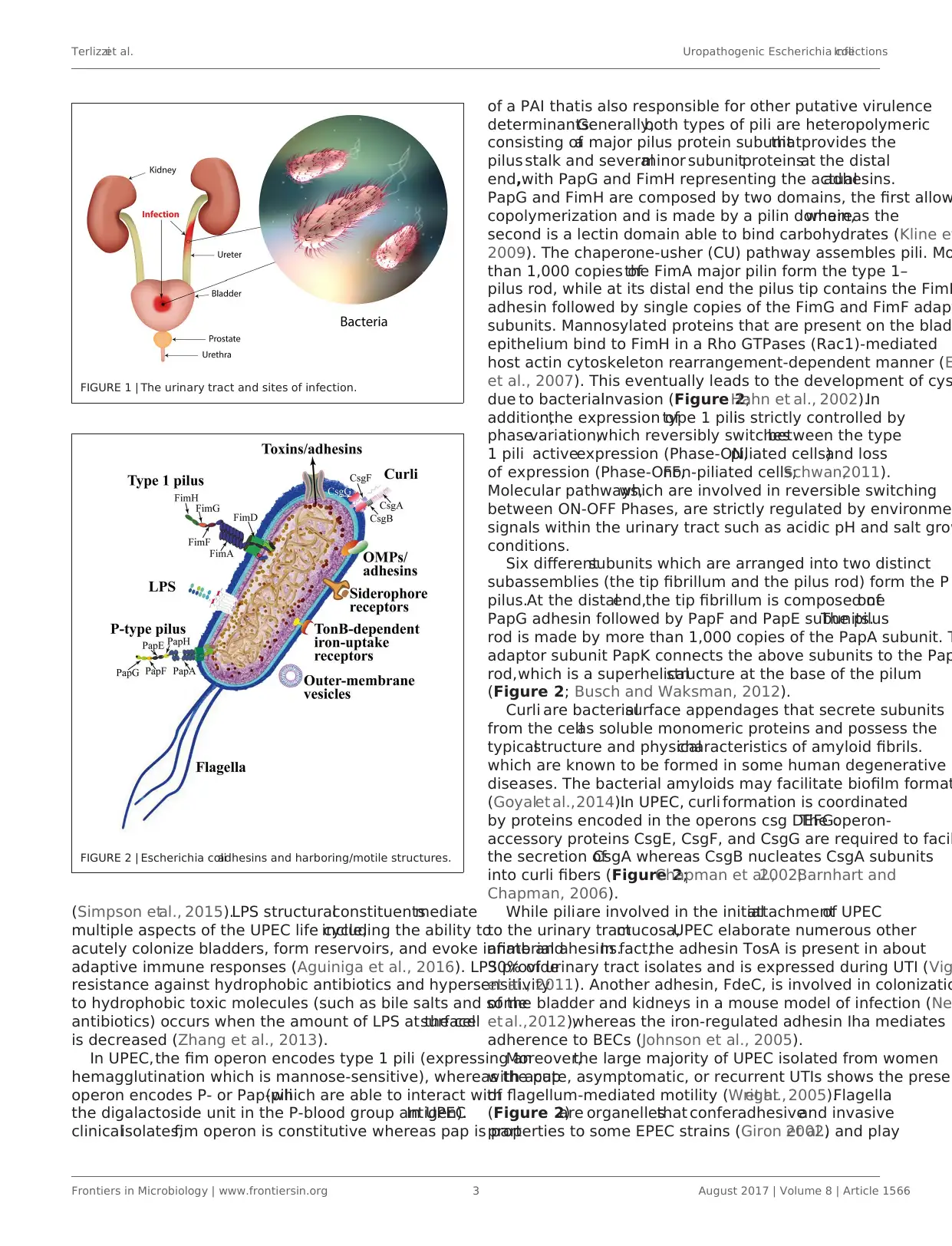
Terlizziet al. Uropathogenic Escherichia coliInfections
FIGURE 1 | The urinary tract and sites of infection.
FIGURE 2 | Escherichia coliadhesins and harboring/motile structures.
(Simpson etal., 2015).LPS structuralconstituentsmediate
multiple aspects of the UPEC life cycle,including the ability to
acutely colonize bladders, form reservoirs, and evoke innate and
adaptive immune responses (Aguiniga et al., 2016). LPS provide
resistance against hydrophobic antibiotics and hypersensitivity
to hydrophobic toxic molecules (such as bile salts and some
antibiotics) occurs when the amount of LPS at the cellsurface
is decreased (Zhang et al., 2013).
In UPEC,the fim operon encodes type 1 pili (expressing an
hemagglutination which is mannose-sensitive), whereas the pap
operon encodes P- or Pap-pili(which are able to interact with
the digalactoside unit in the P-blood group antigen).In UPEC
clinicalisolates,fim operon is constitutive whereas pap is part
of a PAI thatis also responsible for other putative virulence
determinants.Generally,both types of pili are heteropolymeric
consisting ofa major pilus protein subunitthatprovides the
pilus stalk and severalminor subunitproteinsat the distal
end,with PapG and FimH representing the actualadhesins.
PapG and FimH are composed by two domains, the first allow
copolymerization and is made by a pilin domain,whereas the
second is a lectin domain able to bind carbohydrates (Kline e
2009). The chaperone-usher (CU) pathway assembles pili. Mo
than 1,000 copies ofthe FimA major pilin form the type 1–
pilus rod, while at its distal end the pilus tip contains the FimH
adhesin followed by single copies of the FimG and FimF adap
subunits. Mannosylated proteins that are present on the blad
epithelium bind to FimH in a Rho GTPases (Rac1)-mediated
host actin cytoskeleton rearrangement-dependent manner (E
et al., 2007). This eventually leads to the development of cys
due to bacterialinvasion (Figure 2;Hahn et al., 2002).In
addition,the expression oftype 1 piliis strictly controlled by
phasevariation,which reversibly switchesbetween the type
1 pili activeexpression (Phase-ON,piliated cells)and loss
of expression (Phase-OFF,non-piliated cells;Schwan,2011).
Molecular pathways,which are involved in reversible switching
between ON-OFF Phases, are strictly regulated by environme
signals within the urinary tract such as acidic pH and salt grow
conditions.
Six differentsubunits which are arranged into two distinct
subassemblies (the tip fibrillum and the pilus rod) form the P
pilus.At the distalend,the tip fibrillum is composed ofone
PapG adhesin followed by PapF and PapE subunits.The pilus
rod is made by more than 1,000 copies of the PapA subunit. T
adaptor subunit PapK connects the above subunits to the Pap
rod,which is a superhelicalstructure at the base of the pilum
(Figure 2; Busch and Waksman, 2012).
Curli are bacterialsurface appendages that secrete subunits
from the cellas soluble monomeric proteins and possess the
typicalstructure and physicalcharacteristics of amyloid fibrils.
which are known to be formed in some human degenerative
diseases. The bacterial amyloids may facilitate biofilm format
(Goyalet al.,2014).In UPEC, curli formation is coordinated
by proteins encoded in the operons csg DEFG.The operon-
accessory proteins CsgE, CsgF, and CsgG are required to facil
the secretion ofCsgA whereas CsgB nucleates CsgA subunits
into curli fibers (Figure 2;Chapman et al.,2002;Barnhart and
Chapman, 2006).
While piliare involved in the initialattachmentof UPEC
to the urinary tractmucosa,UPEC elaborate numerous other
afimbrial ahesins.In fact,the adhesin TosA is present in about
30% of urinary tract isolates and is expressed during UTI (Vig
et al., 2011). Another adhesin, FdeC, is involved in colonizatio
of the bladder and kidneys in a mouse model of infection (Ne
et al.,2012),whereas the iron-regulated adhesin Iha mediates
adherence to BECs (Johnson et al., 2005).
Moreover,the large majority of UPEC isolated from women
with acute, asymptomatic, or recurrent UTIs shows the presen
of flagellum-mediated motility (Wrightet al., 2005).Flagella
(Figure 2)are organellesthat conferadhesiveand invasive
properties to some EPEC strains (Giron et al.,2002) and play
Frontiers in Microbiology | www.frontiersin.org 3 August 2017 | Volume 8 | Article 1566
FIGURE 1 | The urinary tract and sites of infection.
FIGURE 2 | Escherichia coliadhesins and harboring/motile structures.
(Simpson etal., 2015).LPS structuralconstituentsmediate
multiple aspects of the UPEC life cycle,including the ability to
acutely colonize bladders, form reservoirs, and evoke innate and
adaptive immune responses (Aguiniga et al., 2016). LPS provide
resistance against hydrophobic antibiotics and hypersensitivity
to hydrophobic toxic molecules (such as bile salts and some
antibiotics) occurs when the amount of LPS at the cellsurface
is decreased (Zhang et al., 2013).
In UPEC,the fim operon encodes type 1 pili (expressing an
hemagglutination which is mannose-sensitive), whereas the pap
operon encodes P- or Pap-pili(which are able to interact with
the digalactoside unit in the P-blood group antigen).In UPEC
clinicalisolates,fim operon is constitutive whereas pap is part
of a PAI thatis also responsible for other putative virulence
determinants.Generally,both types of pili are heteropolymeric
consisting ofa major pilus protein subunitthatprovides the
pilus stalk and severalminor subunitproteinsat the distal
end,with PapG and FimH representing the actualadhesins.
PapG and FimH are composed by two domains, the first allow
copolymerization and is made by a pilin domain,whereas the
second is a lectin domain able to bind carbohydrates (Kline e
2009). The chaperone-usher (CU) pathway assembles pili. Mo
than 1,000 copies ofthe FimA major pilin form the type 1–
pilus rod, while at its distal end the pilus tip contains the FimH
adhesin followed by single copies of the FimG and FimF adap
subunits. Mannosylated proteins that are present on the blad
epithelium bind to FimH in a Rho GTPases (Rac1)-mediated
host actin cytoskeleton rearrangement-dependent manner (E
et al., 2007). This eventually leads to the development of cys
due to bacterialinvasion (Figure 2;Hahn et al., 2002).In
addition,the expression oftype 1 piliis strictly controlled by
phasevariation,which reversibly switchesbetween the type
1 pili activeexpression (Phase-ON,piliated cells)and loss
of expression (Phase-OFF,non-piliated cells;Schwan,2011).
Molecular pathways,which are involved in reversible switching
between ON-OFF Phases, are strictly regulated by environme
signals within the urinary tract such as acidic pH and salt grow
conditions.
Six differentsubunits which are arranged into two distinct
subassemblies (the tip fibrillum and the pilus rod) form the P
pilus.At the distalend,the tip fibrillum is composed ofone
PapG adhesin followed by PapF and PapE subunits.The pilus
rod is made by more than 1,000 copies of the PapA subunit. T
adaptor subunit PapK connects the above subunits to the Pap
rod,which is a superhelicalstructure at the base of the pilum
(Figure 2; Busch and Waksman, 2012).
Curli are bacterialsurface appendages that secrete subunits
from the cellas soluble monomeric proteins and possess the
typicalstructure and physicalcharacteristics of amyloid fibrils.
which are known to be formed in some human degenerative
diseases. The bacterial amyloids may facilitate biofilm format
(Goyalet al.,2014).In UPEC, curli formation is coordinated
by proteins encoded in the operons csg DEFG.The operon-
accessory proteins CsgE, CsgF, and CsgG are required to facil
the secretion ofCsgA whereas CsgB nucleates CsgA subunits
into curli fibers (Figure 2;Chapman et al.,2002;Barnhart and
Chapman, 2006).
While piliare involved in the initialattachmentof UPEC
to the urinary tractmucosa,UPEC elaborate numerous other
afimbrial ahesins.In fact,the adhesin TosA is present in about
30% of urinary tract isolates and is expressed during UTI (Vig
et al., 2011). Another adhesin, FdeC, is involved in colonizatio
of the bladder and kidneys in a mouse model of infection (Ne
et al.,2012),whereas the iron-regulated adhesin Iha mediates
adherence to BECs (Johnson et al., 2005).
Moreover,the large majority of UPEC isolated from women
with acute, asymptomatic, or recurrent UTIs shows the presen
of flagellum-mediated motility (Wrightet al., 2005).Flagella
(Figure 2)are organellesthat conferadhesiveand invasive
properties to some EPEC strains (Giron et al.,2002) and play
Frontiers in Microbiology | www.frontiersin.org 3 August 2017 | Volume 8 | Article 1566
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Terlizziet al. Uropathogenic Escherichia coliInfections
a key role in the dynamic of biofilms (Pratt and Kolter,1998).
It was recently reported that during biofilm formation,flagella
play different roles such as adherence, maturation, and dispersal
as shown by gene expression and regulation during the growth
phase (Nakamura et al., 2016).
On the other hand,UPEC toxins play different pathogenetic
roles during infection. The α-hemolysin is in fact associated with
renaldamage and scarring,induces Ca2+ oscillations in renal
tubular epithelial cells,thereby potentially enhancing ascension
and colonization of ureters and kidney parenchyma by disrupting
the normalflow of urine.Recently (Nagamatsu etal.,2015),
α-hemolysin wasfound to induce proinflammatory Caspase-
1/Caspase-4-dependentcell death in bladderepithelialcells,
resulting in cell exfoliation (see below).
UPEC toxins,adhesins,enzymes,and non-protein antigens
like LPS are not released as soluble molecules;rather,they are
associated with outer-membrane vesicles,which bud off the
surface ofGram-negative bacteria during allstages ofgrowth
(Figure 2;Ellis and Kuehn,2010).The formation of membrane
vesicles is considered a “smart” way to protect bacterialtoxins
and an efficient system to deliver them into host cell (Wiles et al.,
2008).
Iron acquisition is a criticalrequirement for UPEC survival
in an environmentthatis iron-limited asthe urinary tract
(Skaar,2010).Thus,is not suprising thatIBC UPEC show
upregulation ofredundantsystems for the acquisition ofiron
(Reigstad etal.,2007).In this regard,siderophores are small-
molecule iron chelators that are produced by UPEC strains to
scavenge ferric iron (Fe3+), thus UPEC express yersiniabactin,
salmochelin,and aerobactin.Siderophore receptors require the
TonB cytoplasmic membrane-localized complex,a high-affinity
iron acquisition system that allows binding and chelation of iron
at the cell surface to promote its uptake (O’Brien et al., 2016).
However,uroepithelialcells, to preventbacterialiron
scavenging, upregulate genes for the transferrin receptor and for
lipocalin 2.
Lastly, further UPEC factors associated with colonization have
been linked to the regulation of metabolic pathways mediated by
two-component signaling systems (TCSs). TCSs are main signal
transduction pathwaysby which bacteria sense and respond
to a wide array ofenvironmentalstimuli,including quorum
sensingsignals,nutrients,antibiotics.TCSs are composed
by a membrane-bound sensorhistidinekinase(HK) and a
cytoplasmic response regulator (RR) that functions by regulating
gene expression (Stock etal.,2000).Among UPEC-associated
TCSs involved in UTI pathogenesis,the BarA/UvrY system has
been described to regulate switching between glycolytic and
gluconeogenic pathways (Tomenius et al., 2006) the EvgS/EvgA
and PhoQ/PhoP systems have been involved in acid resistance
(Eguchi et al.,2011), while the function of KguS/KguR is in the
controlof the utilization ofα-ketoglutarate.In this way they
facilate the adaptation of UPEC in the urinary tract (Cai et al.,
2013).
The importanceof the abovedescribed UPEC virulence
factors in UTI pathogenesis has been further supported, in recent
years, by the application of multiple “omics” technologies aimed
at investigating the UPEC genomic diversity,the globalgene
expression in different models of infection both in vitro and in
vivo,and to define the occurrence ofUPEC-specific proteins
as new candidate therapeutic and vaccine targets (as recentl
reviewed by Lo et al., 2017).
Next-generationsequencing(NGS) technologiesare
providingrapid low-costdetermination ofUPEC genomes
useful to monitor outbreaks,epidemiology of emerging strains,
as wellas evolution ofresistance (Petty etal.,2014;Stoesser
et al.,2016).On the other hand,analysis ofdifferentUPEC
genomes and the comparison with the E. coli genomic databa
revealed the plasticity of UPEC pan genome, and the presenc
UPEC-specific PAIs genes predicted to encode putative virulen
factors, such as pilus proteins, adhesins, and iron-uptake syst
(Moriel et al., 2016).
Transcriptomics investigations by both microarrays and NG
based RNA sequencing (RNA-seq), on the other hand, has led
the identification of virulence and fitness UPEC genes, expres
during different in vitro and in vivo infection-relevant conditio
In this regard,RNA-seq-based transcriptome analysis of mouse
macrophages infected in vitro with two UPEC strains, allowed
identify strain-specific differentially expressed genes associat
to the survivalin macrophages,such as those involved in the
responses to oxidative stress,as wellas those involved in the
initial adhesion of UPEC to cells, such as multiple flagella gen
(Mavromatis et al.,2015).Moreover,the global gene expression
of different UPEC strains has been investigated by RNA-seq o
urine samples collected from UTI patients. These transcriptom
studies defined the global transcription profile for UPEC durin
UTI, highlighted the high genomic diversity of different UPEC
strains,and confirmed,on a global scale,the expression during
UTI of severalgenes encoding virulence factors.In fact,it has
been observed the transcription ofgenes associated with the
UPEC’sadhesion to the uroepithelium (type 1 and P pili),
of genesinvolved in iron uptake(enterobactin,aerobactin,
yersiniabactin,and salmochelin),of genesencodingtoxins
(hemolysins nad cytotoxic factors),as well as those involved in
copper efflux (Bieleckiet al.,2014;Subashchandrabose etal.,
2014).
High-resolution liquid chromatograph-mass
spectrometry/mass spectrometry (LC-MS/MS)-based technolo
has been applied toidentifyand characterizethe surface
proteome ofUPEC isolatesand of strainsgrown in human
urine (Wurpel et al., 2015, 2016). These studies identified seve
expressed proteinshighly conserved among differentstrains,
thus representing the core surface proteome ofUPEC. UPEC
core surface proteins,such as integralOuter Membrane (OM)
proteins (e.g.,OmpA,OmpC,OmpF) and severaliron-uptake
proteins,were in factdetected in more than 80% ofstrains
(Wurpelet al.,2015).Clearly,characterization of those UPEC
surfaceproteinsthat are conserved among differentstrains
and immunogenic is an essentialstep for identifying potential
vaccine candidates and new therapeutic targets (Cash, 2014)
Moreover,new insights into spatialchanges in the UPEC
proteome under experimentalconditions mimicking bacterial
growth in the urinary tract, have been provided by MALDI TOF
IMS-based proteome profiling of differentially expressed prote
within UPEC biofilms.The application ofthis technique,that
Frontiers in Microbiology | www.frontiersin.org 4 August 2017 | Volume 8 | Article 1566
a key role in the dynamic of biofilms (Pratt and Kolter,1998).
It was recently reported that during biofilm formation,flagella
play different roles such as adherence, maturation, and dispersal
as shown by gene expression and regulation during the growth
phase (Nakamura et al., 2016).
On the other hand,UPEC toxins play different pathogenetic
roles during infection. The α-hemolysin is in fact associated with
renaldamage and scarring,induces Ca2+ oscillations in renal
tubular epithelial cells,thereby potentially enhancing ascension
and colonization of ureters and kidney parenchyma by disrupting
the normalflow of urine.Recently (Nagamatsu etal.,2015),
α-hemolysin wasfound to induce proinflammatory Caspase-
1/Caspase-4-dependentcell death in bladderepithelialcells,
resulting in cell exfoliation (see below).
UPEC toxins,adhesins,enzymes,and non-protein antigens
like LPS are not released as soluble molecules;rather,they are
associated with outer-membrane vesicles,which bud off the
surface ofGram-negative bacteria during allstages ofgrowth
(Figure 2;Ellis and Kuehn,2010).The formation of membrane
vesicles is considered a “smart” way to protect bacterialtoxins
and an efficient system to deliver them into host cell (Wiles et al.,
2008).
Iron acquisition is a criticalrequirement for UPEC survival
in an environmentthatis iron-limited asthe urinary tract
(Skaar,2010).Thus,is not suprising thatIBC UPEC show
upregulation ofredundantsystems for the acquisition ofiron
(Reigstad etal.,2007).In this regard,siderophores are small-
molecule iron chelators that are produced by UPEC strains to
scavenge ferric iron (Fe3+), thus UPEC express yersiniabactin,
salmochelin,and aerobactin.Siderophore receptors require the
TonB cytoplasmic membrane-localized complex,a high-affinity
iron acquisition system that allows binding and chelation of iron
at the cell surface to promote its uptake (O’Brien et al., 2016).
However,uroepithelialcells, to preventbacterialiron
scavenging, upregulate genes for the transferrin receptor and for
lipocalin 2.
Lastly, further UPEC factors associated with colonization have
been linked to the regulation of metabolic pathways mediated by
two-component signaling systems (TCSs). TCSs are main signal
transduction pathwaysby which bacteria sense and respond
to a wide array ofenvironmentalstimuli,including quorum
sensingsignals,nutrients,antibiotics.TCSs are composed
by a membrane-bound sensorhistidinekinase(HK) and a
cytoplasmic response regulator (RR) that functions by regulating
gene expression (Stock etal.,2000).Among UPEC-associated
TCSs involved in UTI pathogenesis,the BarA/UvrY system has
been described to regulate switching between glycolytic and
gluconeogenic pathways (Tomenius et al., 2006) the EvgS/EvgA
and PhoQ/PhoP systems have been involved in acid resistance
(Eguchi et al.,2011), while the function of KguS/KguR is in the
controlof the utilization ofα-ketoglutarate.In this way they
facilate the adaptation of UPEC in the urinary tract (Cai et al.,
2013).
The importanceof the abovedescribed UPEC virulence
factors in UTI pathogenesis has been further supported, in recent
years, by the application of multiple “omics” technologies aimed
at investigating the UPEC genomic diversity,the globalgene
expression in different models of infection both in vitro and in
vivo,and to define the occurrence ofUPEC-specific proteins
as new candidate therapeutic and vaccine targets (as recentl
reviewed by Lo et al., 2017).
Next-generationsequencing(NGS) technologiesare
providingrapid low-costdetermination ofUPEC genomes
useful to monitor outbreaks,epidemiology of emerging strains,
as wellas evolution ofresistance (Petty etal.,2014;Stoesser
et al.,2016).On the other hand,analysis ofdifferentUPEC
genomes and the comparison with the E. coli genomic databa
revealed the plasticity of UPEC pan genome, and the presenc
UPEC-specific PAIs genes predicted to encode putative virulen
factors, such as pilus proteins, adhesins, and iron-uptake syst
(Moriel et al., 2016).
Transcriptomics investigations by both microarrays and NG
based RNA sequencing (RNA-seq), on the other hand, has led
the identification of virulence and fitness UPEC genes, expres
during different in vitro and in vivo infection-relevant conditio
In this regard,RNA-seq-based transcriptome analysis of mouse
macrophages infected in vitro with two UPEC strains, allowed
identify strain-specific differentially expressed genes associat
to the survivalin macrophages,such as those involved in the
responses to oxidative stress,as wellas those involved in the
initial adhesion of UPEC to cells, such as multiple flagella gen
(Mavromatis et al.,2015).Moreover,the global gene expression
of different UPEC strains has been investigated by RNA-seq o
urine samples collected from UTI patients. These transcriptom
studies defined the global transcription profile for UPEC durin
UTI, highlighted the high genomic diversity of different UPEC
strains,and confirmed,on a global scale,the expression during
UTI of severalgenes encoding virulence factors.In fact,it has
been observed the transcription ofgenes associated with the
UPEC’sadhesion to the uroepithelium (type 1 and P pili),
of genesinvolved in iron uptake(enterobactin,aerobactin,
yersiniabactin,and salmochelin),of genesencodingtoxins
(hemolysins nad cytotoxic factors),as well as those involved in
copper efflux (Bieleckiet al.,2014;Subashchandrabose etal.,
2014).
High-resolution liquid chromatograph-mass
spectrometry/mass spectrometry (LC-MS/MS)-based technolo
has been applied toidentifyand characterizethe surface
proteome ofUPEC isolatesand of strainsgrown in human
urine (Wurpel et al., 2015, 2016). These studies identified seve
expressed proteinshighly conserved among differentstrains,
thus representing the core surface proteome ofUPEC. UPEC
core surface proteins,such as integralOuter Membrane (OM)
proteins (e.g.,OmpA,OmpC,OmpF) and severaliron-uptake
proteins,were in factdetected in more than 80% ofstrains
(Wurpelet al.,2015).Clearly,characterization of those UPEC
surfaceproteinsthat are conserved among differentstrains
and immunogenic is an essentialstep for identifying potential
vaccine candidates and new therapeutic targets (Cash, 2014)
Moreover,new insights into spatialchanges in the UPEC
proteome under experimentalconditions mimicking bacterial
growth in the urinary tract, have been provided by MALDI TOF
IMS-based proteome profiling of differentially expressed prote
within UPEC biofilms.The application ofthis technique,that
Frontiers in Microbiology | www.frontiersin.org 4 August 2017 | Volume 8 | Article 1566
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Terlizziet al. Uropathogenic Escherichia coliInfections
allows for in situ two-dimensional assessment of protein spatial
distribution and abundance, revealed the occurrence of different
bacterial subpopulations within biofilms: a type-1 pili-expressing
cells localized atthe air-exposed region and a curli-equipped
population localized to the underlying air-liquid interface (Floyd
et al., 2015).
Together,all the above mentioned “omics” approaches have
allowed a greatdealof new information to be available and
that is enabling a more comprehensive understanding of UPEC’s
pathogenic mechanisms.
THE BLADDER EPITHELIUM SHOWS
SELF-DEFENSE MECHANISMS AGAINST
INVADING BACTERIA
The most commonly targeted site of UTIs is the bladder.The
bladder epithelium possesses powerfulbarriers and the BECs
show antibacterial activities.Despite their properties,BECs and
the bladder epithelium are often circumvented by UPEC (Wu
et al., 2017). As discussed, the progressive ascending colonization
of bacteria contaminatesthe urethra and the origin ofthis
infection is usually from the gut (Kaper et al., 2004). Owing to the
presence of urine, that represents an ideal growth broth, bacteria
proliferate in a relatively short time lapse,while the flushing of
urine during urination removes most of the invading bacteria.
However,bacterialstrains are able ofbinding tightly to BECs
lining the bladder using fimbrial organelles (Duncan et al., 2004;
Chahales and Thanassi, 2015).
The multilayeredbladderepithelium isalso known as
“transitionalepithelium” and itis composed by three layers:
basalcelllayer (5–10 μm in diameter),intermediate celllayer
(20 μm in diameter),and superficialapicallayerwith large
hexagonal cells (diameters of 25–250 μm), which are also termed
“umbrella cells.” A basement membrane lies underneath the basal
epithelium (Figures 3A,F). The umbrella cells play a prominent
role in maintaining a barrier against most substances found in
urine,and show a number of properties,including specialized
membrane lipids,asymmetric unitmembrane particles,and a
plasmalemma with stiff plaques.These plaques may cover up
to 90% ofthe urothelialcellsurface,with each plaque being
composed of nearly 1,000 subunits. These subunits are made by
proteins (uroplakins, UPs), which serve as the major receptors for
UPEC adherence to the host cell and are localized within plaques
on the apical membranes of the mature umbrella cells (Veranic
et al.,2004).There is a correlation between the glycosylation
changesin UPs and the differentpathologicalconditionsof
the urothelium such UTI and interstitialcystitis (Birder,2005;
Katnik-Prastowska et al., 2014; Habuka et al., 2015).
The fusiform vesicles (FVs) are unique cytoplasmic organelles
contained in theumbrellacells.FVs deliverpreassembled
crystallinearraysof UP proteinsto the apicalcell surface
of urothelialumbrella cells.DifferentRab GTPasesfunction
as regulatorsof specific stepsin membrane traffic pathways
and are localized to the cytosolic face ofspecific intracellular
membranes.Rab27b,is a smallGTPase regulating intracellular
vesicle movement which is expressed at an extraordinary high
level (0.1% of total protein) in urothelium. The Rab27b+ FVs
involved in the storage of extra membrane which are necessa
when urine accumulates and causes bladder expansion (Wan
et al.,2016).In order to enter epithelialcells,UPEC coopt the
superficialepithelialcells by expoiting their bladder volume-
regulating properties by stimulating the exocytosis of fusiform
vesicles right where the bacterialattach.The adherent bacteria
are then internalized when these membranes are subsequent
retracted into cells (Figure 3B;Wu et al.,2017).UPEC have
been found to reside within Rab27b/CD63/Caveolin-1-positive
fusiform vesicles(O’Brien etal., 2016).Internalized UPEC
becomeencased in Rab27b+ fusiform vesicleswithin the
cytosolof the superficialepithelium (Figure 3B;Bishop etal.,
2007). Replication of internalized UPEC bacteria rapidly occur
resulting in the maturation of IBCs,a structure that possesses
biofilm-like properties which is protected from innate defense
and antibiotics (Justice et al., 2006; Goller and Seed, 2010). F
with lysosomes is thus impaired, because internalized bacteri
mostly encased in Rab27b+ compartments.
Defensemechanismsof bladderepithelialcells against
intrusion of bacterial include receptors such as toll-like recept
(e.g., TLR2, TLR4, TLR5, and TLR11) that are able to promptly
recognize intruding bacteria (Larue etal.,2013).After UPEC
encapsulation within RAB27b+ vesicles in BECs,intracellular
UPEC are recognized by TLR4 which increasesintracellular
cyclic AMP (cAMP) levels(Figure 3B).This triggersthe
exocytosisof RAB27b+ vesiclesharboringUPEC and the
intracellular bacterialexpulsion back into the bladder lumen
(Figure 3C).
However,someUPEC break theRAB27b+ vacuoleand
cannotbe expelled into theurine;thus,thesebacteriaare
targeted by autophagy and delivered into the lysosomes,where
theyactivelyneutralizethe pH by reducing theiracidicity
and degradative potential(Abraham and Miao,2015).These
malfunctioning lysosomes are sensed by a lysosomaltransient
receptor potential mucolipin 3 Ca2+ channel (TRPML3), which
is localized on the membrane of lysosomes (Miao et al.,2015).
The activation ofthis Ca2+channelrapidly fluxesout into
the cytosolthe Ca2+ stored in the lysosome,which induces
the spontaneous expulsion into the extracellular space ofthe
defective lysosomes and its contents (Figure 3D).
Pathogensensingby TLR4 inducesthe productionof
various soluble factors which are secreted by BECs,including
antimicrobial peptides (AMP, such as cathelicidin and β-defen
1; Sun et al., 2013; Chromek, 2015), antimicrobial proteins [s
as pentraxin 3 (PTX3); (Uzun et al., 2016)] and chemokines [s
as CXC-chemokine ligand 1 (CXCL1) and CC-chemokine ligand
5 (CCR5);Schiwon et al.,2014;Figure 3E].Attachment to the
urothelium or bacterial lysis are inhibited by these antimicrob
peptides, which are also induced when bacteria succeed to at
to the urothelium (Spencer etal.,2014).Moreover,excretion
in the urine of uromodulin,a major high mannose-containing
glycoprotein, exerts a protective effects against UTI by compe
with the binding of UPEC FimH to uroplakin Ia (Pak et al., 200
When all these export mechanisms fail to clear the urothel
from the invading UPEC, BECs activate the last line of defense
Acute infections are commonly associated with of the exfoliat
Frontiers in Microbiology | www.frontiersin.org 5 August 2017 | Volume 8 | Article 1566
allows for in situ two-dimensional assessment of protein spatial
distribution and abundance, revealed the occurrence of different
bacterial subpopulations within biofilms: a type-1 pili-expressing
cells localized atthe air-exposed region and a curli-equipped
population localized to the underlying air-liquid interface (Floyd
et al., 2015).
Together,all the above mentioned “omics” approaches have
allowed a greatdealof new information to be available and
that is enabling a more comprehensive understanding of UPEC’s
pathogenic mechanisms.
THE BLADDER EPITHELIUM SHOWS
SELF-DEFENSE MECHANISMS AGAINST
INVADING BACTERIA
The most commonly targeted site of UTIs is the bladder.The
bladder epithelium possesses powerfulbarriers and the BECs
show antibacterial activities.Despite their properties,BECs and
the bladder epithelium are often circumvented by UPEC (Wu
et al., 2017). As discussed, the progressive ascending colonization
of bacteria contaminatesthe urethra and the origin ofthis
infection is usually from the gut (Kaper et al., 2004). Owing to the
presence of urine, that represents an ideal growth broth, bacteria
proliferate in a relatively short time lapse,while the flushing of
urine during urination removes most of the invading bacteria.
However,bacterialstrains are able ofbinding tightly to BECs
lining the bladder using fimbrial organelles (Duncan et al., 2004;
Chahales and Thanassi, 2015).
The multilayeredbladderepithelium isalso known as
“transitionalepithelium” and itis composed by three layers:
basalcelllayer (5–10 μm in diameter),intermediate celllayer
(20 μm in diameter),and superficialapicallayerwith large
hexagonal cells (diameters of 25–250 μm), which are also termed
“umbrella cells.” A basement membrane lies underneath the basal
epithelium (Figures 3A,F). The umbrella cells play a prominent
role in maintaining a barrier against most substances found in
urine,and show a number of properties,including specialized
membrane lipids,asymmetric unitmembrane particles,and a
plasmalemma with stiff plaques.These plaques may cover up
to 90% ofthe urothelialcellsurface,with each plaque being
composed of nearly 1,000 subunits. These subunits are made by
proteins (uroplakins, UPs), which serve as the major receptors for
UPEC adherence to the host cell and are localized within plaques
on the apical membranes of the mature umbrella cells (Veranic
et al.,2004).There is a correlation between the glycosylation
changesin UPs and the differentpathologicalconditionsof
the urothelium such UTI and interstitialcystitis (Birder,2005;
Katnik-Prastowska et al., 2014; Habuka et al., 2015).
The fusiform vesicles (FVs) are unique cytoplasmic organelles
contained in theumbrellacells.FVs deliverpreassembled
crystallinearraysof UP proteinsto the apicalcell surface
of urothelialumbrella cells.DifferentRab GTPasesfunction
as regulatorsof specific stepsin membrane traffic pathways
and are localized to the cytosolic face ofspecific intracellular
membranes.Rab27b,is a smallGTPase regulating intracellular
vesicle movement which is expressed at an extraordinary high
level (0.1% of total protein) in urothelium. The Rab27b+ FVs
involved in the storage of extra membrane which are necessa
when urine accumulates and causes bladder expansion (Wan
et al.,2016).In order to enter epithelialcells,UPEC coopt the
superficialepithelialcells by expoiting their bladder volume-
regulating properties by stimulating the exocytosis of fusiform
vesicles right where the bacterialattach.The adherent bacteria
are then internalized when these membranes are subsequent
retracted into cells (Figure 3B;Wu et al.,2017).UPEC have
been found to reside within Rab27b/CD63/Caveolin-1-positive
fusiform vesicles(O’Brien etal., 2016).Internalized UPEC
becomeencased in Rab27b+ fusiform vesicleswithin the
cytosolof the superficialepithelium (Figure 3B;Bishop etal.,
2007). Replication of internalized UPEC bacteria rapidly occur
resulting in the maturation of IBCs,a structure that possesses
biofilm-like properties which is protected from innate defense
and antibiotics (Justice et al., 2006; Goller and Seed, 2010). F
with lysosomes is thus impaired, because internalized bacteri
mostly encased in Rab27b+ compartments.
Defensemechanismsof bladderepithelialcells against
intrusion of bacterial include receptors such as toll-like recept
(e.g., TLR2, TLR4, TLR5, and TLR11) that are able to promptly
recognize intruding bacteria (Larue etal.,2013).After UPEC
encapsulation within RAB27b+ vesicles in BECs,intracellular
UPEC are recognized by TLR4 which increasesintracellular
cyclic AMP (cAMP) levels(Figure 3B).This triggersthe
exocytosisof RAB27b+ vesiclesharboringUPEC and the
intracellular bacterialexpulsion back into the bladder lumen
(Figure 3C).
However,someUPEC break theRAB27b+ vacuoleand
cannotbe expelled into theurine;thus,thesebacteriaare
targeted by autophagy and delivered into the lysosomes,where
theyactivelyneutralizethe pH by reducing theiracidicity
and degradative potential(Abraham and Miao,2015).These
malfunctioning lysosomes are sensed by a lysosomaltransient
receptor potential mucolipin 3 Ca2+ channel (TRPML3), which
is localized on the membrane of lysosomes (Miao et al.,2015).
The activation ofthis Ca2+channelrapidly fluxesout into
the cytosolthe Ca2+ stored in the lysosome,which induces
the spontaneous expulsion into the extracellular space ofthe
defective lysosomes and its contents (Figure 3D).
Pathogensensingby TLR4 inducesthe productionof
various soluble factors which are secreted by BECs,including
antimicrobial peptides (AMP, such as cathelicidin and β-defen
1; Sun et al., 2013; Chromek, 2015), antimicrobial proteins [s
as pentraxin 3 (PTX3); (Uzun et al., 2016)] and chemokines [s
as CXC-chemokine ligand 1 (CXCL1) and CC-chemokine ligand
5 (CCR5);Schiwon et al.,2014;Figure 3E].Attachment to the
urothelium or bacterial lysis are inhibited by these antimicrob
peptides, which are also induced when bacteria succeed to at
to the urothelium (Spencer etal.,2014).Moreover,excretion
in the urine of uromodulin,a major high mannose-containing
glycoprotein, exerts a protective effects against UTI by compe
with the binding of UPEC FimH to uroplakin Ia (Pak et al., 200
When all these export mechanisms fail to clear the urothel
from the invading UPEC, BECs activate the last line of defense
Acute infections are commonly associated with of the exfoliat
Frontiers in Microbiology | www.frontiersin.org 5 August 2017 | Volume 8 | Article 1566
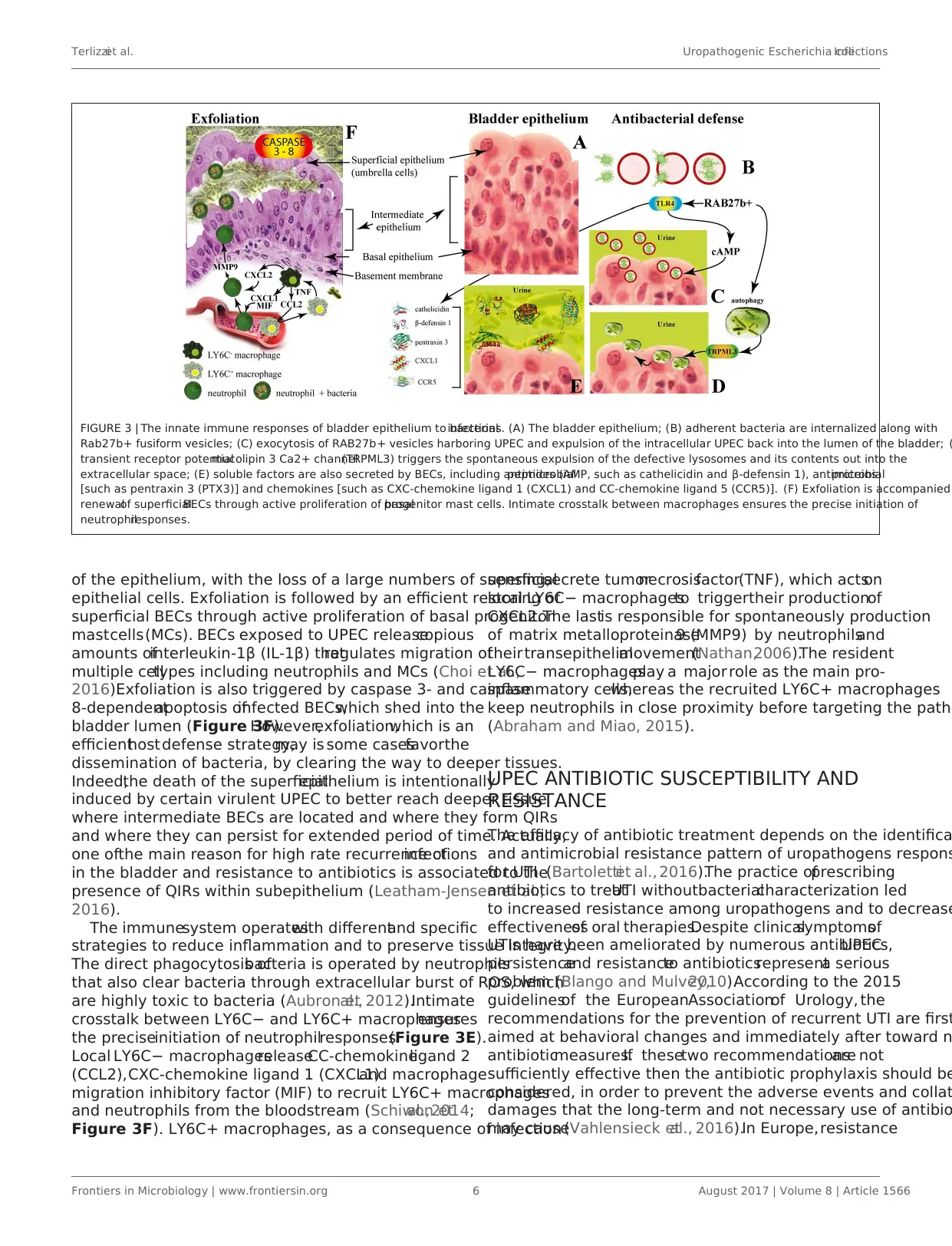
Terlizziet al. Uropathogenic Escherichia coliInfections
FIGURE 3 | The innate immune responses of bladder epithelium to bacterialinfections. (A) The bladder epithelium; (B) adherent bacteria are internalized along with
Rab27b+ fusiform vesicles; (C) exocytosis of RAB27b+ vesicles harboring UPEC and expulsion of the intracellular UPEC back into the lumen of the bladder; (
transient receptor potentialmucolipin 3 Ca2+ channel(TRPML3) triggers the spontaneous expulsion of the defective lysosomes and its contents out into the
extracellular space; (E) soluble factors are also secreted by BECs, including antimicrobialpeptides (AMP, such as cathelicidin and β-defensin 1), antimicrobialproteins
[such as pentraxin 3 (PTX3)] and chemokines [such as CXC-chemokine ligand 1 (CXCL1) and CC-chemokine ligand 5 (CCR5)]. (F) Exfoliation is accompanied
renewalof superficialBECs through active proliferation of basalprogenitor mast cells. Intimate crosstalk between macrophages ensures the precise initiation of
neutrophilresponses.
of the epithelium, with the loss of a large numbers of superficial
epithelial cells. Exfoliation is followed by an efficient restoring of
superficial BECs through active proliferation of basal progenitor
mastcells(MCs). BECs exposed to UPEC releasecopious
amounts ofinterleukin-1β (IL-1β) thatregulates migration of
multiple celltypes including neutrophils and MCs (Choi et al.,
2016).Exfoliation is also triggered by caspase 3- and caspase
8-dependentapoptosis ofinfected BECs,which shed into the
bladder lumen (Figure 3F).However,exfoliation,which is an
efficienthost defense strategy,may is some casesfavorthe
dissemination of bacteria, by clearing the way to deeper tissues.
Indeed,the death of the superficialepithelium is intentionally
induced by certain virulent UPEC to better reach deeper tissue
where intermediate BECs are located and where they form QIRs
and where they can persist for extended period of time. Actually,
one ofthe main reason for high rate recurrence ofinfections
in the bladder and resistance to antibiotics is associated to the
presence of QIRs within subepithelium (Leatham-Jensen et al.,
2016).
The immunesystem operateswith differentand specific
strategies to reduce inflammation and to preserve tissue integrity.
The direct phagocytosis ofbacteria is operated by neutrophils
that also clear bacteria through extracellular burst of ROS, which
are highly toxic to bacteria (Aubron etal., 2012).Intimate
crosstalk between LY6C− and LY6C+ macrophagesensures
the preciseinitiation of neutrophilresponses(Figure 3E).
Local LY6C− macrophagesreleaseCC-chemokineligand 2
(CCL2),CXC-chemokine ligand 1 (CXCL1)and macrophage
migration inhibitory factor (MIF) to recruit LY6C+ macrophages
and neutrophils from the bloodstream (Schiwon etal.,2014;
Figure 3F). LY6C+ macrophages, as a consequence of infection
sensing,secrete tumornecrosisfactor(TNF), which actson
local LY6C− macrophagesto triggertheir productionof
CXCL2.The lastis responsible for spontaneously production
of matrix metalloproteinase9 (MMP9) by neutrophilsand
theirtransepithelialmovement(Nathan,2006).The resident
LY6C− macrophagesplay a major role as the main pro-
inflammatory cells,whereas the recruited LY6C+ macrophages
keep neutrophils in close proximity before targeting the patho
(Abraham and Miao, 2015).
UPEC ANTIBIOTIC SUSCEPTIBILITY AND
RESISTANCE
The efficacy of antibiotic treatment depends on the identifica
and antimicrobial resistance pattern of uropathogens respons
for UTI (Bartolettiet al., 2016).The practice ofprescribing
antibiotics to treatUTI withoutbacterialcharacterization led
to increased resistance among uropathogens and to decrease
effectivenessof oral therapies.Despite clinicalsymptomsof
UTIs have been ameliorated by numerous antibiotics,UPEC
persistenceand resistanceto antibioticsrepresenta serious
problem (Blango and Mulvey,2010).According to the 2015
guidelinesof the EuropeanAssociationof Urology, the
recommendations for the prevention of recurrent UTI are first
aimed at behavioral changes and immediately after toward n
antibioticmeasures.If thesetwo recommendationsare not
sufficiently effective then the antibiotic prophylaxis should be
considered, in order to prevent the adverse events and collat
damages that the long-term and not necessary use of antibio
may cause(Vahlensieck etal., 2016).In Europe,resistance
Frontiers in Microbiology | www.frontiersin.org 6 August 2017 | Volume 8 | Article 1566
FIGURE 3 | The innate immune responses of bladder epithelium to bacterialinfections. (A) The bladder epithelium; (B) adherent bacteria are internalized along with
Rab27b+ fusiform vesicles; (C) exocytosis of RAB27b+ vesicles harboring UPEC and expulsion of the intracellular UPEC back into the lumen of the bladder; (
transient receptor potentialmucolipin 3 Ca2+ channel(TRPML3) triggers the spontaneous expulsion of the defective lysosomes and its contents out into the
extracellular space; (E) soluble factors are also secreted by BECs, including antimicrobialpeptides (AMP, such as cathelicidin and β-defensin 1), antimicrobialproteins
[such as pentraxin 3 (PTX3)] and chemokines [such as CXC-chemokine ligand 1 (CXCL1) and CC-chemokine ligand 5 (CCR5)]. (F) Exfoliation is accompanied
renewalof superficialBECs through active proliferation of basalprogenitor mast cells. Intimate crosstalk between macrophages ensures the precise initiation of
neutrophilresponses.
of the epithelium, with the loss of a large numbers of superficial
epithelial cells. Exfoliation is followed by an efficient restoring of
superficial BECs through active proliferation of basal progenitor
mastcells(MCs). BECs exposed to UPEC releasecopious
amounts ofinterleukin-1β (IL-1β) thatregulates migration of
multiple celltypes including neutrophils and MCs (Choi et al.,
2016).Exfoliation is also triggered by caspase 3- and caspase
8-dependentapoptosis ofinfected BECs,which shed into the
bladder lumen (Figure 3F).However,exfoliation,which is an
efficienthost defense strategy,may is some casesfavorthe
dissemination of bacteria, by clearing the way to deeper tissues.
Indeed,the death of the superficialepithelium is intentionally
induced by certain virulent UPEC to better reach deeper tissue
where intermediate BECs are located and where they form QIRs
and where they can persist for extended period of time. Actually,
one ofthe main reason for high rate recurrence ofinfections
in the bladder and resistance to antibiotics is associated to the
presence of QIRs within subepithelium (Leatham-Jensen et al.,
2016).
The immunesystem operateswith differentand specific
strategies to reduce inflammation and to preserve tissue integrity.
The direct phagocytosis ofbacteria is operated by neutrophils
that also clear bacteria through extracellular burst of ROS, which
are highly toxic to bacteria (Aubron etal., 2012).Intimate
crosstalk between LY6C− and LY6C+ macrophagesensures
the preciseinitiation of neutrophilresponses(Figure 3E).
Local LY6C− macrophagesreleaseCC-chemokineligand 2
(CCL2),CXC-chemokine ligand 1 (CXCL1)and macrophage
migration inhibitory factor (MIF) to recruit LY6C+ macrophages
and neutrophils from the bloodstream (Schiwon etal.,2014;
Figure 3F). LY6C+ macrophages, as a consequence of infection
sensing,secrete tumornecrosisfactor(TNF), which actson
local LY6C− macrophagesto triggertheir productionof
CXCL2.The lastis responsible for spontaneously production
of matrix metalloproteinase9 (MMP9) by neutrophilsand
theirtransepithelialmovement(Nathan,2006).The resident
LY6C− macrophagesplay a major role as the main pro-
inflammatory cells,whereas the recruited LY6C+ macrophages
keep neutrophils in close proximity before targeting the patho
(Abraham and Miao, 2015).
UPEC ANTIBIOTIC SUSCEPTIBILITY AND
RESISTANCE
The efficacy of antibiotic treatment depends on the identifica
and antimicrobial resistance pattern of uropathogens respons
for UTI (Bartolettiet al., 2016).The practice ofprescribing
antibiotics to treatUTI withoutbacterialcharacterization led
to increased resistance among uropathogens and to decrease
effectivenessof oral therapies.Despite clinicalsymptomsof
UTIs have been ameliorated by numerous antibiotics,UPEC
persistenceand resistanceto antibioticsrepresenta serious
problem (Blango and Mulvey,2010).According to the 2015
guidelinesof the EuropeanAssociationof Urology, the
recommendations for the prevention of recurrent UTI are first
aimed at behavioral changes and immediately after toward n
antibioticmeasures.If thesetwo recommendationsare not
sufficiently effective then the antibiotic prophylaxis should be
considered, in order to prevent the adverse events and collat
damages that the long-term and not necessary use of antibio
may cause(Vahlensieck etal., 2016).In Europe,resistance
Frontiers in Microbiology | www.frontiersin.org 6 August 2017 | Volume 8 | Article 1566
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Terlizziet al. Uropathogenic Escherichia coliInfections
to UPEC isolatesshowsaverage valuesof 11.8% forthird-
generation cephalosporins and 22.3% for fluoroquinolones.In
the U.S., fluoroquinolone-resistant UPEC represented the 31.3%
of isolates among hospitalized patients between the years 2007
and 2010 (Edelsberg et al., 2014). These data confirm the general
consideration thatnumberof effective antibiotic compounds
availabilityand the prevalenceof antibioticresistanceare
worsening,as demonstrated by an increased number of clinical
studies (Bartoletti et al., 2016).
Antimicrobialprophylaxisfor women with recurrentUTI
include,for example,50 mg or 100 mg ofnitrofurantoin once
a day;100 mg of Trimethoprim (TMP) once a day;40/200 mg
TMP/sulfamethoxazole (co-trimoxazole)once a day orthree
timesa week;3 g of fosfomycin trometamolevery 10 days
and,during pregnancy,for example,125–250 mg of cephalexin
or cefaclor 250 mg once a day (Grabe etal., 2015;Giancola
et al., 2017).Among otherantibiotics,imipenem represents
the bestefficientantibiotic againstall UPEC strains(100%),
followedby ertapenem (99.98%),amikacin(99.94%),and
nitrofurantoin (99.91%). Carbapenems like imipenem represent
the bestoption for the treatmentof extended-spectrum beta-
lactamase (ESBL) strains (Idilet al.,2016).UPEC strains are
also susceptible to ciprofloxacin (Tosun et al., 2016), cefotaxime,
piperacillin/tazobactam (Dizbayet al., 2016),azithromycin,
doxycycline and ceftriaxone (Saha et al., 2015). However, several
UPEC isolates are resistantto ampicillin,oral first-generation
cephalosporins,TMP-sulfamethoxazole (Moya-Dionisio etal.,
2016),cefuroxime (Chang etal., 2016),cotrimoxazole (Saha
et al.,2015),amoxicillin-clavulanate,nalidixic acid,cefradine,
and aminopenicillins (Narchi and Al-Hamdani,2010).In some
cases,the combined effectof differentantibiotics prompted a
significant increment in susceptibility, as found for triclosan with
amoxicillin and gentamicin (Wignall et al., 2008). A retrospective
analysis has identified ciprofloxacin as the most used antibiotic
for empiricaltherapies(76% ofcases;Parish and Holliday,
2012).
Due to ecologicalside effects,the oral cephalosporins
and fluoroquinolones are no longer recommended as routine
treatments,except for specific clinicalsituations.Furthermore,
the worldwide incrementof UPEC strainsresistantto TMP
questions its use with or without a sulfonamide as an effective
prophylactic agent(Idil et al., 2016).High urinary levelsof
levofloxacin are not enough to cure UTIs and the combination
of ceftolozane/tazobactam was more effective as an alternative
treatmentin settingsof increased fluoroquinolone resistance
(Huntington et al.,2016).Increased resistance of UPEC strain
isolatesagainstampicillin (96.42 %),tetracycline (85.71 %),
amikacin (71.42 %),ciprofloxacin (67.85 %),and gentamycin
(58.71 %)hasbeen found in pregnantwomen with history
of recurrentUTIs (Habibi and Khameneie,2016).Finally,
numerousgovernmentagenciesadvise againstthe long-term
use ofprophylactic nitrofurantoin because ofrare butserious
pulmonary and hepatic adverse effects (Vahlensieck et al., 2016).
New antibiotics, such as colistin (Cui et al., 2016), finafloxacin,
and cefiderocol (S-649266), which are currently in early clinical
development, might be useful in the treatment of UTIs (Zacché
and Giarenis, 2016).
Figure 4shows the structureformulaeof the most
representative antibiotics for which UPEC resistance has been
demonstrated (red background) and those showing susceptib
to UPEC (green background).The yellow background shows
antibiotics that already show resistance in some UPEC strains
ALTERNATIVE ANTIMICROBIAL REMEDIES
Antibiotics willcontinue to be an unavoidable source for the
prevention ofUTIs on a case-by-casebasis.However,the
excessive use of antibiotics and the long-term interference w
intestinal microbiota, require to search for alternative remedi
A plethoraof moleculeshas been tested to reduceUPEC
infectionsby exploiting theirability eitherto stimulatethe
immune system or to interfere with the UPEC ability to adher
and invade the urothelium. Here we briefly summarize the m
effective alternative remedies to fight UPECs.
Vaccines
The developmentof new strategies to fightUTI has focused
on the development of vaccines based on bacterial componen
with the aim of identifying specific UPEC factors for potential
use as vaccine antigens (McLellan and Hunstad,2016).Among
candidate antigens,potential targets are adhesins,antimicrobial
peptides (AMPs),and siderophores (Spaulding and Hultgren,
2016). However, the use of vaccines may alter the proteobac
populations ofE. coliin the gutand may find a difficultway
to reach the bladder lumen.Furthermore,vaccine use might be
more efficientto treatupper rather than lower urinary tracts
(McLellan and Hunstad,2016).Recently,healthy adult women
with a history of recurrent UTI where the subject of a multicen
phase 1b trial where a single intramuscular injection of either
bioconjugate vaccine containing the O-antigens of four E.coli
serotypes (ExPEC4V) or placebo were administered. Vaccinat
induced significantIgG responses for allserotypes;moreover,
the vaccine group showed significantly lower UTIs caused by
UPEC of any serotype when compared with the placebo group
(Huttner et al.,2017).In a meta-analysis of about 900 patients,
the oral vaccineOM-89 (Uro-VaxomR ) reduced themean
number ofUTIs by half,whereas a vaginalvaccine (Urovac)
showed a scanty reduction in recurrentUTIs and caused a
vaginalirritation in nearly 28% ofpatients (Beerepootet al.,
2013).An alternative strategy to elicitprotective immunity is
to selectas antigens smallmolecules,rather than proteins or
peptides.The use of siderophore-protein conjugates was found
to elicitimmune responses targeted to bacterialsiderophores
and to successfully protectagainstUTI (Mike etal.,2016).A
reverse-vaccinology approach in combination with proteomic
and genomics was used to identify putative broadly protectiv
vaccine antigens (Moriel et al., 2016). Currently, no UTI vacci
are approved in the United States but among current strategi
immunotherapeutic formulation OM-89 (marketed in Europe b
EurimPharm GmbH as UroVaxom), which is a bacterial extrac
prepared from 18 strains of E. coli, is really promising (Neto e
2016).The identification ofpotentialvaccine targets has been
recently reviewed (O’Brien et al.,2016;Poolman and Wacker,
2016).
Frontiers in Microbiology | www.frontiersin.org 7 August 2017 | Volume 8 | Article 1566
to UPEC isolatesshowsaverage valuesof 11.8% forthird-
generation cephalosporins and 22.3% for fluoroquinolones.In
the U.S., fluoroquinolone-resistant UPEC represented the 31.3%
of isolates among hospitalized patients between the years 2007
and 2010 (Edelsberg et al., 2014). These data confirm the general
consideration thatnumberof effective antibiotic compounds
availabilityand the prevalenceof antibioticresistanceare
worsening,as demonstrated by an increased number of clinical
studies (Bartoletti et al., 2016).
Antimicrobialprophylaxisfor women with recurrentUTI
include,for example,50 mg or 100 mg ofnitrofurantoin once
a day;100 mg of Trimethoprim (TMP) once a day;40/200 mg
TMP/sulfamethoxazole (co-trimoxazole)once a day orthree
timesa week;3 g of fosfomycin trometamolevery 10 days
and,during pregnancy,for example,125–250 mg of cephalexin
or cefaclor 250 mg once a day (Grabe etal., 2015;Giancola
et al., 2017).Among otherantibiotics,imipenem represents
the bestefficientantibiotic againstall UPEC strains(100%),
followedby ertapenem (99.98%),amikacin(99.94%),and
nitrofurantoin (99.91%). Carbapenems like imipenem represent
the bestoption for the treatmentof extended-spectrum beta-
lactamase (ESBL) strains (Idilet al.,2016).UPEC strains are
also susceptible to ciprofloxacin (Tosun et al., 2016), cefotaxime,
piperacillin/tazobactam (Dizbayet al., 2016),azithromycin,
doxycycline and ceftriaxone (Saha et al., 2015). However, several
UPEC isolates are resistantto ampicillin,oral first-generation
cephalosporins,TMP-sulfamethoxazole (Moya-Dionisio etal.,
2016),cefuroxime (Chang etal., 2016),cotrimoxazole (Saha
et al.,2015),amoxicillin-clavulanate,nalidixic acid,cefradine,
and aminopenicillins (Narchi and Al-Hamdani,2010).In some
cases,the combined effectof differentantibiotics prompted a
significant increment in susceptibility, as found for triclosan with
amoxicillin and gentamicin (Wignall et al., 2008). A retrospective
analysis has identified ciprofloxacin as the most used antibiotic
for empiricaltherapies(76% ofcases;Parish and Holliday,
2012).
Due to ecologicalside effects,the oral cephalosporins
and fluoroquinolones are no longer recommended as routine
treatments,except for specific clinicalsituations.Furthermore,
the worldwide incrementof UPEC strainsresistantto TMP
questions its use with or without a sulfonamide as an effective
prophylactic agent(Idil et al., 2016).High urinary levelsof
levofloxacin are not enough to cure UTIs and the combination
of ceftolozane/tazobactam was more effective as an alternative
treatmentin settingsof increased fluoroquinolone resistance
(Huntington et al.,2016).Increased resistance of UPEC strain
isolatesagainstampicillin (96.42 %),tetracycline (85.71 %),
amikacin (71.42 %),ciprofloxacin (67.85 %),and gentamycin
(58.71 %)hasbeen found in pregnantwomen with history
of recurrentUTIs (Habibi and Khameneie,2016).Finally,
numerousgovernmentagenciesadvise againstthe long-term
use ofprophylactic nitrofurantoin because ofrare butserious
pulmonary and hepatic adverse effects (Vahlensieck et al., 2016).
New antibiotics, such as colistin (Cui et al., 2016), finafloxacin,
and cefiderocol (S-649266), which are currently in early clinical
development, might be useful in the treatment of UTIs (Zacché
and Giarenis, 2016).
Figure 4shows the structureformulaeof the most
representative antibiotics for which UPEC resistance has been
demonstrated (red background) and those showing susceptib
to UPEC (green background).The yellow background shows
antibiotics that already show resistance in some UPEC strains
ALTERNATIVE ANTIMICROBIAL REMEDIES
Antibiotics willcontinue to be an unavoidable source for the
prevention ofUTIs on a case-by-casebasis.However,the
excessive use of antibiotics and the long-term interference w
intestinal microbiota, require to search for alternative remedi
A plethoraof moleculeshas been tested to reduceUPEC
infectionsby exploiting theirability eitherto stimulatethe
immune system or to interfere with the UPEC ability to adher
and invade the urothelium. Here we briefly summarize the m
effective alternative remedies to fight UPECs.
Vaccines
The developmentof new strategies to fightUTI has focused
on the development of vaccines based on bacterial componen
with the aim of identifying specific UPEC factors for potential
use as vaccine antigens (McLellan and Hunstad,2016).Among
candidate antigens,potential targets are adhesins,antimicrobial
peptides (AMPs),and siderophores (Spaulding and Hultgren,
2016). However, the use of vaccines may alter the proteobac
populations ofE. coliin the gutand may find a difficultway
to reach the bladder lumen.Furthermore,vaccine use might be
more efficientto treatupper rather than lower urinary tracts
(McLellan and Hunstad,2016).Recently,healthy adult women
with a history of recurrent UTI where the subject of a multicen
phase 1b trial where a single intramuscular injection of either
bioconjugate vaccine containing the O-antigens of four E.coli
serotypes (ExPEC4V) or placebo were administered. Vaccinat
induced significantIgG responses for allserotypes;moreover,
the vaccine group showed significantly lower UTIs caused by
UPEC of any serotype when compared with the placebo group
(Huttner et al.,2017).In a meta-analysis of about 900 patients,
the oral vaccineOM-89 (Uro-VaxomR ) reduced themean
number ofUTIs by half,whereas a vaginalvaccine (Urovac)
showed a scanty reduction in recurrentUTIs and caused a
vaginalirritation in nearly 28% ofpatients (Beerepootet al.,
2013).An alternative strategy to elicitprotective immunity is
to selectas antigens smallmolecules,rather than proteins or
peptides.The use of siderophore-protein conjugates was found
to elicitimmune responses targeted to bacterialsiderophores
and to successfully protectagainstUTI (Mike etal.,2016).A
reverse-vaccinology approach in combination with proteomic
and genomics was used to identify putative broadly protectiv
vaccine antigens (Moriel et al., 2016). Currently, no UTI vacci
are approved in the United States but among current strategi
immunotherapeutic formulation OM-89 (marketed in Europe b
EurimPharm GmbH as UroVaxom), which is a bacterial extrac
prepared from 18 strains of E. coli, is really promising (Neto e
2016).The identification ofpotentialvaccine targets has been
recently reviewed (O’Brien et al.,2016;Poolman and Wacker,
2016).
Frontiers in Microbiology | www.frontiersin.org 7 August 2017 | Volume 8 | Article 1566
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
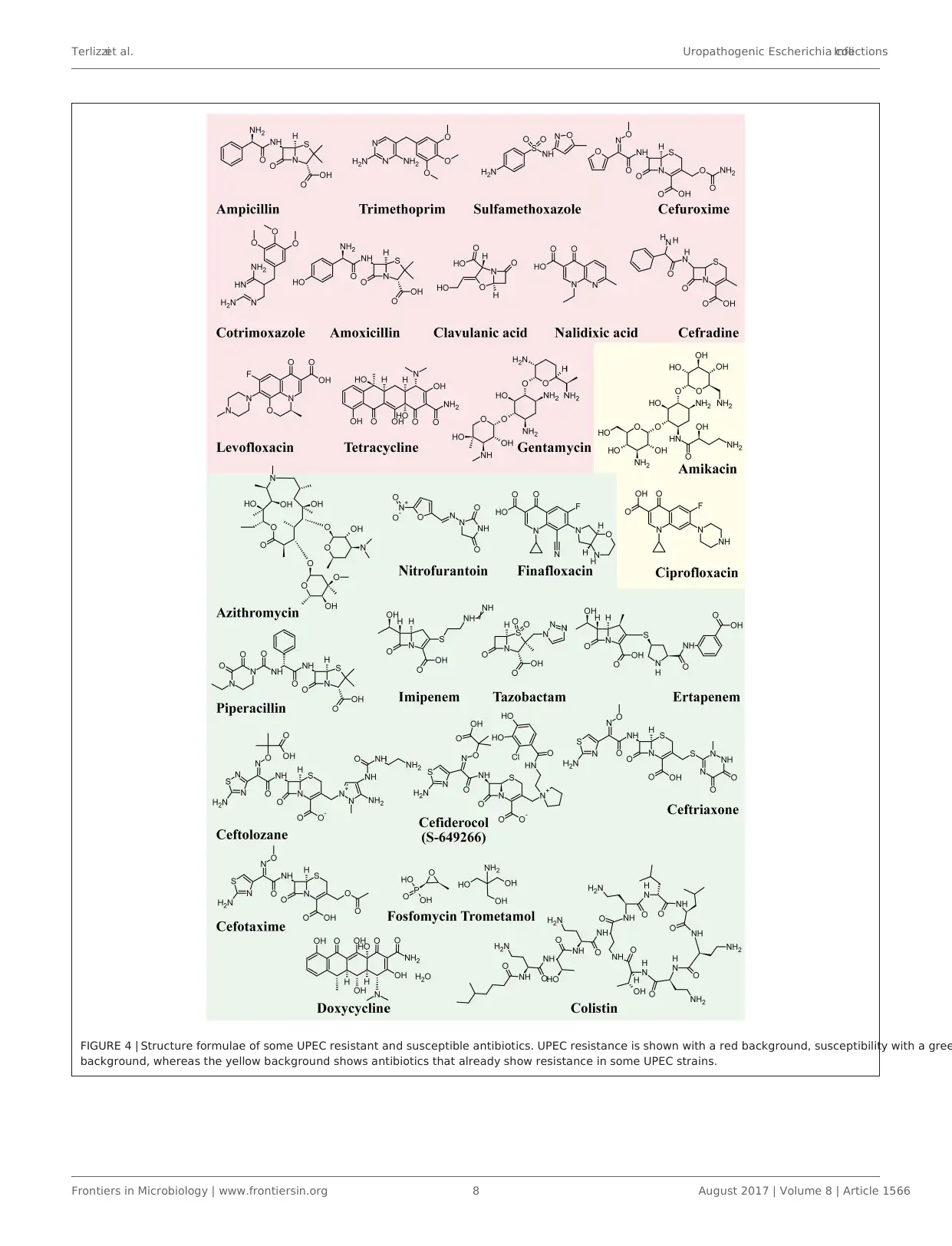
Terlizziet al. Uropathogenic Escherichia coliInfections
FIGURE 4 | Structure formulae of some UPEC resistant and susceptible antibiotics. UPEC resistance is shown with a red background, susceptibility with a gree
background, whereas the yellow background shows antibiotics that already show resistance in some UPEC strains.
Frontiers in Microbiology | www.frontiersin.org 8 August 2017 | Volume 8 | Article 1566
FIGURE 4 | Structure formulae of some UPEC resistant and susceptible antibiotics. UPEC resistance is shown with a red background, susceptibility with a gree
background, whereas the yellow background shows antibiotics that already show resistance in some UPEC strains.
Frontiers in Microbiology | www.frontiersin.org 8 August 2017 | Volume 8 | Article 1566
Terlizziet al. Uropathogenic Escherichia coliInfections
Probiotics
Women with recurrentUTIs often show alterationsin their
vaginal or periurethral microbiota (Czaja et al., 2009). Probiotics
have been extensively used as alternative approaches to reduce
recurrent UTIs (Zacché and Giarenis, 2016). Several Lactobacilli
strains showed UPEC inhibitory activity between that of sensitive
and resistant antibiotics (Shim et al.,2016).One of the major
rolesof Lactobacilliis theirability to clearpotentialUPEC
reservoirs,thus preventing recurrences.Lactobacillus-mediated
protection from UTI is notclear,and may involve hydrogen
peroxide,surfactants,and anti-adhesive molecules production
(O’Brien etal.,2016).However,contrasting results have been
reported. For instance, Lactobacilli prophylaxis did not decrease
the rate of UTI recurrence in several open randomized trials with
women who had a UTI caused by UPEC (Kontiokari et al., 2001;
Beerepoot et al., 2013; Schwenger et al., 2015). On the other hand,
Lactobacilli were shown to actively stimulate the immune system
by up- and down-regulation of NF-κB activity (Karlsson et al.,
2012), and to colonize and protect the vagina (Reid, 2000), thus
suggesting some sort of indirect-cooperative function.
Estrogens
The vaginalepithelium andits acidic microenvironment
provide substantialinhibition ofbacterialgrowth ofenteric
microorganisms.Estrogenis an importantmodulatorof
urothelium cellgrowth and differentiation.Estrogenmay
constitute a risk factor for infections in young women; however,
after menopause the low estradiollevels have been related to
recurrent infections (Mody and Juthani-Mehta, 2014). Estrogen
applicationmodulatestwo epithelialdefensemechanisms:
induction of AMPs and reduction of epithelial exfoliation (Luthje
et al., 2013).Furthermore,increased epithelialintegrity and
higher expression of AMPs may reduce the formation of QIRs
as the sourceof recurrentinfections(Luthjeand Brauner,
2016).However,oral estrogen therapy failed to be effective
at reducing UTI risk compared with placebo,whereas vaginal
estrogen application reduced UTI (Perrotta et al., 2008; Matul
et al.,2016).Vaginal estrogen therapy was found to be effective
in preventing recurrent UTI ofpostmenopausalwomen when
used in combination with hyaluronic acid,chondroitin sulfate,
curcumin,and quercetin administered peros (Torella etal.,
2016).
Pilicides and Curlicides
The requirement of pili for adhesion by UPEC makes inhibitors
of the assembling chaperone-usher pathway (CUP) as potenti
targets to reduce UTI (Aberg and Almqvist,2007).Therefore,
pilicides represent an interesting alternative to antibiotics.Two
classes of pilicides have been developed:amino acid derivatives
and pyridinones (Svensson et al., 2001). In laboratory and clin
E. coli strains,these compounds have been demonstrated to be
able to reduce by almost90% hemagglutination mediated by
either type 1 or P-pili adherence to BECs and biofilm formatio
mediated by type Ipili (Pinkneret al., 2006).One of these
pilicides,ec240,was found to decrease motility and dysregulate
CUP pili, including type 1, P, and S pili (Greene et al., 2014).
Curli-mediated biofilm formation requires a specific assemb
machinery (Chapman et al.,2002).Curlicides are inhibitors of
both type 1 pilus production and curli biogenesis.Compounds
derived from the peptidomimetic scaffold thatshow pilicide
activity can preventboth Aβ aggregation and curliformation
(Chorellet al.,2011).A small-molecule curlicide (FN075) was
shown to inhibit both type 1 pilus production and curli biogen
by reducing the biofilm and virulence of UPEC in a mouse mo
of experimental cystitis (Cegelski et al., 2009).
Figure 5 depicts the chemical structure of some pilicides a
curlicides.
D-Mannose and D-Mannose-Derived FimH
Antagonists
One of the main strategies to reduce UPEC infection is targeti
bacterialadhesion by inhibiting,for instance,FimH. By using
FIGURE 5 | Structure formulae of pilicide scaffold, some bioactive pilicides, and the curlicide FN075.
Frontiers in Microbiology | www.frontiersin.org 9 August 2017 | Volume 8 | Article 1566
Probiotics
Women with recurrentUTIs often show alterationsin their
vaginal or periurethral microbiota (Czaja et al., 2009). Probiotics
have been extensively used as alternative approaches to reduce
recurrent UTIs (Zacché and Giarenis, 2016). Several Lactobacilli
strains showed UPEC inhibitory activity between that of sensitive
and resistant antibiotics (Shim et al.,2016).One of the major
rolesof Lactobacilliis theirability to clearpotentialUPEC
reservoirs,thus preventing recurrences.Lactobacillus-mediated
protection from UTI is notclear,and may involve hydrogen
peroxide,surfactants,and anti-adhesive molecules production
(O’Brien etal.,2016).However,contrasting results have been
reported. For instance, Lactobacilli prophylaxis did not decrease
the rate of UTI recurrence in several open randomized trials with
women who had a UTI caused by UPEC (Kontiokari et al., 2001;
Beerepoot et al., 2013; Schwenger et al., 2015). On the other hand,
Lactobacilli were shown to actively stimulate the immune system
by up- and down-regulation of NF-κB activity (Karlsson et al.,
2012), and to colonize and protect the vagina (Reid, 2000), thus
suggesting some sort of indirect-cooperative function.
Estrogens
The vaginalepithelium andits acidic microenvironment
provide substantialinhibition ofbacterialgrowth ofenteric
microorganisms.Estrogenis an importantmodulatorof
urothelium cellgrowth and differentiation.Estrogenmay
constitute a risk factor for infections in young women; however,
after menopause the low estradiollevels have been related to
recurrent infections (Mody and Juthani-Mehta, 2014). Estrogen
applicationmodulatestwo epithelialdefensemechanisms:
induction of AMPs and reduction of epithelial exfoliation (Luthje
et al., 2013).Furthermore,increased epithelialintegrity and
higher expression of AMPs may reduce the formation of QIRs
as the sourceof recurrentinfections(Luthjeand Brauner,
2016).However,oral estrogen therapy failed to be effective
at reducing UTI risk compared with placebo,whereas vaginal
estrogen application reduced UTI (Perrotta et al., 2008; Matul
et al.,2016).Vaginal estrogen therapy was found to be effective
in preventing recurrent UTI ofpostmenopausalwomen when
used in combination with hyaluronic acid,chondroitin sulfate,
curcumin,and quercetin administered peros (Torella etal.,
2016).
Pilicides and Curlicides
The requirement of pili for adhesion by UPEC makes inhibitors
of the assembling chaperone-usher pathway (CUP) as potenti
targets to reduce UTI (Aberg and Almqvist,2007).Therefore,
pilicides represent an interesting alternative to antibiotics.Two
classes of pilicides have been developed:amino acid derivatives
and pyridinones (Svensson et al., 2001). In laboratory and clin
E. coli strains,these compounds have been demonstrated to be
able to reduce by almost90% hemagglutination mediated by
either type 1 or P-pili adherence to BECs and biofilm formatio
mediated by type Ipili (Pinkneret al., 2006).One of these
pilicides,ec240,was found to decrease motility and dysregulate
CUP pili, including type 1, P, and S pili (Greene et al., 2014).
Curli-mediated biofilm formation requires a specific assemb
machinery (Chapman et al.,2002).Curlicides are inhibitors of
both type 1 pilus production and curli biogenesis.Compounds
derived from the peptidomimetic scaffold thatshow pilicide
activity can preventboth Aβ aggregation and curliformation
(Chorellet al.,2011).A small-molecule curlicide (FN075) was
shown to inhibit both type 1 pilus production and curli biogen
by reducing the biofilm and virulence of UPEC in a mouse mo
of experimental cystitis (Cegelski et al., 2009).
Figure 5 depicts the chemical structure of some pilicides a
curlicides.
D-Mannose and D-Mannose-Derived FimH
Antagonists
One of the main strategies to reduce UPEC infection is targeti
bacterialadhesion by inhibiting,for instance,FimH. By using
FIGURE 5 | Structure formulae of pilicide scaffold, some bioactive pilicides, and the curlicide FN075.
Frontiers in Microbiology | www.frontiersin.org 9 August 2017 | Volume 8 | Article 1566
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
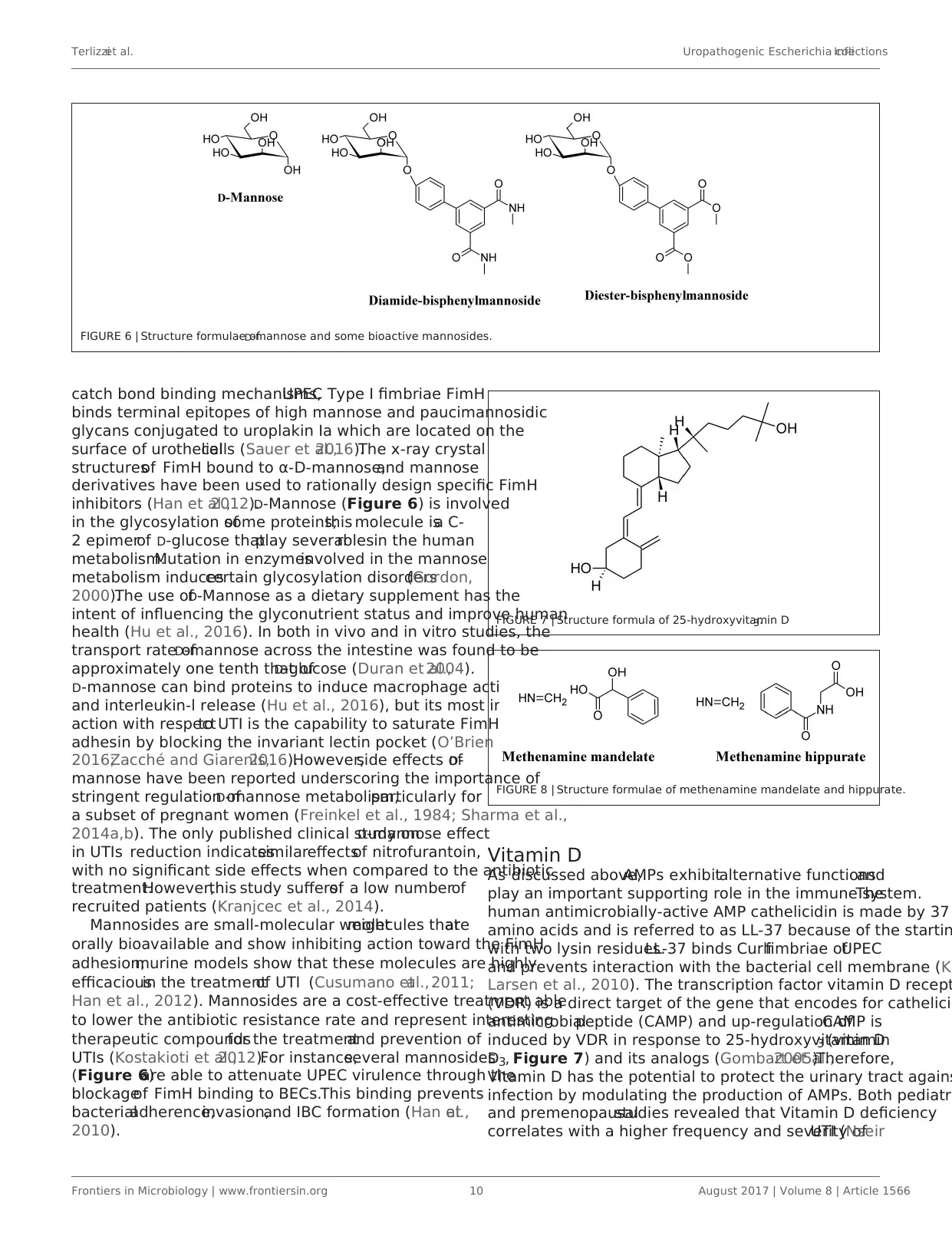
Terlizziet al. Uropathogenic Escherichia coliInfections
FIGURE 6 | Structure formulae ofD-mannose and some bioactive mannosides.
catch bond binding mechanisms,UPEC Type I fimbriae FimH
binds terminal epitopes of high mannose and paucimannosidic
glycans conjugated to uroplakin Ia which are located on the
surface of urothelialcells (Sauer et al.,2016).The x-ray crystal
structuresof FimH bound to α-D-mannose,and mannose
derivatives have been used to rationally design specific FimH
inhibitors (Han et al.,2012).D-Mannose (Figure 6) is involved
in the glycosylation ofsome proteins;this molecule isa C-
2 epimerof D-glucose thatplay severalrolesin the human
metabolism.Mutation in enzymesinvolved in the mannose
metabolism inducescertain glycosylation disorders(Gordon,
2000).The use ofD-Mannose as a dietary supplement has the
intent of influencing the glyconutrient status and improve human
health (Hu et al., 2016). In both in vivo and in vitro studies, the
transport rate ofD-mannose across the intestine was found to be
approximately one tenth that ofD-glucose (Duran et al.,2004).
D-mannose can bind proteins to induce macrophage activation
and interleukin-l release (Hu et al., 2016), but its most important
action with respectto UTI is the capability to saturate FimH
adhesin by blocking the invariant lectin pocket (O’Brien et al.,
2016;Zacché and Giarenis,2016).However,side effects ofD-
mannose have been reported underscoring the importance of
stringent regulation ofD-mannose metabolism,particularly for
a subset of pregnant women (Freinkel et al., 1984; Sharma et al.,
2014a,b). The only published clinical study onD-mannose effect
in UTIs reduction indicatessimilareffectsof nitrofurantoin,
with no significant side effects when compared to the antibiotic
treatment.However,this study suffersof a low numberof
recruited patients (Kranjcec et al., 2014).
Mannosides are small-molecular weightmolecules thatare
orally bioavailable and show inhibiting action toward the FimH
adhesion;murine models show that these molecules are highly
efficaciousin the treatmentof UTI (Cusumano etal., 2011;
Han et al., 2012). Mannosides are a cost-effective treatment able
to lower the antibiotic resistance rate and represent interesting
therapeutic compoundsfor the treatmentand prevention of
UTIs (Kostakioti et al.,2012).For instance,several mannosides
(Figure 6)are able to attenuate UPEC virulence through the
blockageof FimH binding to BECs.This binding prevents
bacterialadherence,invasion,and IBC formation (Han etal.,
2010).
FIGURE 7 | Structure formula of 25-hydroxyvitamin D3.
FIGURE 8 | Structure formulae of methenamine mandelate and hippurate.
Vitamin D
As discussed above,AMPs exhibitalternative functionsand
play an important supporting role in the immune system.The
human antimicrobially-active AMP cathelicidin is made by 37
amino acids and is referred to as LL-37 because of the startin
with two lysin residues.LL-37 binds Curlifimbriae ofUPEC
and prevents interaction with the bacterial cell membrane (Ka
Larsen et al., 2010). The transcription factor vitamin D recept
(VDR) is a direct target of the gene that encodes for cathelicid
antimicrobialpeptide (CAMP) and up-regulation ofCAMP is
induced by VDR in response to 25-hydroxyvitamin D3 (vitamin
D3, Figure 7) and its analogs (Gombart et al.,2005).Therefore,
Vitamin D has the potential to protect the urinary tract agains
infection by modulating the production of AMPs. Both pediatr
and premenopausalstudies revealed that Vitamin D deficiency
correlates with a higher frequency and severity ofUTI (Nseir
Frontiers in Microbiology | www.frontiersin.org 10 August 2017 | Volume 8 | Article 1566
FIGURE 6 | Structure formulae ofD-mannose and some bioactive mannosides.
catch bond binding mechanisms,UPEC Type I fimbriae FimH
binds terminal epitopes of high mannose and paucimannosidic
glycans conjugated to uroplakin Ia which are located on the
surface of urothelialcells (Sauer et al.,2016).The x-ray crystal
structuresof FimH bound to α-D-mannose,and mannose
derivatives have been used to rationally design specific FimH
inhibitors (Han et al.,2012).D-Mannose (Figure 6) is involved
in the glycosylation ofsome proteins;this molecule isa C-
2 epimerof D-glucose thatplay severalrolesin the human
metabolism.Mutation in enzymesinvolved in the mannose
metabolism inducescertain glycosylation disorders(Gordon,
2000).The use ofD-Mannose as a dietary supplement has the
intent of influencing the glyconutrient status and improve human
health (Hu et al., 2016). In both in vivo and in vitro studies, the
transport rate ofD-mannose across the intestine was found to be
approximately one tenth that ofD-glucose (Duran et al.,2004).
D-mannose can bind proteins to induce macrophage activation
and interleukin-l release (Hu et al., 2016), but its most important
action with respectto UTI is the capability to saturate FimH
adhesin by blocking the invariant lectin pocket (O’Brien et al.,
2016;Zacché and Giarenis,2016).However,side effects ofD-
mannose have been reported underscoring the importance of
stringent regulation ofD-mannose metabolism,particularly for
a subset of pregnant women (Freinkel et al., 1984; Sharma et al.,
2014a,b). The only published clinical study onD-mannose effect
in UTIs reduction indicatessimilareffectsof nitrofurantoin,
with no significant side effects when compared to the antibiotic
treatment.However,this study suffersof a low numberof
recruited patients (Kranjcec et al., 2014).
Mannosides are small-molecular weightmolecules thatare
orally bioavailable and show inhibiting action toward the FimH
adhesion;murine models show that these molecules are highly
efficaciousin the treatmentof UTI (Cusumano etal., 2011;
Han et al., 2012). Mannosides are a cost-effective treatment able
to lower the antibiotic resistance rate and represent interesting
therapeutic compoundsfor the treatmentand prevention of
UTIs (Kostakioti et al.,2012).For instance,several mannosides
(Figure 6)are able to attenuate UPEC virulence through the
blockageof FimH binding to BECs.This binding prevents
bacterialadherence,invasion,and IBC formation (Han etal.,
2010).
FIGURE 7 | Structure formula of 25-hydroxyvitamin D3.
FIGURE 8 | Structure formulae of methenamine mandelate and hippurate.
Vitamin D
As discussed above,AMPs exhibitalternative functionsand
play an important supporting role in the immune system.The
human antimicrobially-active AMP cathelicidin is made by 37
amino acids and is referred to as LL-37 because of the startin
with two lysin residues.LL-37 binds Curlifimbriae ofUPEC
and prevents interaction with the bacterial cell membrane (Ka
Larsen et al., 2010). The transcription factor vitamin D recept
(VDR) is a direct target of the gene that encodes for cathelicid
antimicrobialpeptide (CAMP) and up-regulation ofCAMP is
induced by VDR in response to 25-hydroxyvitamin D3 (vitamin
D3, Figure 7) and its analogs (Gombart et al.,2005).Therefore,
Vitamin D has the potential to protect the urinary tract agains
infection by modulating the production of AMPs. Both pediatr
and premenopausalstudies revealed that Vitamin D deficiency
correlates with a higher frequency and severity ofUTI (Nseir
Frontiers in Microbiology | www.frontiersin.org 10 August 2017 | Volume 8 | Article 1566
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
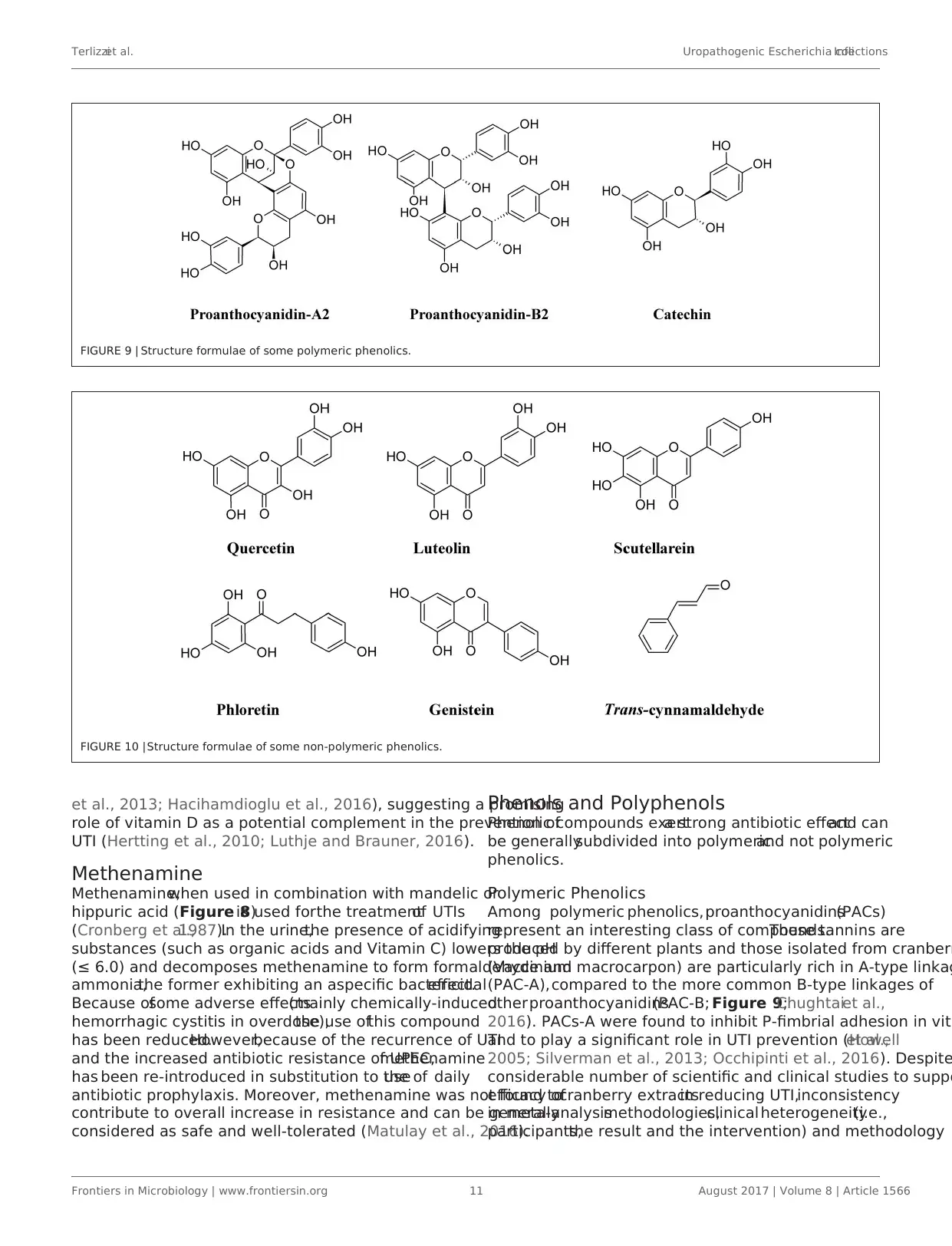
Terlizziet al. Uropathogenic Escherichia coliInfections
FIGURE 9 | Structure formulae of some polymeric phenolics.
FIGURE 10 |Structure formulae of some non-polymeric phenolics.
et al., 2013; Hacihamdioglu et al., 2016), suggesting a promising
role of vitamin D as a potential complement in the prevention of
UTI (Hertting et al., 2010; Luthje and Brauner, 2016).
Methenamine
Methenamine,when used in combination with mandelic or
hippuric acid (Figure 8)is used forthe treatmentof UTIs
(Cronberg et al.,1987).In the urine,the presence of acidifying
substances (such as organic acids and Vitamin C) lowers the pH
(≤ 6.0) and decomposes methenamine to form formaldehyde and
ammonia,the former exhibiting an aspecific bactericidaleffect.
Because ofsome adverse effects(mainly chemically-induced
hemorrhagic cystitis in overdose),the use ofthis compound
has been reduced.However,because of the recurrence of UTI
and the increased antibiotic resistance of UPEC,methenamine
has been re-introduced in substitution to theuse of daily
antibiotic prophylaxis. Moreover, methenamine was not found to
contribute to overall increase in resistance and can be generally
considered as safe and well-tolerated (Matulay et al., 2016).
Phenols and Polyphenols
Phenolic compounds exerta strong antibiotic effectand can
be generallysubdivided into polymericand not polymeric
phenolics.
Polymeric Phenolics
Among polymeric phenolics,proanthocyanidins(PACs)
represent an interesting class of compounds.These tannins are
produced by different plants and those isolated from cranberr
(Vaccinium macrocarpon) are particularly rich in A-type linkag
(PAC-A),compared to the more common B-type linkages of
otherproanthocyanidins(PAC-B; Figure 9;Chughtaiet al.,
2016). PACs-A were found to inhibit P-fimbrial adhesion in vitr
and to play a significant role in UTI prevention (Howellet al.,
2005; Silverman et al., 2013; Occhipinti et al., 2016). Despite
considerable number of scientific and clinical studies to suppo
efficacy ofcranberry extractsin reducing UTI,inconsistency
in meta-analysismethodologies,clinical heterogeneity(i.e.,
participants,the result and the intervention) and methodology
Frontiers in Microbiology | www.frontiersin.org 11 August 2017 | Volume 8 | Article 1566
FIGURE 9 | Structure formulae of some polymeric phenolics.
FIGURE 10 |Structure formulae of some non-polymeric phenolics.
et al., 2013; Hacihamdioglu et al., 2016), suggesting a promising
role of vitamin D as a potential complement in the prevention of
UTI (Hertting et al., 2010; Luthje and Brauner, 2016).
Methenamine
Methenamine,when used in combination with mandelic or
hippuric acid (Figure 8)is used forthe treatmentof UTIs
(Cronberg et al.,1987).In the urine,the presence of acidifying
substances (such as organic acids and Vitamin C) lowers the pH
(≤ 6.0) and decomposes methenamine to form formaldehyde and
ammonia,the former exhibiting an aspecific bactericidaleffect.
Because ofsome adverse effects(mainly chemically-induced
hemorrhagic cystitis in overdose),the use ofthis compound
has been reduced.However,because of the recurrence of UTI
and the increased antibiotic resistance of UPEC,methenamine
has been re-introduced in substitution to theuse of daily
antibiotic prophylaxis. Moreover, methenamine was not found to
contribute to overall increase in resistance and can be generally
considered as safe and well-tolerated (Matulay et al., 2016).
Phenols and Polyphenols
Phenolic compounds exerta strong antibiotic effectand can
be generallysubdivided into polymericand not polymeric
phenolics.
Polymeric Phenolics
Among polymeric phenolics,proanthocyanidins(PACs)
represent an interesting class of compounds.These tannins are
produced by different plants and those isolated from cranberr
(Vaccinium macrocarpon) are particularly rich in A-type linkag
(PAC-A),compared to the more common B-type linkages of
otherproanthocyanidins(PAC-B; Figure 9;Chughtaiet al.,
2016). PACs-A were found to inhibit P-fimbrial adhesion in vitr
and to play a significant role in UTI prevention (Howellet al.,
2005; Silverman et al., 2013; Occhipinti et al., 2016). Despite
considerable number of scientific and clinical studies to suppo
efficacy ofcranberry extractsin reducing UTI,inconsistency
in meta-analysismethodologies,clinical heterogeneity(i.e.,
participants,the result and the intervention) and methodology
Frontiers in Microbiology | www.frontiersin.org 11 August 2017 | Volume 8 | Article 1566
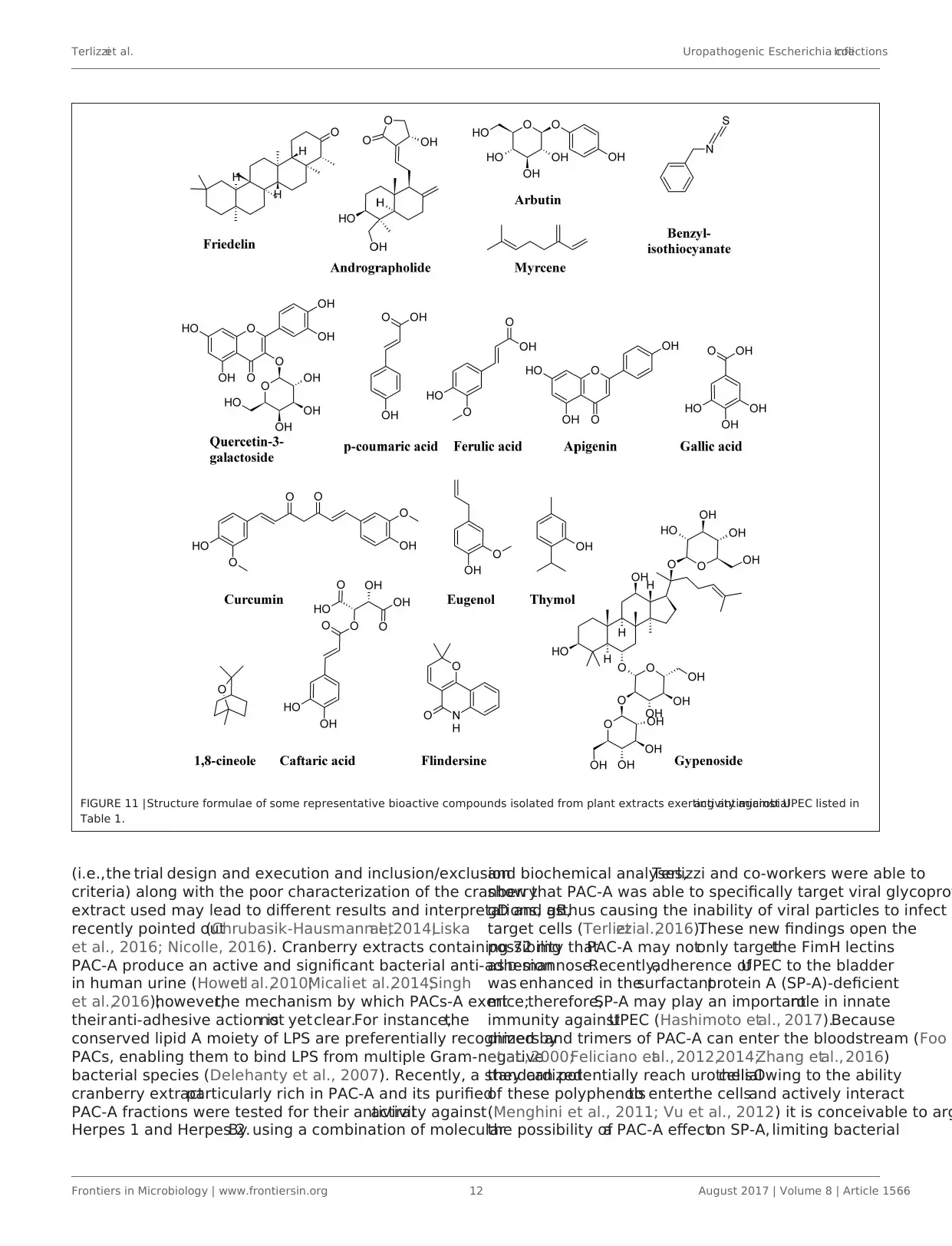
Terlizziet al. Uropathogenic Escherichia coliInfections
FIGURE 11 |Structure formulae of some representative bioactive compounds isolated from plant extracts exerting antimicrobialactivity against UPEC listed in
Table 1.
(i.e.,the trial design and execution and inclusion/exclusion
criteria) along with the poor characterization of the cranberry
extract used may lead to different results and interpretations, as
recently pointed out(Chrubasik-Hausmann etal.,2014;Liska
et al., 2016; Nicolle, 2016). Cranberry extracts containing 72 mg
PAC-A produce an active and significant bacterial anti-adhesion
in human urine (Howellet al.,2010;Micaliet al.,2014;Singh
et al.,2016);however,the mechanism by which PACs-A exert
theiranti-adhesive action isnot yetclear.For instance,the
conserved lipid A moiety of LPS are preferentially recognized by
PACs, enabling them to bind LPS from multiple Gram-negative
bacterial species (Delehanty et al., 2007). Recently, a standardized
cranberry extractparticularly rich in PAC-A and its purified
PAC-A fractions were tested for their antiviralactivity against
Herpes 1 and Herpes 2.By using a combination of molecular
and biochemical analyses,Terlizzi and co-workers were able to
show that PAC-A was able to specifically target viral glycopro
gD and gB,thus causing the inability of viral particles to infect
target cells (Terlizziet al.,2016).These new findings open the
possibility thatPAC-A may notonly targetthe FimH lectins
as D-mannose.Recently,adherence ofUPEC to the bladder
was enhanced in thesurfactantprotein A (SP-A)-deficient
mice;therefore,SP-A may play an importantrole in innate
immunity againstUPEC (Hashimoto etal., 2017).Because
dimers and trimers of PAC-A can enter the bloodstream (Foo
et al., 2000;Feliciano etal., 2012,2014;Zhang etal., 2016)
they can potentially reach urothelialcells.Owing to the ability
of these polyphenolsto enterthe cellsand actively interact
(Menghini et al., 2011; Vu et al., 2012) it is conceivable to arg
the possibility ofa PAC-A effecton SP-A, limiting bacterial
Frontiers in Microbiology | www.frontiersin.org 12 August 2017 | Volume 8 | Article 1566
FIGURE 11 |Structure formulae of some representative bioactive compounds isolated from plant extracts exerting antimicrobialactivity against UPEC listed in
Table 1.
(i.e.,the trial design and execution and inclusion/exclusion
criteria) along with the poor characterization of the cranberry
extract used may lead to different results and interpretations, as
recently pointed out(Chrubasik-Hausmann etal.,2014;Liska
et al., 2016; Nicolle, 2016). Cranberry extracts containing 72 mg
PAC-A produce an active and significant bacterial anti-adhesion
in human urine (Howellet al.,2010;Micaliet al.,2014;Singh
et al.,2016);however,the mechanism by which PACs-A exert
theiranti-adhesive action isnot yetclear.For instance,the
conserved lipid A moiety of LPS are preferentially recognized by
PACs, enabling them to bind LPS from multiple Gram-negative
bacterial species (Delehanty et al., 2007). Recently, a standardized
cranberry extractparticularly rich in PAC-A and its purified
PAC-A fractions were tested for their antiviralactivity against
Herpes 1 and Herpes 2.By using a combination of molecular
and biochemical analyses,Terlizzi and co-workers were able to
show that PAC-A was able to specifically target viral glycopro
gD and gB,thus causing the inability of viral particles to infect
target cells (Terlizziet al.,2016).These new findings open the
possibility thatPAC-A may notonly targetthe FimH lectins
as D-mannose.Recently,adherence ofUPEC to the bladder
was enhanced in thesurfactantprotein A (SP-A)-deficient
mice;therefore,SP-A may play an importantrole in innate
immunity againstUPEC (Hashimoto etal., 2017).Because
dimers and trimers of PAC-A can enter the bloodstream (Foo
et al., 2000;Feliciano etal., 2012,2014;Zhang etal., 2016)
they can potentially reach urothelialcells.Owing to the ability
of these polyphenolsto enterthe cellsand actively interact
(Menghini et al., 2011; Vu et al., 2012) it is conceivable to arg
the possibility ofa PAC-A effecton SP-A, limiting bacterial
Frontiers in Microbiology | www.frontiersin.org 12 August 2017 | Volume 8 | Article 1566
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 23
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





