Conversion of Vegetable Oil to Hydrocarbons: A Literature Review
VerifiedAdded on 2022/08/19
|11
|1728
|14
Literature Review
AI Summary
This literature review examines the conversion of vegetable oil into hydrocarbons, a crucial area of research for renewable fuels. It explores four primary methods: hydroprocessing, pyrolysis, catalytic cracking, and hydrolysis followed by decarboxylation, with a specific focus on comparing their efficiency, yields, and operating conditions. The review analyzes the advantages and disadvantages of each approach, considering factors such as feedstock availability, energy costs, and catalyst requirements. The goal is to determine the most promising method, particularly focusing on the hydrolysis then decarboxylation route. The review compares conversion rates, product yields, catalyst effectiveness, and the temperature and pressure demands of each process, drawing on a range of published research to critically assess the literature and identify the most viable and sustainable strategies for converting vegetable oil into valuable hydrocarbon fuels. The analysis aims to highlight the superior benefits of the hydrolysis then decarboxylation method in terms of feedstock availability, energy efficiency, and overall cost-effectiveness.

Conversion of vegetable oil to hydrocarbons
1 | P a g e
1 | P a g e
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Content’s table
Number
of content
Name of content Location of
page
number
1. Objectives 3
2. About the literature 4
3. Task-1 Hydroprocessing 5
4. Task-2 Pyrolysis 6
5. Task-3 Catalytic cracking 7
6. Task-4 Hydrolysis then decarboxylation 8,9
7. Conclusion 10
8 References 11
2 | P a g e
Number
of content
Name of content Location of
page
number
1. Objectives 3
2. About the literature 4
3. Task-1 Hydroprocessing 5
4. Task-2 Pyrolysis 6
5. Task-3 Catalytic cracking 7
6. Task-4 Hydrolysis then decarboxylation 8,9
7. Conclusion 10
8 References 11
2 | P a g e

Objectives
The main goal of writing this literature review is the conversion of vegetable oil in
hydrocarbon in these terms-
Hydroprocessing.
Pyrolysis.
Cracking.
Hydrolysis then de-carboxylation.
About the literature
3 | P a g e
The main goal of writing this literature review is the conversion of vegetable oil in
hydrocarbon in these terms-
Hydroprocessing.
Pyrolysis.
Cracking.
Hydrolysis then de-carboxylation.
About the literature
3 | P a g e
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

At most research of the literature is focussed onvproducing of biodiesel one supplement of
petroleum based diesel. This process is done in four steps- hydroprocessing, pyrolysis,
catalytic cracking and decarboxylation. The process is compared on the base of ability of
producing bio fuels that is used in spark ignition engine. As a biofuel feedstock, the edible
oils have been investigated that is compared with sources of food so we need to switch to non
edible oils. By the mega research, this is clear that transesterification is not adequate on its
own. The process Hydrocracking is an ideal solution because it can produce together high
quality bio jet fuel and bio gasoline using single catalyst.
Task-1 Hydroprocessing
4 | P a g e
petroleum based diesel. This process is done in four steps- hydroprocessing, pyrolysis,
catalytic cracking and decarboxylation. The process is compared on the base of ability of
producing bio fuels that is used in spark ignition engine. As a biofuel feedstock, the edible
oils have been investigated that is compared with sources of food so we need to switch to non
edible oils. By the mega research, this is clear that transesterification is not adequate on its
own. The process Hydrocracking is an ideal solution because it can produce together high
quality bio jet fuel and bio gasoline using single catalyst.
Task-1 Hydroprocessing
4 | P a g e
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
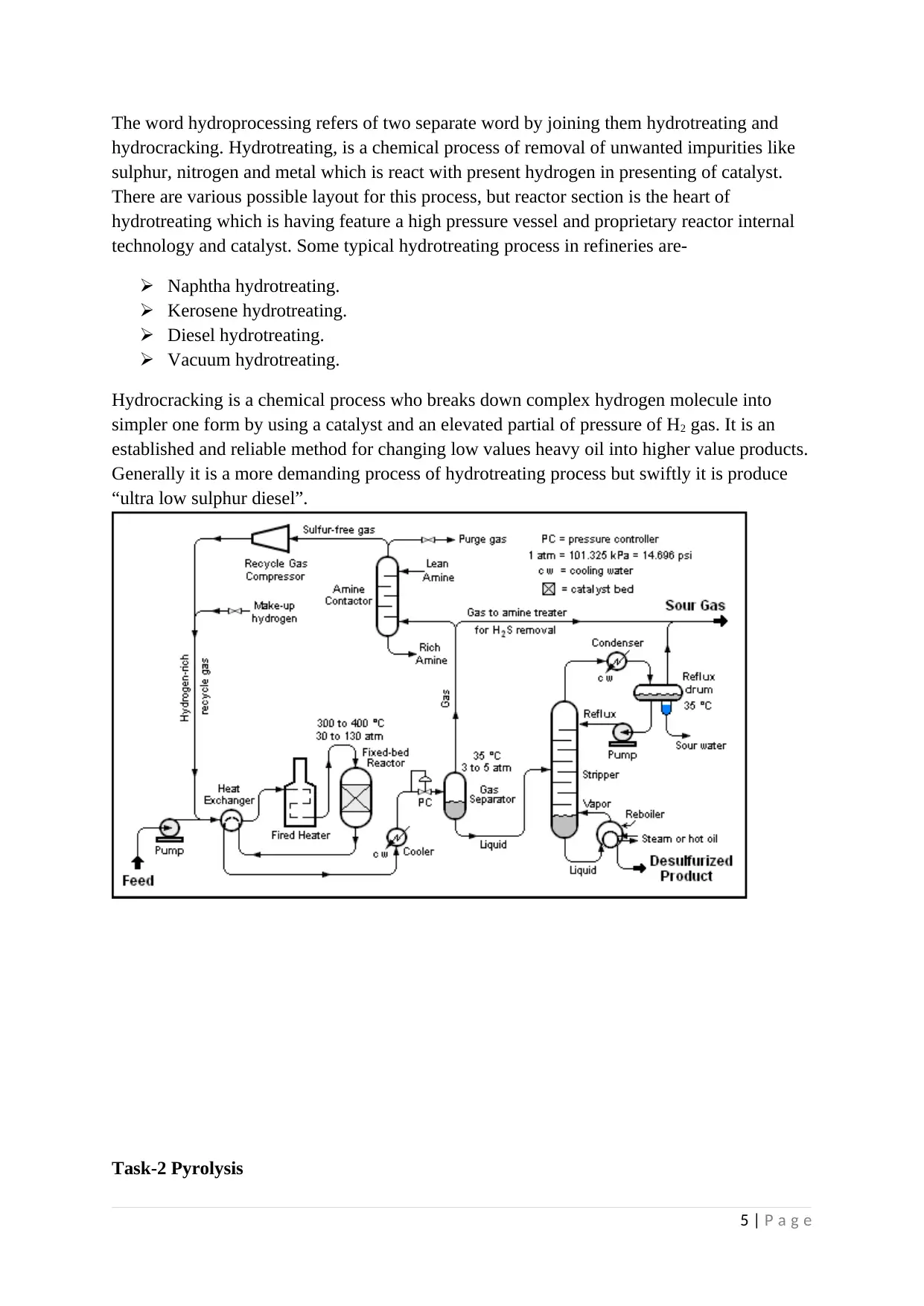
The word hydroprocessing refers of two separate word by joining them hydrotreating and
hydrocracking. Hydrotreating, is a chemical process of removal of unwanted impurities like
sulphur, nitrogen and metal which is react with present hydrogen in presenting of catalyst.
There are various possible layout for this process, but reactor section is the heart of
hydrotreating which is having feature a high pressure vessel and proprietary reactor internal
technology and catalyst. Some typical hydrotreating process in refineries are-
Naphtha hydrotreating.
Kerosene hydrotreating.
Diesel hydrotreating.
Vacuum hydrotreating.
Hydrocracking is a chemical process who breaks down complex hydrogen molecule into
simpler one form by using a catalyst and an elevated partial of pressure of H2 gas. It is an
established and reliable method for changing low values heavy oil into higher value products.
Generally it is a more demanding process of hydrotreating process but swiftly it is produce
“ultra low sulphur diesel”.
Task-2 Pyrolysis
5 | P a g e
hydrocracking. Hydrotreating, is a chemical process of removal of unwanted impurities like
sulphur, nitrogen and metal which is react with present hydrogen in presenting of catalyst.
There are various possible layout for this process, but reactor section is the heart of
hydrotreating which is having feature a high pressure vessel and proprietary reactor internal
technology and catalyst. Some typical hydrotreating process in refineries are-
Naphtha hydrotreating.
Kerosene hydrotreating.
Diesel hydrotreating.
Vacuum hydrotreating.
Hydrocracking is a chemical process who breaks down complex hydrogen molecule into
simpler one form by using a catalyst and an elevated partial of pressure of H2 gas. It is an
established and reliable method for changing low values heavy oil into higher value products.
Generally it is a more demanding process of hydrotreating process but swiftly it is produce
“ultra low sulphur diesel”.
Task-2 Pyrolysis
5 | P a g e
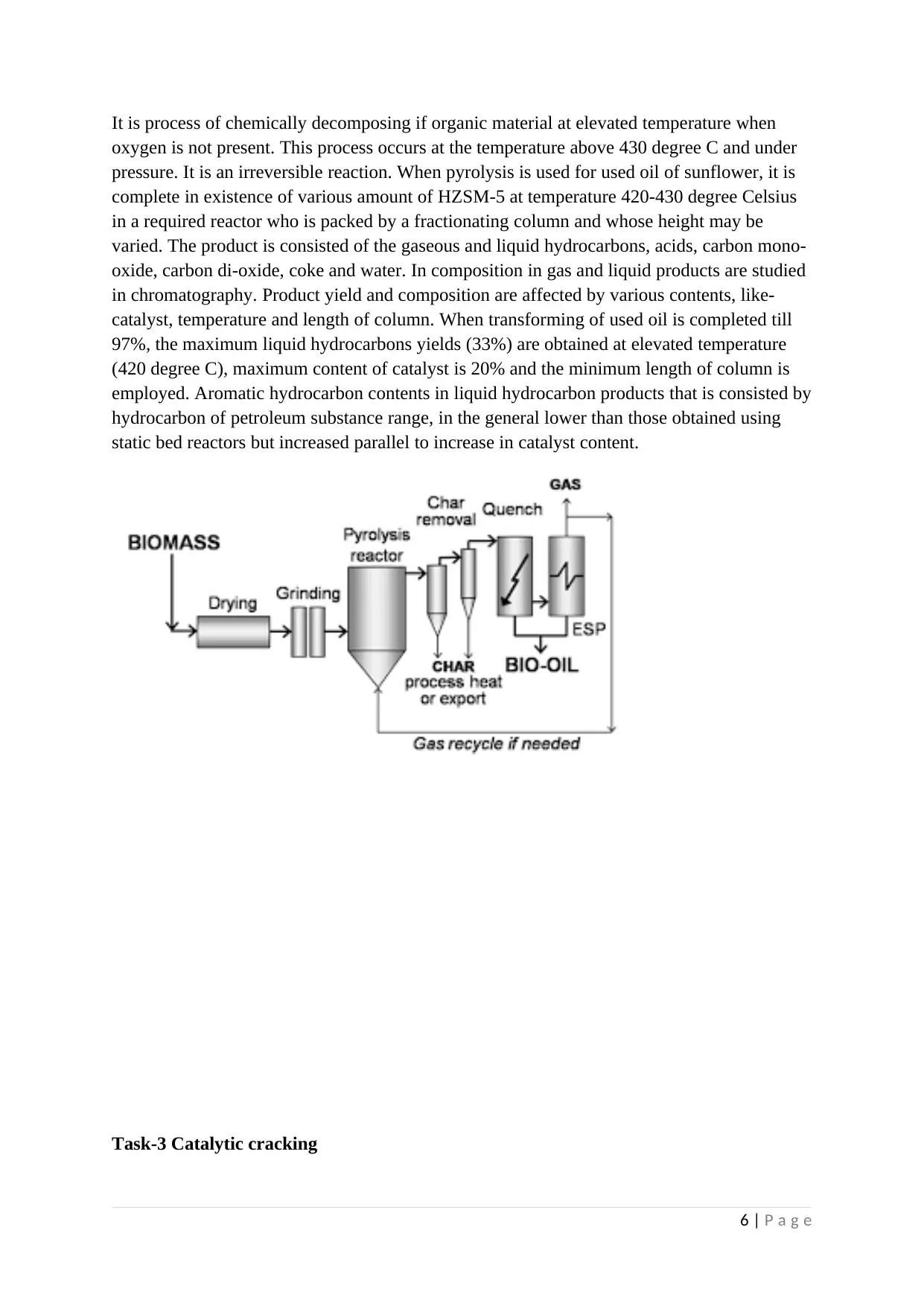
It is process of chemically decomposing if organic material at elevated temperature when
oxygen is not present. This process occurs at the temperature above 430 degree C and under
pressure. It is an irreversible reaction. When pyrolysis is used for used oil of sunflower, it is
complete in existence of various amount of HZSM-5 at temperature 420-430 degree Celsius
in a required reactor who is packed by a fractionating column and whose height may be
varied. The product is consisted of the gaseous and liquid hydrocarbons, acids, carbon mono-
oxide, carbon di-oxide, coke and water. In composition in gas and liquid products are studied
in chromatography. Product yield and composition are affected by various contents, like-
catalyst, temperature and length of column. When transforming of used oil is completed till
97%, the maximum liquid hydrocarbons yields (33%) are obtained at elevated temperature
(420 degree C), maximum content of catalyst is 20% and the minimum length of column is
employed. Aromatic hydrocarbon contents in liquid hydrocarbon products that is consisted by
hydrocarbon of petroleum substance range, in the general lower than those obtained using
static bed reactors but increased parallel to increase in catalyst content.
Task-3 Catalytic cracking
6 | P a g e
oxygen is not present. This process occurs at the temperature above 430 degree C and under
pressure. It is an irreversible reaction. When pyrolysis is used for used oil of sunflower, it is
complete in existence of various amount of HZSM-5 at temperature 420-430 degree Celsius
in a required reactor who is packed by a fractionating column and whose height may be
varied. The product is consisted of the gaseous and liquid hydrocarbons, acids, carbon mono-
oxide, carbon di-oxide, coke and water. In composition in gas and liquid products are studied
in chromatography. Product yield and composition are affected by various contents, like-
catalyst, temperature and length of column. When transforming of used oil is completed till
97%, the maximum liquid hydrocarbons yields (33%) are obtained at elevated temperature
(420 degree C), maximum content of catalyst is 20% and the minimum length of column is
employed. Aromatic hydrocarbon contents in liquid hydrocarbon products that is consisted by
hydrocarbon of petroleum substance range, in the general lower than those obtained using
static bed reactors but increased parallel to increase in catalyst content.
Task-3 Catalytic cracking
6 | P a g e
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

For preparing of liquid fuel of hydrocarbon the process of catalytic cracking of non edible
oils is suggested as an alternative method. The aluminium mixed SBA-15 mesoporous
substance is prepared by two separate method, in comparing their cracking phenomenon in
production of gasoline by waste oil of palm. Catalyst is ready by direct synthesis method
(AISBA) disorder like pore size distribution and there catalyst is ready by post synthesis
method (AGSBA), which is having a very narrow size like pore distribution. Both catalyst
give comparable result but in with regard AGSBA shows higher result and yields of
petroleum substance fraction as compared with AISBA. It can assign cause of better thermal
stability of AGSBA method.
Task-4 Hydrolysis then decarboxylation
7 | P a g e
oils is suggested as an alternative method. The aluminium mixed SBA-15 mesoporous
substance is prepared by two separate method, in comparing their cracking phenomenon in
production of gasoline by waste oil of palm. Catalyst is ready by direct synthesis method
(AISBA) disorder like pore size distribution and there catalyst is ready by post synthesis
method (AGSBA), which is having a very narrow size like pore distribution. Both catalyst
give comparable result but in with regard AGSBA shows higher result and yields of
petroleum substance fraction as compared with AISBA. It can assign cause of better thermal
stability of AGSBA method.
Task-4 Hydrolysis then decarboxylation
7 | P a g e
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
The process of deoxygenation, it covers all the chemical reaction involved in the removing of
O2 from molecule. Decarboxylation covers chemical reaction of deoxygenation where as CO2
is removed. In fatty acid it would be yields olefins, CO, and H2O in case of 1 and paraffins
and CO2 when 2 occurs. If hydrogen gas is not consumed in this reaction, DCO can be
proceed when H2 free deoxygenetion chemical reaction.
Hydrodeoxygenation, in this molecule of oxygen is removed from a molecule by using H2. In
fatty acids, its means lowering oxidation state of carbon atom of carboxylic group with use of
hydrogen in form hydrocarbon and water.
Decarboxylation is chemical process of removing a carboxyl group and release CO2. In this
group of CO2 is replaced by H2.
It does not react easily for common carboxylic acid. Carboxylic acid contains a beta carbonyl
group decarboxylation at a little temperature. Acetoacitic and malonic acid both are the
compound of this.
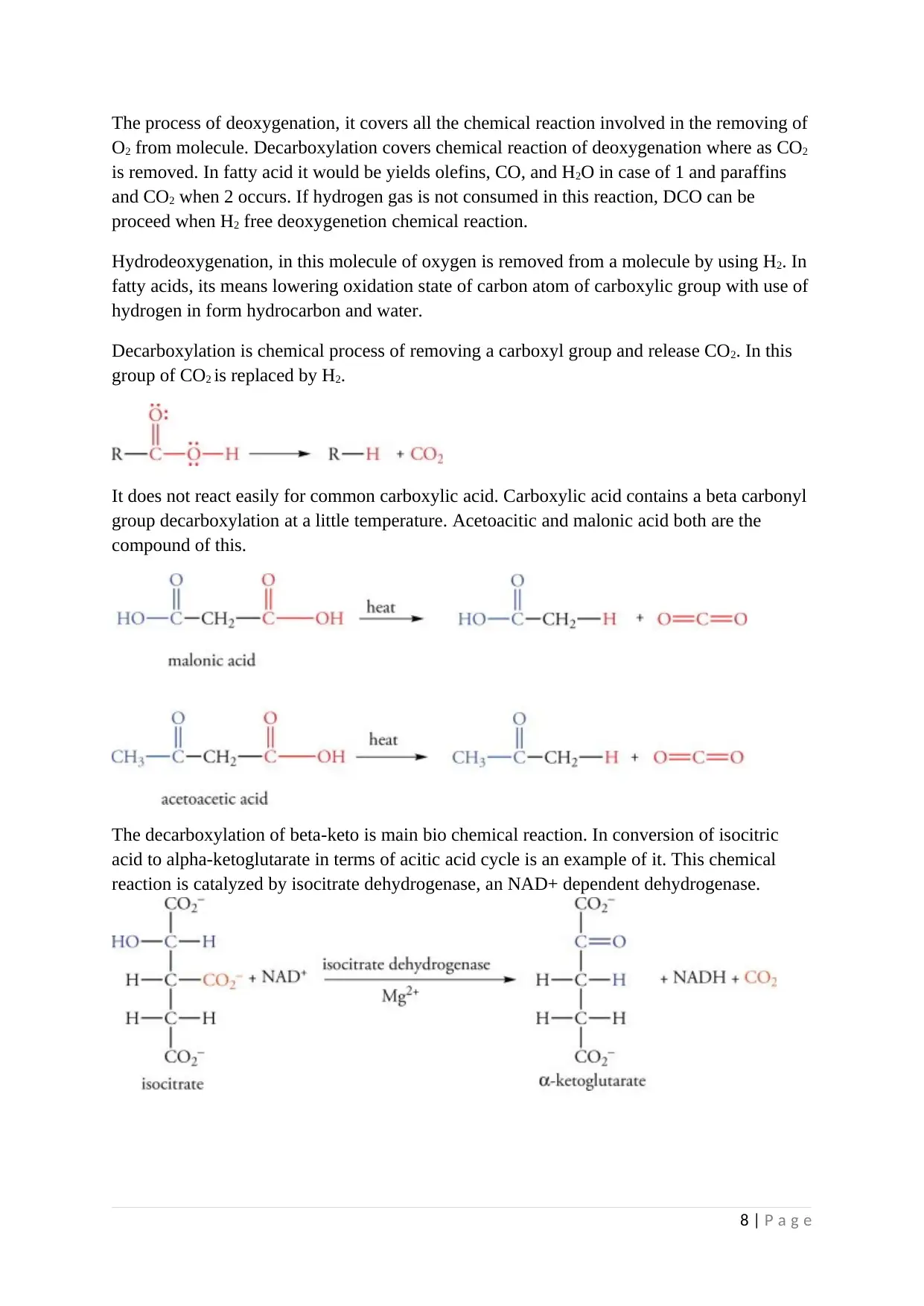
The decarboxylation of beta-keto is main bio chemical reaction. In conversion of isocitric
acid to alpha-ketoglutarate in terms of acitic acid cycle is an example of it. This chemical
reaction is catalyzed by isocitrate dehydrogenase, an NAD+ dependent dehydrogenase.
8 | P a g e
O2 from molecule. Decarboxylation covers chemical reaction of deoxygenation where as CO2
is removed. In fatty acid it would be yields olefins, CO, and H2O in case of 1 and paraffins
and CO2 when 2 occurs. If hydrogen gas is not consumed in this reaction, DCO can be
proceed when H2 free deoxygenetion chemical reaction.
Hydrodeoxygenation, in this molecule of oxygen is removed from a molecule by using H2. In
fatty acids, its means lowering oxidation state of carbon atom of carboxylic group with use of
hydrogen in form hydrocarbon and water.
Decarboxylation is chemical process of removing a carboxyl group and release CO2. In this
group of CO2 is replaced by H2.
It does not react easily for common carboxylic acid. Carboxylic acid contains a beta carbonyl
group decarboxylation at a little temperature. Acetoacitic and malonic acid both are the
compound of this.
The decarboxylation of beta-keto is main bio chemical reaction. In conversion of isocitric
acid to alpha-ketoglutarate in terms of acitic acid cycle is an example of it. This chemical
reaction is catalyzed by isocitrate dehydrogenase, an NAD+ dependent dehydrogenase.
8 | P a g e
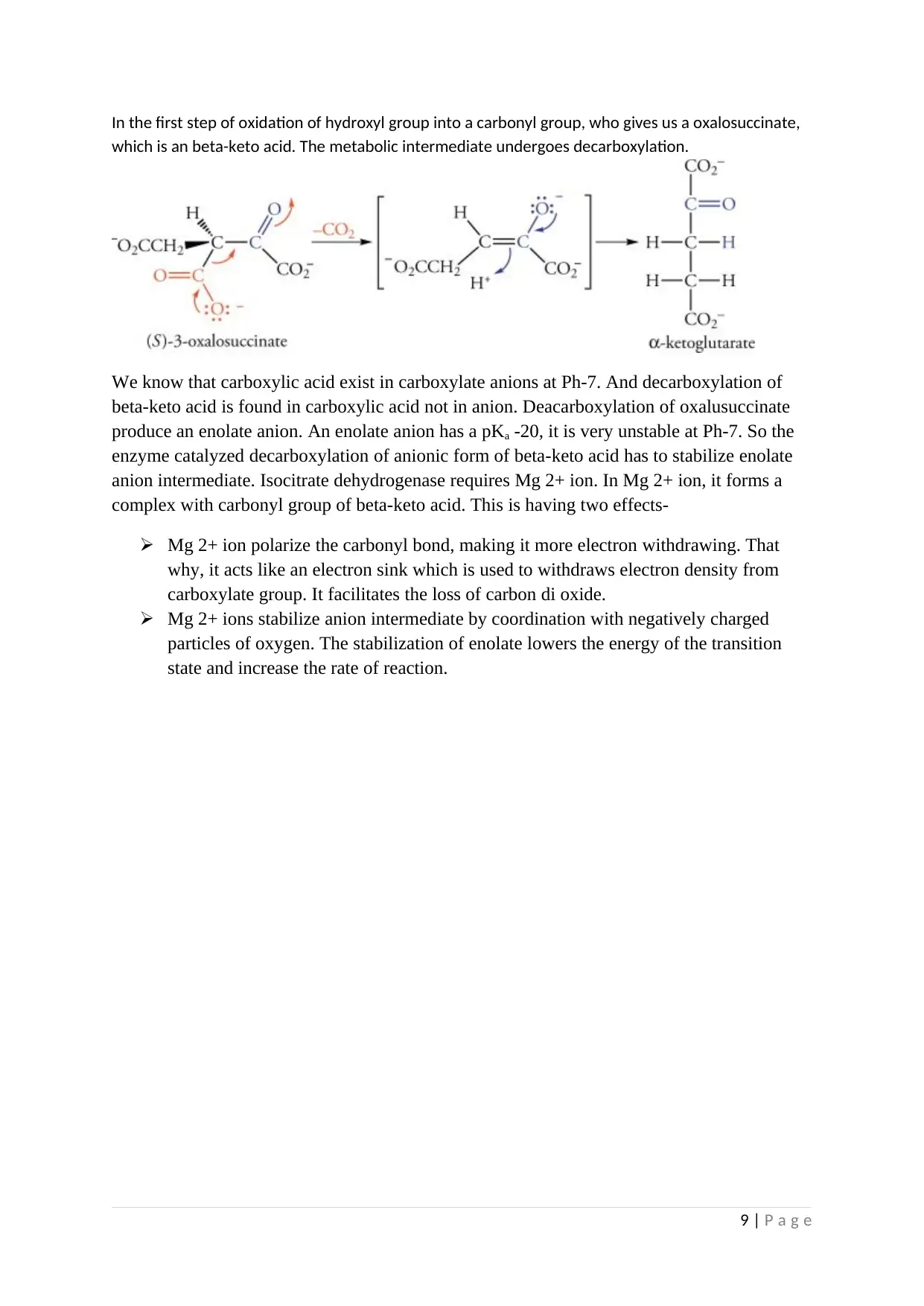
In the first step of oxidation of hydroxyl group into a carbonyl group, who gives us a oxalosuccinate,
which is an beta-keto acid. The metabolic intermediate undergoes decarboxylation.
We know that carboxylic acid exist in carboxylate anions at Ph-7. And decarboxylation of
beta-keto acid is found in carboxylic acid not in anion. Deacarboxylation of oxalusuccinate
produce an enolate anion. An enolate anion has a pKa -20, it is very unstable at Ph-7. So the
enzyme catalyzed decarboxylation of anionic form of beta-keto acid has to stabilize enolate
anion intermediate. Isocitrate dehydrogenase requires Mg 2+ ion. In Mg 2+ ion, it forms a
complex with carbonyl group of beta-keto acid. This is having two effects-
Mg 2+ ion polarize the carbonyl bond, making it more electron withdrawing. That
why, it acts like an electron sink which is used to withdraws electron density from
carboxylate group. It facilitates the loss of carbon di oxide.
Mg 2+ ions stabilize anion intermediate by coordination with negatively charged
particles of oxygen. The stabilization of enolate lowers the energy of the transition
state and increase the rate of reaction.
9 | P a g e
which is an beta-keto acid. The metabolic intermediate undergoes decarboxylation.
We know that carboxylic acid exist in carboxylate anions at Ph-7. And decarboxylation of
beta-keto acid is found in carboxylic acid not in anion. Deacarboxylation of oxalusuccinate
produce an enolate anion. An enolate anion has a pKa -20, it is very unstable at Ph-7. So the
enzyme catalyzed decarboxylation of anionic form of beta-keto acid has to stabilize enolate
anion intermediate. Isocitrate dehydrogenase requires Mg 2+ ion. In Mg 2+ ion, it forms a
complex with carbonyl group of beta-keto acid. This is having two effects-
Mg 2+ ion polarize the carbonyl bond, making it more electron withdrawing. That
why, it acts like an electron sink which is used to withdraws electron density from
carboxylate group. It facilitates the loss of carbon di oxide.
Mg 2+ ions stabilize anion intermediate by coordination with negatively charged
particles of oxygen. The stabilization of enolate lowers the energy of the transition
state and increase the rate of reaction.
9 | P a g e
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Conclusion
Decarboxylation chemical reaction are common in nature and they can be react in lots of
ways which include decarboxylation of keto acid, conversion of amino acid and carbohydrate
synthesis. Various decarboxylase use cofactors like metals ion. It is safe process to
concluding the existence of hydrogen appears essential to obtaining high deoxygenation
activities. Although saturated compound are selectively converted into hydrocarbon under
inert atmosphere.
10 | P a g e
Decarboxylation chemical reaction are common in nature and they can be react in lots of
ways which include decarboxylation of keto acid, conversion of amino acid and carbohydrate
synthesis. Various decarboxylase use cofactors like metals ion. It is safe process to
concluding the existence of hydrogen appears essential to obtaining high deoxygenation
activities. Although saturated compound are selectively converted into hydrocarbon under
inert atmosphere.
10 | P a g e
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References
Alcala, A. and Bridgwater, A. V., 2013. Upgrading fast pyrolysis liquids: Blends of biodiesel
and pyrolysis oil. Fuel, 109, pp. 417-426.
Balat, M., 2007, Production of biodiesel from vegetable oils: A survey. Energy Sources, Part
A. 29(10), pp 895-913.
Demirbas,A., 2002. Diesel fuel from vegetable oil via transesterification and soap pyrolysis,
Energy Sources, 24(9), pp. 835-841.
Demirbas, A., 2003. Biodiesel fuel from vegetable oils via catalytic and non catalytic
supercritical alcohal transesterifications and other methods: A survey. Energy conversion and
management. 44(13), pp. 2093-2109.
Demirbas, A., 2010. New biorenewable fuels from vegetable oil. Energy sources, Part A:
recovery, utilization, and environmental effects. 32(7), pp. 628-636.
Lappi, H., Alen, R., 2009. Production of vegetable oil-based biofuel thermochemical
behaviour of fatty and sodium salts during pyrolysis. Journal of analytical and applied
pyrolysis, 86(2), pp. 274-280.
Lappi, H., Alen, R., 2011. Pyrolysis of vegetable oil soaps-palm, olive, rapessed and carter
oils. Journal of analytical and Applied pyrolysis: 91(1)-pp. 154-158.
Lima, D. G., Soares, V. C., Ribeiro, E B., Carvalho, D. A., Cardoso, E.C., Rassi, F. C.,
Mundim, K. C., Rubim, J. C. And Suarez, P. A., 2004. Diesel like fuel obtaining by pyrolysis
of vegetable oils. Journal of Analytical and Applied pyrolysis, 71(2). Pp. 987-996.
Sensoz, S., Angin, D. And Yorgun, S., 2000. Influence of particle size on the pyrolysis of
rapessed(brassica napus): fuel properties of bio-oil. Biomass and Bioenergy.19(4), pp. 271-
279.
Vinh, T. Q., Loan, N.T.T., Yang, X.Y. and Su, B.L., 2011 preparation of bio-fuels by
catalytic cracking reaction of vegetable oil sludge. Fuel . 90(3), pp. 1069-1075.
Wang, Y., Fan, L., Cao, L., Zhao, Y., Liu, Y. And Ruan, R., 2017. Catalytic co-pyrolysis of
waste vegetable oil and high density polyethylene for hydrocarbon fuel production. Waste
management, 61, pp. 276-282.
Zhenvi, C., Xing, J., Shuyuan, L. I. And Li, L., 2004. Thermodynamics calculation of the
pyrolysis of vegetable oils. Energy Sources. 26(9). pp. 849-896.
11 | P a g e
Alcala, A. and Bridgwater, A. V., 2013. Upgrading fast pyrolysis liquids: Blends of biodiesel
and pyrolysis oil. Fuel, 109, pp. 417-426.
Balat, M., 2007, Production of biodiesel from vegetable oils: A survey. Energy Sources, Part
A. 29(10), pp 895-913.
Demirbas,A., 2002. Diesel fuel from vegetable oil via transesterification and soap pyrolysis,
Energy Sources, 24(9), pp. 835-841.
Demirbas, A., 2003. Biodiesel fuel from vegetable oils via catalytic and non catalytic
supercritical alcohal transesterifications and other methods: A survey. Energy conversion and
management. 44(13), pp. 2093-2109.
Demirbas, A., 2010. New biorenewable fuels from vegetable oil. Energy sources, Part A:
recovery, utilization, and environmental effects. 32(7), pp. 628-636.
Lappi, H., Alen, R., 2009. Production of vegetable oil-based biofuel thermochemical
behaviour of fatty and sodium salts during pyrolysis. Journal of analytical and applied
pyrolysis, 86(2), pp. 274-280.
Lappi, H., Alen, R., 2011. Pyrolysis of vegetable oil soaps-palm, olive, rapessed and carter
oils. Journal of analytical and Applied pyrolysis: 91(1)-pp. 154-158.
Lima, D. G., Soares, V. C., Ribeiro, E B., Carvalho, D. A., Cardoso, E.C., Rassi, F. C.,
Mundim, K. C., Rubim, J. C. And Suarez, P. A., 2004. Diesel like fuel obtaining by pyrolysis
of vegetable oils. Journal of Analytical and Applied pyrolysis, 71(2). Pp. 987-996.
Sensoz, S., Angin, D. And Yorgun, S., 2000. Influence of particle size on the pyrolysis of
rapessed(brassica napus): fuel properties of bio-oil. Biomass and Bioenergy.19(4), pp. 271-
279.
Vinh, T. Q., Loan, N.T.T., Yang, X.Y. and Su, B.L., 2011 preparation of bio-fuels by
catalytic cracking reaction of vegetable oil sludge. Fuel . 90(3), pp. 1069-1075.
Wang, Y., Fan, L., Cao, L., Zhao, Y., Liu, Y. And Ruan, R., 2017. Catalytic co-pyrolysis of
waste vegetable oil and high density polyethylene for hydrocarbon fuel production. Waste
management, 61, pp. 276-282.
Zhenvi, C., Xing, J., Shuyuan, L. I. And Li, L., 2004. Thermodynamics calculation of the
pyrolysis of vegetable oils. Energy Sources. 26(9). pp. 849-896.
11 | P a g e
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


