Financial Analysis of Vectura Pharmaceutical Company Report
VerifiedAdded on 2021/05/30
|14
|3522
|21
Report
AI Summary
This report provides a detailed financial analysis of Vectura Pharmaceutical Company, a leading UK-based pharmaceutical company. It examines the company's strategy, focusing on maximizing value and leveraging its technology to address airways diseases. The report delves into Vectura's product portfolio, categorizing them into inhaled, non-inhaled, and oral products, and analyzes the revenue generated from each segment, including key products like Flutiform, Seebri Breezhaler, Ultibro Breezhaler, and GSK Ellipta products. It also reviews the company's financial performance, including revenue streams from royalties, product sales, and development services, comparing the financial results of 2017 and 2016. The report discusses employee compensation, retirement obligations, and key financial metrics, offering insights into Vectura's financial health and strategic direction within the pharmaceutical industry.

Vectura Pharmaceutical Company 1
Financial Decision Making: Vectura Pharmaceutical Company
By Student’s Name
Code+ course name
Professor’s name
University name
City, State
Date
Financial Decision Making: Vectura Pharmaceutical Company
By Student’s Name
Code+ course name
Professor’s name
University name
City, State
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Vectura Pharmaceutical Company 2
Financial Decision Making: Vectura Pharmaceutical Company
Introduction
About Vectura location
The Vectura is a leading company in Britain pharmaceutical industry and it is based in
Chippenham, UK. The company was founded by David Anthony. Besides the UK market, the
company also operates in France, Germany and United States of America. It is also listed as a
stock exchange company in the London Stock Exchange market (Apte, 2014, p. 45). The Vectura
begun in 1997 as a pharmaceutical company. 1n 1999 after acquiring a coordinated drug
development Vectura became a drug formulation study Centre. In 2007, Vectura was listed as an
investment market. To increase its market share and profitability, Vectura merged with
Skyepharma in 2016 under the name Vectura (The merged company was not renamed). The
company engages in commercialization, development, and research of therapeutic products.
Besides, development of pharmaceutical products, the company is also a founder of inhaled
therapies aimed at treating respiratory ailments also known as airways diseases. Vectura is
among the leading companies globally that has expertise in developing, designing and delivering
a wide range of products.
With vast capabilities and expertise, the company has the ability to utilize resources, dry powder,
solutions, and suspension. Moreover, Vectura has approximately 140 device engineers, and
biotechnological and pharmaceutical scientists who engage in the development of a wide range
of products. With a deep experience and vertical integrated capability (VIC), Vectura will
provide differentiated and unique medicines which meet specific need of both its partners and
clients. Vectura will be a strong company in the UK market. Besides over 2000 partners in the
Financial Decision Making: Vectura Pharmaceutical Company
Introduction
About Vectura location
The Vectura is a leading company in Britain pharmaceutical industry and it is based in
Chippenham, UK. The company was founded by David Anthony. Besides the UK market, the
company also operates in France, Germany and United States of America. It is also listed as a
stock exchange company in the London Stock Exchange market (Apte, 2014, p. 45). The Vectura
begun in 1997 as a pharmaceutical company. 1n 1999 after acquiring a coordinated drug
development Vectura became a drug formulation study Centre. In 2007, Vectura was listed as an
investment market. To increase its market share and profitability, Vectura merged with
Skyepharma in 2016 under the name Vectura (The merged company was not renamed). The
company engages in commercialization, development, and research of therapeutic products.
Besides, development of pharmaceutical products, the company is also a founder of inhaled
therapies aimed at treating respiratory ailments also known as airways diseases. Vectura is
among the leading companies globally that has expertise in developing, designing and delivering
a wide range of products.
With vast capabilities and expertise, the company has the ability to utilize resources, dry powder,
solutions, and suspension. Moreover, Vectura has approximately 140 device engineers, and
biotechnological and pharmaceutical scientists who engage in the development of a wide range
of products. With a deep experience and vertical integrated capability (VIC), Vectura will
provide differentiated and unique medicines which meet specific need of both its partners and
clients. Vectura will be a strong company in the UK market. Besides over 2000 partners in the

Vectura Pharmaceutical Company 3
supply chain, Vectura has at least 478 workers working on a permanent basis (Inamdar, 2014, p.
567)
A. Company Strategy
The Vectura’s strategy is based on maximizing value, leverage its skills and unique technology.
The company aims at changing the lives of patients with airways diseases through enhancing
performance and delivery of inhaled products. The objective will be fulfilled by offering quality
generic products as an alternative to branded therapies. Airways diseases comprise of illnesses
such as COPD, cystic fibrosis, and asthma (Jackhotiya, 2018, p. 154). According to a study by
Jackhotiya (2018, p. 154), the number of patients suffering from airways diseases is expected to
increase in One hundred million in the next six years. At the moment the market is valued at
$40bn and is expected to increase to $56bn by the year 2025.
In 2018, the company aims at maintaining its strong cash flow and profitability from in-market
sales. This will help to reinvest in new opportunities in the market as a way of wealth
maximization and offering solutions to a larger market. Generic drugs and enhanced therapies
are new products in the market and Vectura will use this as a competitive advantage to attract
more consumers. The company also considers Merger and acquisitions as a strategy to increase
their market growth and expansion.
Vectura is operating within a large airways market and the company’s growth directly depends
on the performance of its products in the market. Likewise, Vectura has a potential of increasing
its revenue from business research and development. In 2018, the cost of a Research
&Development sector of the company is expected to reduce from $ 65 million to $ 55 million.
supply chain, Vectura has at least 478 workers working on a permanent basis (Inamdar, 2014, p.
567)
A. Company Strategy
The Vectura’s strategy is based on maximizing value, leverage its skills and unique technology.
The company aims at changing the lives of patients with airways diseases through enhancing
performance and delivery of inhaled products. The objective will be fulfilled by offering quality
generic products as an alternative to branded therapies. Airways diseases comprise of illnesses
such as COPD, cystic fibrosis, and asthma (Jackhotiya, 2018, p. 154). According to a study by
Jackhotiya (2018, p. 154), the number of patients suffering from airways diseases is expected to
increase in One hundred million in the next six years. At the moment the market is valued at
$40bn and is expected to increase to $56bn by the year 2025.
In 2018, the company aims at maintaining its strong cash flow and profitability from in-market
sales. This will help to reinvest in new opportunities in the market as a way of wealth
maximization and offering solutions to a larger market. Generic drugs and enhanced therapies
are new products in the market and Vectura will use this as a competitive advantage to attract
more consumers. The company also considers Merger and acquisitions as a strategy to increase
their market growth and expansion.
Vectura is operating within a large airways market and the company’s growth directly depends
on the performance of its products in the market. Likewise, Vectura has a potential of increasing
its revenue from business research and development. In 2018, the cost of a Research
&Development sector of the company is expected to reduce from $ 65 million to $ 55 million.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Vectura Pharmaceutical Company 4
The Expenditure on R&D activities is expected to reduce further from $55 million to $45 million
in 2019.
With a focusing on increasing their sales. The company’s sales were $103.7 million and is
projected to grow by 10% in 2018. Moreover, reducing the total expenditures is another option
of increasing profitability. However, the Capital expenditure is expected to increase from $10
million to $15 million due to the proposed expansions and innovative activities in 2018 (Kohok,
2015, p. 234).
B. Products and services
Vectura being a revenue-generating company has a variety of product. The products are
categorized into three segments namely oral, inhaled and non-inhaled i.e. ten oral products, two
non-inhaled products, and eight inhaled products. The products are marketed and sold by
partners of global relationship and diverse portfolio in clinical development. Our key partners are
Novartis, Hikma, Mundipharma, Baxter, Sandoz, UCB, GSK, Bayer, Ablynx, Kyorin, Almirall,
Chiesi, Janssen, Tianjin, and Dynavax. The company acquires royalties from the selling seven
products while revenue is realized from selling Flutiform to Kyorin and Mundipharma
companies (Kulkarni, 2017, p. 345).
I. Inhaled products
a) Flutiform
Flutiform is a combination of ICS (inhaled anti-inflammatory and LABA (a bronchodilator) in a
pMDI device. The revenue earned from selling the products to Kyorin and Mundipharma
companies it is expected to increase to $30m in future. Currently, the product is sold in the UK,
but the market is expected to expand into the European countries and the US. At the end of 2017,
The Expenditure on R&D activities is expected to reduce further from $55 million to $45 million
in 2019.
With a focusing on increasing their sales. The company’s sales were $103.7 million and is
projected to grow by 10% in 2018. Moreover, reducing the total expenditures is another option
of increasing profitability. However, the Capital expenditure is expected to increase from $10
million to $15 million due to the proposed expansions and innovative activities in 2018 (Kohok,
2015, p. 234).
B. Products and services
Vectura being a revenue-generating company has a variety of product. The products are
categorized into three segments namely oral, inhaled and non-inhaled i.e. ten oral products, two
non-inhaled products, and eight inhaled products. The products are marketed and sold by
partners of global relationship and diverse portfolio in clinical development. Our key partners are
Novartis, Hikma, Mundipharma, Baxter, Sandoz, UCB, GSK, Bayer, Ablynx, Kyorin, Almirall,
Chiesi, Janssen, Tianjin, and Dynavax. The company acquires royalties from the selling seven
products while revenue is realized from selling Flutiform to Kyorin and Mundipharma
companies (Kulkarni, 2017, p. 345).
I. Inhaled products
a) Flutiform
Flutiform is a combination of ICS (inhaled anti-inflammatory and LABA (a bronchodilator) in a
pMDI device. The revenue earned from selling the products to Kyorin and Mundipharma
companies it is expected to increase to $30m in future. Currently, the product is sold in the UK,
but the market is expected to expand into the European countries and the US. At the end of 2017,
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Vectura Pharmaceutical Company 5
the sales of Flutiform increased by 11.8% to $206.2 million thus generating $68.5 million
revenue and royalty for the company. The revenue increased from destocking reported
previously in the supply market. The product is marketed by partners in the ROW and Europe.
Upon approval by DCP and MHRA, the launch of enhanced flutiform k-haler will help in the
market expansion and increased revenue (Pandey, 2013, p. 432).
b) Seebri Breezhaler and Ultibro Breezhaler
Currently, Seebri Breezhaler and Ultibro Breezhaler are substantial products launched outside
US. In 2017 Novartis reported a total sale of $562M thereafter Vectura earned $17.3M of
royalties. These products are supplied by Novartis in ROW and EU.
Ultibro Breezhaler is a dual inhaled bronchodilator (LABA/LAMA) which is meant to relieve
patients with COPD. This product has been launched in more than one hundred countries such as
Europe, Japan, and China. Net sales increase by 12% annually due to restocking. In 2017, a
positive growth of 18% was recorded in translating to 76.3% IMS. The growth was as a result of
GOLD changes, FLASH study, and FLAME study, which recommended LABA/LAMA as a
reliever of symptomatic patients. In 2017, Ultibro Breezhaler recorded a net sale $151 million.
c) GSK Ellipta products.
The company earns royalties from Breo, Anoro and Increase Ellipta products. Vectura partners
in the sales of GSK Ellipta products are GSK and Skypharma. Earnings from the product was
subject to a $9 million annual Cap in 2017. The product has been established in 35 countries with
a reported sales of $3.8 million in 2017 compared to $4.9 million in 2016. The reduction in the
revenues arose as a result of restocking in the supply chain (Pritchard, et al., 2018, p. 34).
the sales of Flutiform increased by 11.8% to $206.2 million thus generating $68.5 million
revenue and royalty for the company. The revenue increased from destocking reported
previously in the supply market. The product is marketed by partners in the ROW and Europe.
Upon approval by DCP and MHRA, the launch of enhanced flutiform k-haler will help in the
market expansion and increased revenue (Pandey, 2013, p. 432).
b) Seebri Breezhaler and Ultibro Breezhaler
Currently, Seebri Breezhaler and Ultibro Breezhaler are substantial products launched outside
US. In 2017 Novartis reported a total sale of $562M thereafter Vectura earned $17.3M of
royalties. These products are supplied by Novartis in ROW and EU.
Ultibro Breezhaler is a dual inhaled bronchodilator (LABA/LAMA) which is meant to relieve
patients with COPD. This product has been launched in more than one hundred countries such as
Europe, Japan, and China. Net sales increase by 12% annually due to restocking. In 2017, a
positive growth of 18% was recorded in translating to 76.3% IMS. The growth was as a result of
GOLD changes, FLASH study, and FLAME study, which recommended LABA/LAMA as a
reliever of symptomatic patients. In 2017, Ultibro Breezhaler recorded a net sale $151 million.
c) GSK Ellipta products.
The company earns royalties from Breo, Anoro and Increase Ellipta products. Vectura partners
in the sales of GSK Ellipta products are GSK and Skypharma. Earnings from the product was
subject to a $9 million annual Cap in 2017. The product has been established in 35 countries with
a reported sales of $3.8 million in 2017 compared to $4.9 million in 2016. The reduction in the
revenues arose as a result of restocking in the supply chain (Pritchard, et al., 2018, p. 34).

Vectura Pharmaceutical Company 6
d) Breelib
Breelib is an alternative drug in treating pulmonary HP. The product was established in Europe
by Bayer Company in 2017. It was then launched in Germany, Poland, Austria, and Portugal
markets. Vectura Company earned approximately $5.0 million in 2017.
II. Non-inhaled and Oral
In addition to the main focus in airways ailments, the company generates revenue from non-
inhaled and oral products. The revenue generated in 2017 was $24.3M in 2017 compared to
$23.7M in 2016.
The company enjoys capabilities, expertise, and technology for manufacturing Oral products.
Investment in the sector is moderate, but it is Vectura expect that the segment will develop fully
within the next three years. The five products established under non-inhaled and oral products
that have been manufactured using Geomatrix technologies are coruno, Madopar DR,
Diclofenactrationpharmuno sular and ZYFLO CR while RAYOS/Lodotra uses Geoclock
chronotechnology (RAO, 2017, p. 53).
i) EXPAREL
The EXPAREL is an injectable dose that provides post-surgical Analgesia. The company
received a 3% share from $ 6.6 million sales in 2017. With new investments and market
expansions, Vectura expects sales to grow by $32 million by the year 2025.
d) Breelib
Breelib is an alternative drug in treating pulmonary HP. The product was established in Europe
by Bayer Company in 2017. It was then launched in Germany, Poland, Austria, and Portugal
markets. Vectura Company earned approximately $5.0 million in 2017.
II. Non-inhaled and Oral
In addition to the main focus in airways ailments, the company generates revenue from non-
inhaled and oral products. The revenue generated in 2017 was $24.3M in 2017 compared to
$23.7M in 2016.
The company enjoys capabilities, expertise, and technology for manufacturing Oral products.
Investment in the sector is moderate, but it is Vectura expect that the segment will develop fully
within the next three years. The five products established under non-inhaled and oral products
that have been manufactured using Geomatrix technologies are coruno, Madopar DR,
Diclofenactrationpharmuno sular and ZYFLO CR while RAYOS/Lodotra uses Geoclock
chronotechnology (RAO, 2017, p. 53).
i) EXPAREL
The EXPAREL is an injectable dose that provides post-surgical Analgesia. The company
received a 3% share from $ 6.6 million sales in 2017. With new investments and market
expansions, Vectura expects sales to grow by $32 million by the year 2025.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Vectura Pharmaceutical Company 7
ii) Advate
Advate treats haemophilia and is sold by the Baxter Company. Vectura earns royalties from the
sales of these products. In 2017, the sale of the product amounted to $1 million. The product is
considered to be at its maturity stage and Vectura does not expect additional royalties (Barr RG,
2010, p. 23).
iii) Solarize
Solarize is an NSAID that treats actinic keratosis exposed from the sun. Vectura earned royalties
amounting to $2.9 million in 2017 compared to $6.7 million in 2016.
iv) Adept
Adept is used when administering surgery to relieve adhesions after abdominal and
gynecological surgery. Royalties earned from this product are minimal, however, the company is
focusing on R&D as an investment strategy is to increase value drivers of Adept in the market.
C. Employees and Retirement obligations
The company has employees who are compensated with equity, besides the overall reward
strategy. This compensation scheme is measured on a fair value at a period of issue. The
compensation also depends on the increase in equity over the period. The company has a pension
scheme for its employees in accordance with Swish law (Chen & Sadatsafavi M. , 2015, p. 545).
The scheme involves guarantees in accordance with IFRS. It is measured as PV for Estimated
cash flows. When the client is curtailed or when the scheme changes, the profit or loss is
reconciled in the consolidated income statement. Vectura provides funds to ESOP trusts for the
ii) Advate
Advate treats haemophilia and is sold by the Baxter Company. Vectura earns royalties from the
sales of these products. In 2017, the sale of the product amounted to $1 million. The product is
considered to be at its maturity stage and Vectura does not expect additional royalties (Barr RG,
2010, p. 23).
iii) Solarize
Solarize is an NSAID that treats actinic keratosis exposed from the sun. Vectura earned royalties
amounting to $2.9 million in 2017 compared to $6.7 million in 2016.
iv) Adept
Adept is used when administering surgery to relieve adhesions after abdominal and
gynecological surgery. Royalties earned from this product are minimal, however, the company is
focusing on R&D as an investment strategy is to increase value drivers of Adept in the market.
C. Employees and Retirement obligations
The company has employees who are compensated with equity, besides the overall reward
strategy. This compensation scheme is measured on a fair value at a period of issue. The
compensation also depends on the increase in equity over the period. The company has a pension
scheme for its employees in accordance with Swish law (Chen & Sadatsafavi M. , 2015, p. 545).
The scheme involves guarantees in accordance with IFRS. It is measured as PV for Estimated
cash flows. When the client is curtailed or when the scheme changes, the profit or loss is
reconciled in the consolidated income statement. Vectura provides funds to ESOP trusts for the
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Vectura Pharmaceutical Company 8
purchase of shares on the market. Employees can also subscribe new issued capital. Shares are
subtracted from reserves.
Workers at French manufacturing and Swiss R&D included 74% due to Skyepharma merger.
Apart from full-time workers, we also have fixed-term contracts with employees to aid in the
delivery and integration, maternity and paternity illness. Remuneration included wages and
salaries, aggregate remuneration, social security cost and payment of pension plans. Other
payments include director’s remuneration and key personnel payments (Frith, et al., 2017, p.
678).
D. Turnover
In the year ended 31st Dec 2017, the company earned 75% of total revenue from the sale of
products (2016) all products were launched in 2000 and generated excess revenue of $2.8 billion
one year. The company realized $148 million in 2017 as revenue which is in line with the
company expectations as compared to $126.5 million 2016.
With the strength in the market performance, delivery strategy and excellence I expect to
maintain revenue in 2018 with R&D investment ranging from $55M to 65M.
E. Financial review
Comparison of the annual financial results for 2017 and 2016 showed distorted results due to
various factors such as change of accounting period, Skypharma merger and reduced revenue
resulting from non-recurring streams. This is information was obtained from audited reports
which have been prepared in accordance with IFRS and accounting policies. The financial
information is important because it allows evaluation of a company’s performance as well as in
decision making.
purchase of shares on the market. Employees can also subscribe new issued capital. Shares are
subtracted from reserves.
Workers at French manufacturing and Swiss R&D included 74% due to Skyepharma merger.
Apart from full-time workers, we also have fixed-term contracts with employees to aid in the
delivery and integration, maternity and paternity illness. Remuneration included wages and
salaries, aggregate remuneration, social security cost and payment of pension plans. Other
payments include director’s remuneration and key personnel payments (Frith, et al., 2017, p.
678).
D. Turnover
In the year ended 31st Dec 2017, the company earned 75% of total revenue from the sale of
products (2016) all products were launched in 2000 and generated excess revenue of $2.8 billion
one year. The company realized $148 million in 2017 as revenue which is in line with the
company expectations as compared to $126.5 million 2016.
With the strength in the market performance, delivery strategy and excellence I expect to
maintain revenue in 2018 with R&D investment ranging from $55M to 65M.
E. Financial review
Comparison of the annual financial results for 2017 and 2016 showed distorted results due to
various factors such as change of accounting period, Skypharma merger and reduced revenue
resulting from non-recurring streams. This is information was obtained from audited reports
which have been prepared in accordance with IFRS and accounting policies. The financial
information is important because it allows evaluation of a company’s performance as well as in
decision making.

Vectura Pharmaceutical Company 9
The statements have been drawn on a pro forma basis in order to show the performance of the
business. There is low income from non-recurring activities relating to R&D development
service and licensing in 2016. Low royalties was also recorded as a result of expiry of several
products before the sales (Gold, 2017, p. 134).
i) Revenue
Vectura generates revenue from royalties, products supply, device revenue, development, service
milestones and signing payments. Underlying revenue improved by 4% showing a strong
performance of products in the market. Income from flutiform and Ultibro rose by 5.3% to
$86.7% million because of increased stocking, and high sales of oral products and EXPAREL.
2017 Revenue Summary
ITEMS Amount ($) in Million
Development services 9
Royalties 52.6
Signing and milestone payments 5.1
Device sales $ Product supply 74.7
Other Revenue 6.6
TOTAL REVENUE 148
Revenue from device sales of product was $74.7 million in 2017 as compared to $72.6 million in
2016. The sales from supplying flutiform to Kyorin and Mundipharma increased from $60.8
million to $63.4 million. This increase is as a result of increased customer demand and
destocking (Watz, 2010, p. 45).
The statements have been drawn on a pro forma basis in order to show the performance of the
business. There is low income from non-recurring activities relating to R&D development
service and licensing in 2016. Low royalties was also recorded as a result of expiry of several
products before the sales (Gold, 2017, p. 134).
i) Revenue
Vectura generates revenue from royalties, products supply, device revenue, development, service
milestones and signing payments. Underlying revenue improved by 4% showing a strong
performance of products in the market. Income from flutiform and Ultibro rose by 5.3% to
$86.7% million because of increased stocking, and high sales of oral products and EXPAREL.
2017 Revenue Summary
ITEMS Amount ($) in Million
Development services 9
Royalties 52.6
Signing and milestone payments 5.1
Device sales $ Product supply 74.7
Other Revenue 6.6
TOTAL REVENUE 148
Revenue from device sales of product was $74.7 million in 2017 as compared to $72.6 million in
2016. The sales from supplying flutiform to Kyorin and Mundipharma increased from $60.8
million to $63.4 million. This increase is as a result of increased customer demand and
destocking (Watz, 2010, p. 45).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Vectura Pharmaceutical Company 10
Royalties
In 2017, royalties grew by 4.6% from non-inhaled and inhaled products. Total royalties from
seebri and Ultibro breezehaler were $17 million in 2017 as compared to $15.6 million in 2016.
Seebri and Ultibro breezehaler net sales were $411million in 2017 posting an improvement of
12%. The increase was as a result of normalizing stock. Royalties from flutiform sales grew as a
result of increased demand of the product in Japan. In 2017, royalties contributed to $5.1 million
revenue as compared to $5 million in 2016. The total sales for Ventura were $206.2 million
resulting to 11.8% increase compared to the total sales in 2016. The increase in sales came from
an agreement between Vectura and Mundipharma which reduced operating cost between the two
companies by 35%. Royalties from Kyorin rose by 18%. Lastly, Moderate contribution was
received from AirFlusal Forspiro. In total, Vectura earned $2.3 million royalties in 2017 as
compared to $1.8M in 2016.
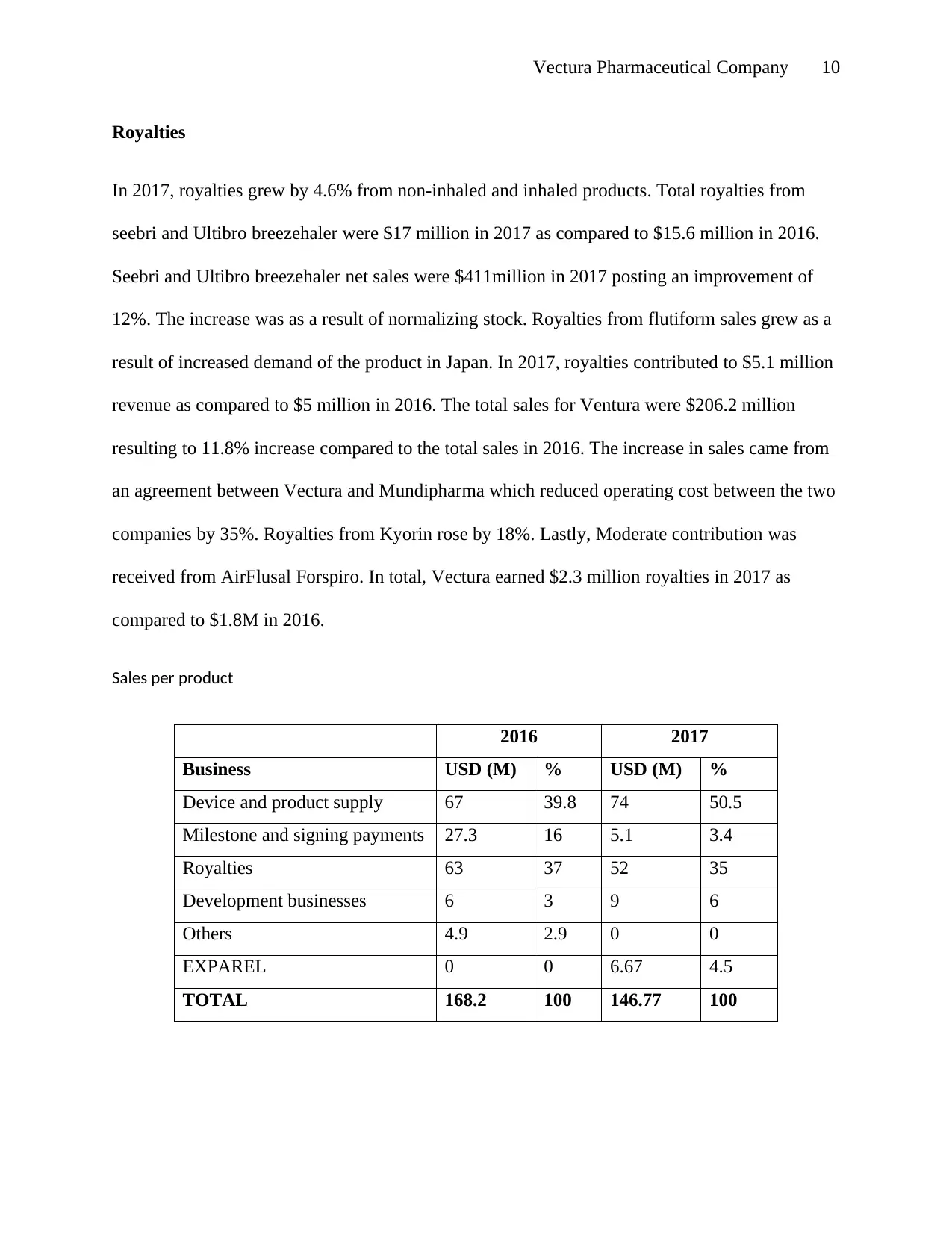
Sales per product
2016 2017
Business USD (M) % USD (M) %
Device and product supply 67 39.8 74 50.5
Milestone and signing payments 27.3 16 5.1 3.4
Royalties 63 37 52 35
Development businesses 6 3 9 6
Others 4.9 2.9 0 0
EXPAREL 0 0 6.67 4.5
TOTAL 168.2 100 146.77 100
Royalties
In 2017, royalties grew by 4.6% from non-inhaled and inhaled products. Total royalties from
seebri and Ultibro breezehaler were $17 million in 2017 as compared to $15.6 million in 2016.
Seebri and Ultibro breezehaler net sales were $411million in 2017 posting an improvement of
12%. The increase was as a result of normalizing stock. Royalties from flutiform sales grew as a
result of increased demand of the product in Japan. In 2017, royalties contributed to $5.1 million
revenue as compared to $5 million in 2016. The total sales for Ventura were $206.2 million
resulting to 11.8% increase compared to the total sales in 2016. The increase in sales came from
an agreement between Vectura and Mundipharma which reduced operating cost between the two
companies by 35%. Royalties from Kyorin rose by 18%. Lastly, Moderate contribution was
received from AirFlusal Forspiro. In total, Vectura earned $2.3 million royalties in 2017 as
compared to $1.8M in 2016.
Sales per product
2016 2017
Business USD (M) % USD (M) %
Device and product supply 67 39.8 74 50.5
Milestone and signing payments 27.3 16 5.1 3.4
Royalties 63 37 52 35
Development businesses 6 3 9 6
Others 4.9 2.9 0 0
EXPAREL 0 0 6.67 4.5
TOTAL 168.2 100 146.77 100
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Vectura Pharmaceutical Company 11
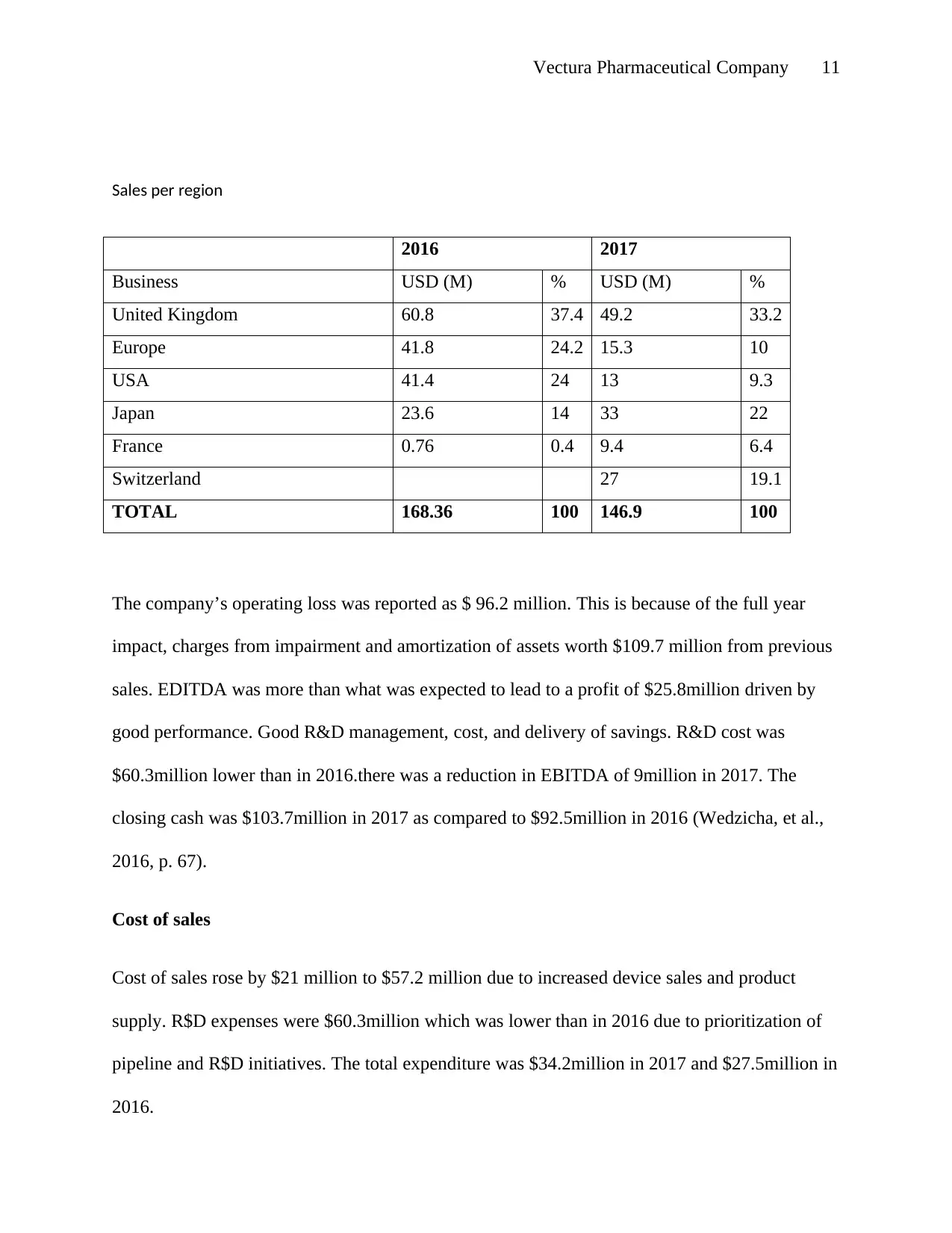
Sales per region
2016 2017
Business USD (M) % USD (M) %
United Kingdom 60.8 37.4 49.2 33.2
Europe 41.8 24.2 15.3 10
USA 41.4 24 13 9.3
Japan 23.6 14 33 22
France 0.76 0.4 9.4 6.4
Switzerland 27 19.1
TOTAL 168.36 100 146.9 100
The company’s operating loss was reported as $ 96.2 million. This is because of the full year
impact, charges from impairment and amortization of assets worth $109.7 million from previous
sales. EDITDA was more than what was expected to lead to a profit of $25.8million driven by
good performance. Good R&D management, cost, and delivery of savings. R&D cost was
$60.3million lower than in 2016.there was a reduction in EBITDA of 9million in 2017. The
closing cash was $103.7million in 2017 as compared to $92.5million in 2016 (Wedzicha, et al.,
2016, p. 67).
Cost of sales
Cost of sales rose by $21 million to $57.2 million due to increased device sales and product
supply. R$D expenses were $60.3million which was lower than in 2016 due to prioritization of
pipeline and R$D initiatives. The total expenditure was $34.2million in 2017 and $27.5million in
2016.
Sales per region
2016 2017
Business USD (M) % USD (M) %
United Kingdom 60.8 37.4 49.2 33.2
Europe 41.8 24.2 15.3 10
USA 41.4 24 13 9.3
Japan 23.6 14 33 22
France 0.76 0.4 9.4 6.4
Switzerland 27 19.1
TOTAL 168.36 100 146.9 100
The company’s operating loss was reported as $ 96.2 million. This is because of the full year
impact, charges from impairment and amortization of assets worth $109.7 million from previous
sales. EDITDA was more than what was expected to lead to a profit of $25.8million driven by
good performance. Good R&D management, cost, and delivery of savings. R&D cost was
$60.3million lower than in 2016.there was a reduction in EBITDA of 9million in 2017. The
closing cash was $103.7million in 2017 as compared to $92.5million in 2016 (Wedzicha, et al.,
2016, p. 67).
Cost of sales
Cost of sales rose by $21 million to $57.2 million due to increased device sales and product
supply. R$D expenses were $60.3million which was lower than in 2016 due to prioritization of
pipeline and R$D initiatives. The total expenditure was $34.2million in 2017 and $27.5million in
2016.

Vectura Pharmaceutical Company 12
Other operating expenditure was $1.7million compared to $1.4million in 2016. Amortization
charge included $109.7million and comprised of $8.7million impairment charge. The Activaero
and skypharma products will be amortized in the coming years. Exceptional costs, after merging,
was $4.5million in 2017. A total of $1.8million was spent on processing legal documents against
GSK concerning Ellipta products and $0.2M for restructuring facilities in France.
Conclusion
From the report 2017 was a year of progress and good financial results have been experienced. In
line with board market expectations as well as inhaled products, ultibro breezhaler and flutiform
have shown good market growth. As much as we struggle to achieve the best there are
challenges being faced by the company and they include failure to secure protection,
commercial limitations by patents or third parties, delays in getting regulatory approval and
dependence. However, this will not hinder our growth, we therefore Even though there are
delays from VR315 generic program from the US the company has seen value from inhaled
generics and therefore need to expand our portfolio for inhaled generics. We also have to
enhance our therapy pipeline and deliver our merger plans fully. Therefore, we have refocused
our investment plans announced January 2018. This is followed by tight financial discipline,
skilled workforce and strong core businesses. We commit to maximizing the value of forecast
sale, differentiated technologies and leveraging our capabilities at a lower risk and cost. The
company has well-defined priorities and expecting significant flows in 2018. In addition, my
strategy is to retain and attract existing and new employees in the company so as to ensure
quality work. I will ensure we have a platform for employee interaction between seniors and
juniors. Leadership is key to success, therefore, I will ensure a better choice of employing staff
with qualification and skills.
Other operating expenditure was $1.7million compared to $1.4million in 2016. Amortization
charge included $109.7million and comprised of $8.7million impairment charge. The Activaero
and skypharma products will be amortized in the coming years. Exceptional costs, after merging,
was $4.5million in 2017. A total of $1.8million was spent on processing legal documents against
GSK concerning Ellipta products and $0.2M for restructuring facilities in France.
Conclusion
From the report 2017 was a year of progress and good financial results have been experienced. In
line with board market expectations as well as inhaled products, ultibro breezhaler and flutiform
have shown good market growth. As much as we struggle to achieve the best there are
challenges being faced by the company and they include failure to secure protection,
commercial limitations by patents or third parties, delays in getting regulatory approval and
dependence. However, this will not hinder our growth, we therefore Even though there are
delays from VR315 generic program from the US the company has seen value from inhaled
generics and therefore need to expand our portfolio for inhaled generics. We also have to
enhance our therapy pipeline and deliver our merger plans fully. Therefore, we have refocused
our investment plans announced January 2018. This is followed by tight financial discipline,
skilled workforce and strong core businesses. We commit to maximizing the value of forecast
sale, differentiated technologies and leveraging our capabilities at a lower risk and cost. The
company has well-defined priorities and expecting significant flows in 2018. In addition, my
strategy is to retain and attract existing and new employees in the company so as to ensure
quality work. I will ensure we have a platform for employee interaction between seniors and
juniors. Leadership is key to success, therefore, I will ensure a better choice of employing staff
with qualification and skills.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 14
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




