Chemistry Experiment: Calibration of Graduated Volumetric Glasswares
VerifiedAdded on 2022/02/04
|8
|1478
|32
Practical Assignment
AI Summary
This practical assignment details a chemistry experiment focused on calibrating volumetric glassware. The experiment involves the calibration of a volumetric pipette, a buret, and a volumetric flask. The procedure includes weighing the glassware, measuring the mass of water delivered or contained, and converting the mass to volume using water density corrections for buoyancy and temperature. The results section presents data from multiple trials, calculates mean volumes, and determines standard deviations. The discussion highlights the importance of calibration for accurate measurements, addresses potential sources of error such as parallax and temperature variations, and emphasizes the need for multiple trials to ensure precision. The experiment concludes with a verification of the true volumes of the glassware and demonstrates proper techniques for using volumetric equipment, referencing relevant literature on analytical chemistry principles and techniques.

EXPERIMENT NO. 1
The Calibration of Graduated Volumetric Glasswares
Lumbo, E. V.
1.0 Introduction
Calibration is a procedure that establishes the relationship between quantity scales
indicated by a measuring instrument under specified condition. It determines accuracy
of the measured data and provides the traceability to the measurement. Every
measuring instrument has its standards in order for it to be said as calibrated (UNIDO,
2006). Volumetric glassware for example is container that can be calibrated. It is done
by measuring the mass of liquid contained in or delivered then computing for its volume
with the use of liquid’s known density. Correction for buoyancy must be considered in
calibration because air buoyancy causes an object’s weight lighter than its weight in
vacuum (Harvey 2000). The objective of this experiment was to study the methods of
calibrating volumetric glassware through quantitative laboratory work.
2.0 Experimental
Volumetric pipet, volumetric flask, and acid buret were the glassware calibrated in this
experiment. The materials used were analytical balance, top loading balance, and
Erlenmeyer flask. The only reagent was water.
The Calibration of Graduated Volumetric Glasswares
Lumbo, E. V.
1.0 Introduction
Calibration is a procedure that establishes the relationship between quantity scales
indicated by a measuring instrument under specified condition. It determines accuracy
of the measured data and provides the traceability to the measurement. Every
measuring instrument has its standards in order for it to be said as calibrated (UNIDO,
2006). Volumetric glassware for example is container that can be calibrated. It is done
by measuring the mass of liquid contained in or delivered then computing for its volume
with the use of liquid’s known density. Correction for buoyancy must be considered in
calibration because air buoyancy causes an object’s weight lighter than its weight in
vacuum (Harvey 2000). The objective of this experiment was to study the methods of
calibrating volumetric glassware through quantitative laboratory work.
2.0 Experimental
Volumetric pipet, volumetric flask, and acid buret were the glassware calibrated in this
experiment. The materials used were analytical balance, top loading balance, and
Erlenmeyer flask. The only reagent was water.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
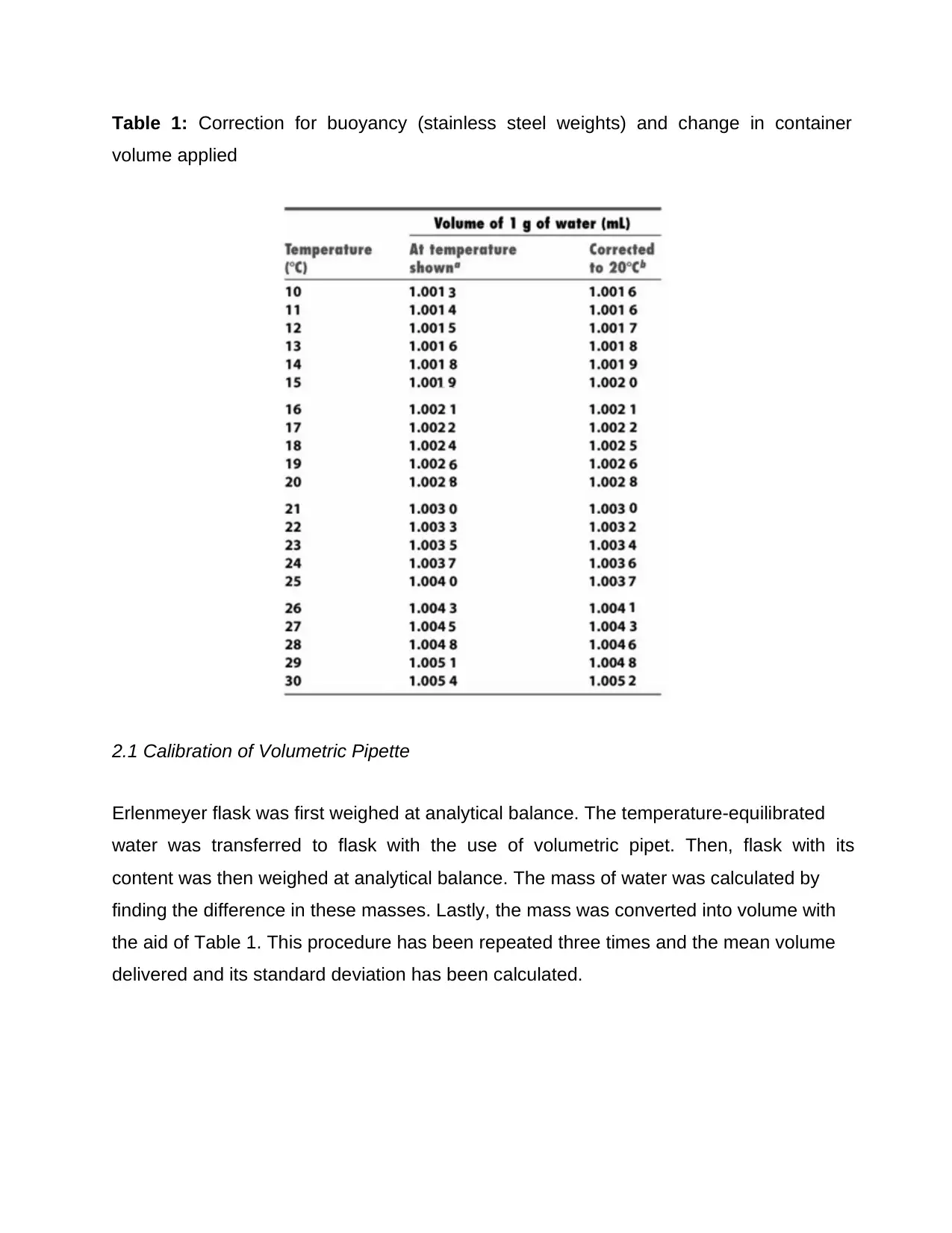
Table 1: Correction for buoyancy (stainless steel weights) and change in container
volume applied
2.1 Calibration of Volumetric Pipette
Erlenmeyer flask was first weighed at analytical balance. The temperature-equilibrated
water was transferred to flask with the use of volumetric pipet. Then, flask with its
content was then weighed at analytical balance. The mass of water was calculated by
finding the difference in these masses. Lastly, the mass was converted into volume with
the aid of Table 1. This procedure has been repeated three times and the mean volume
delivered and its standard deviation has been calculated.
volume applied
2.1 Calibration of Volumetric Pipette
Erlenmeyer flask was first weighed at analytical balance. The temperature-equilibrated
water was transferred to flask with the use of volumetric pipet. Then, flask with its
content was then weighed at analytical balance. The mass of water was calculated by
finding the difference in these masses. Lastly, the mass was converted into volume with
the aid of Table 1. This procedure has been repeated three times and the mean volume
delivered and its standard deviation has been calculated.

2.2 Calibration of Buret
Buret was first filled with temperature-equilibrated water. There should be no air bubble
trapped at the tip of the buret. The water was drained up to the 0.00mL mark. Then the
tip of the buret was touched to the wall of the beaker to remove adhering drop. After 10
minutes, the volume was checked. There should be no change that can be seen. A
125mL Erlenmeyer flask was weighed at analytical balance. Then, 5mL water was
delivered to the flask with the use of buret. The buret was refilled. The flask and its
contents were weighed. The mass of water was calculated by subtracting these
masses. This mass was converted to volume using Table 1. The apparent volume was
subtracted to the true volume obtained from the experiment. This difference was the
correction to the apparent volume to get the true volume. The calibration was repeated
until there is ±0.02mL agreement.
Plot of correction was done by repeating the procedure in the calibration of buret but
instead of just delivering 5mL, it was done by delivering 10mL to the receiver and then
5mL intervals over its entire volume.
2.3 Calibration of Volumetric Flask
Volumetric flask was first weighed at top loading balance. Then it was filled with
temperature-equilibrated water and reweighed. The corrected volume of water was
calculated the same manner as the calibration of volumetric pipet.
Buret was first filled with temperature-equilibrated water. There should be no air bubble
trapped at the tip of the buret. The water was drained up to the 0.00mL mark. Then the
tip of the buret was touched to the wall of the beaker to remove adhering drop. After 10
minutes, the volume was checked. There should be no change that can be seen. A
125mL Erlenmeyer flask was weighed at analytical balance. Then, 5mL water was
delivered to the flask with the use of buret. The buret was refilled. The flask and its
contents were weighed. The mass of water was calculated by subtracting these
masses. This mass was converted to volume using Table 1. The apparent volume was
subtracted to the true volume obtained from the experiment. This difference was the
correction to the apparent volume to get the true volume. The calibration was repeated
until there is ±0.02mL agreement.
Plot of correction was done by repeating the procedure in the calibration of buret but
instead of just delivering 5mL, it was done by delivering 10mL to the receiver and then
5mL intervals over its entire volume.
2.3 Calibration of Volumetric Flask
Volumetric flask was first weighed at top loading balance. Then it was filled with
temperature-equilibrated water and reweighed. The corrected volume of water was
calculated the same manner as the calibration of volumetric pipet.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3.0 Results and Discussions
Volumetric glasswares need to be calibrated from time to time because it expand and
contract at different temperature which can cause error in measurement (Waser, 1964).
Parallax also is another that can cause error in measurement. It happens when
meniscus (marked curvature due to liquid confined in the narrow tube) is viewed above
or below eye level (Skoog et al). The volume appears larger if it is viewed above while it
appears smaller if viewed below. Several trials should be done in determination of true
volume for more accuracy of measurement. There should be a certain agreement
between trials for it to be said accurate (UNIDO, 2006).
A volumetric pipette is used to deliver a fixed single volume between 0.5 to 200mL
(Harris 2007). In this experiment, 10mL volumetric pipette was used. The receiver used
was Erlenmeyer flask with rubber cork. A beaker can also be used as a receiver but
since analytical balance was used for measuring weight and a liquid reagent was
contained, it is safer to use Erlenmeyer flask to avoid spill.
Table 2: Results on trials for calibration of volumetric pipette
Trial 1 Trial 2 Trial 3
Mass of
receiver
126899.1mg 110902.1mg 1405705mg
Mass of
receiver +
10mL water
137189.4mg 121171.3mg 150827.1mg
Mass of water 10.2903g 10.2692g 10.2566g
Volumetric glasswares need to be calibrated from time to time because it expand and
contract at different temperature which can cause error in measurement (Waser, 1964).
Parallax also is another that can cause error in measurement. It happens when
meniscus (marked curvature due to liquid confined in the narrow tube) is viewed above
or below eye level (Skoog et al). The volume appears larger if it is viewed above while it
appears smaller if viewed below. Several trials should be done in determination of true
volume for more accuracy of measurement. There should be a certain agreement
between trials for it to be said accurate (UNIDO, 2006).
A volumetric pipette is used to deliver a fixed single volume between 0.5 to 200mL
(Harris 2007). In this experiment, 10mL volumetric pipette was used. The receiver used
was Erlenmeyer flask with rubber cork. A beaker can also be used as a receiver but
since analytical balance was used for measuring weight and a liquid reagent was
contained, it is safer to use Erlenmeyer flask to avoid spill.
Table 2: Results on trials for calibration of volumetric pipette
Trial 1 Trial 2 Trial 3
Mass of
receiver
126899.1mg 110902.1mg 1405705mg
Mass of
receiver +
10mL water
137189.4mg 121171.3mg 150827.1mg
Mass of water 10.2903g 10.2692g 10.2566g
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
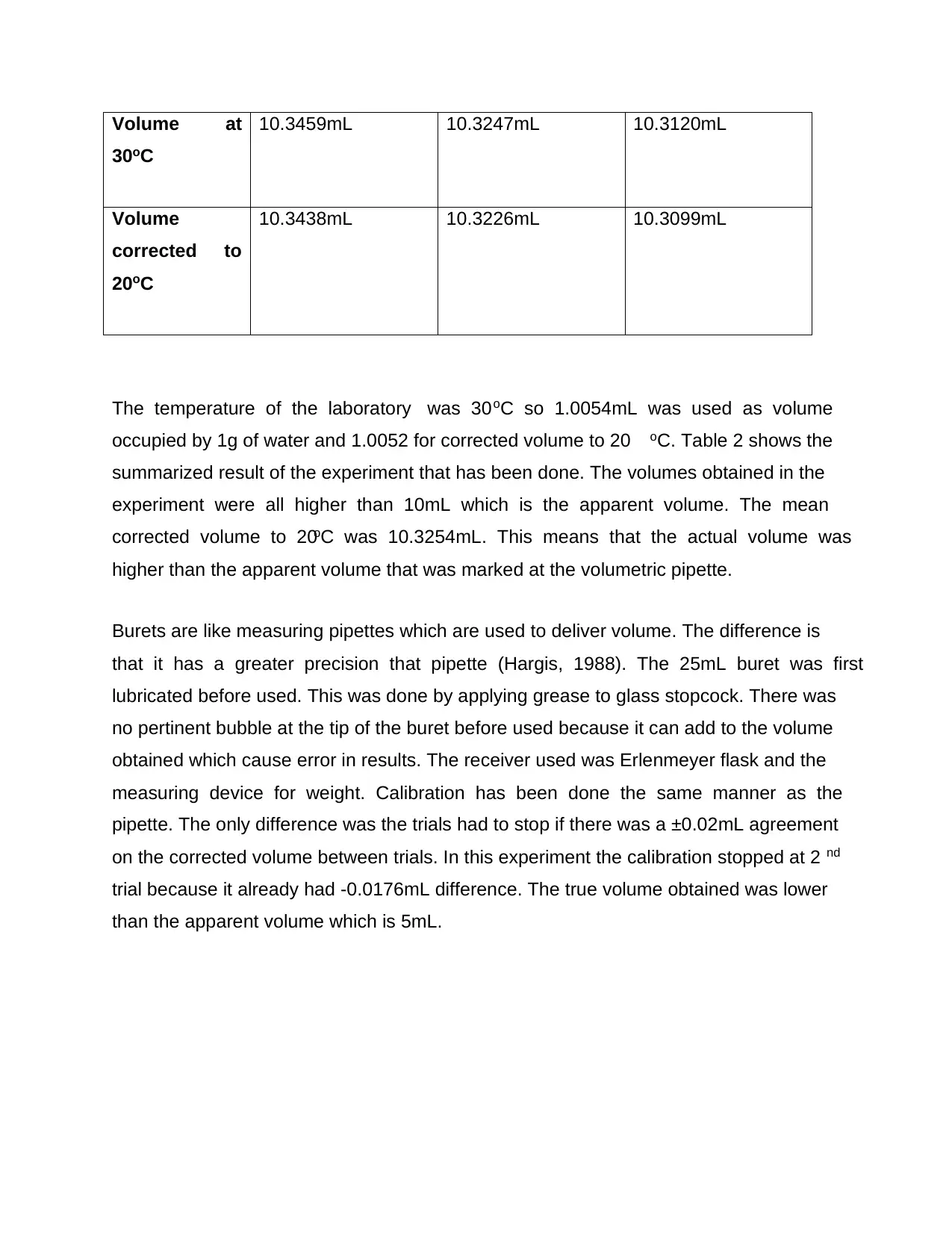
Volume at
30oC
10.3459mL 10.3247mL 10.3120mL
Volume
corrected to
20oC
10.3438mL 10.3226mL 10.3099mL
The temperature of the laboratory was 30oC so 1.0054mL was used as volume
occupied by 1g of water and 1.0052 for corrected volume to 20 oC. Table 2 shows the
summarized result of the experiment that has been done. The volumes obtained in the
experiment were all higher than 10mL which is the apparent volume. The mean
corrected volume to 20oC was 10.3254mL. This means that the actual volume was
higher than the apparent volume that was marked at the volumetric pipette.
Burets are like measuring pipettes which are used to deliver volume. The difference is
that it has a greater precision that pipette (Hargis, 1988). The 25mL buret was first
lubricated before used. This was done by applying grease to glass stopcock. There was
no pertinent bubble at the tip of the buret before used because it can add to the volume
obtained which cause error in results. The receiver used was Erlenmeyer flask and the
measuring device for weight. Calibration has been done the same manner as the
pipette. The only difference was the trials had to stop if there was a ±0.02mL agreement
on the corrected volume between trials. In this experiment the calibration stopped at 2 nd
trial because it already had -0.0176mL difference. The true volume obtained was lower
than the apparent volume which is 5mL.
30oC
10.3459mL 10.3247mL 10.3120mL
Volume
corrected to
20oC
10.3438mL 10.3226mL 10.3099mL
The temperature of the laboratory was 30oC so 1.0054mL was used as volume
occupied by 1g of water and 1.0052 for corrected volume to 20 oC. Table 2 shows the
summarized result of the experiment that has been done. The volumes obtained in the
experiment were all higher than 10mL which is the apparent volume. The mean
corrected volume to 20oC was 10.3254mL. This means that the actual volume was
higher than the apparent volume that was marked at the volumetric pipette.
Burets are like measuring pipettes which are used to deliver volume. The difference is
that it has a greater precision that pipette (Hargis, 1988). The 25mL buret was first
lubricated before used. This was done by applying grease to glass stopcock. There was
no pertinent bubble at the tip of the buret before used because it can add to the volume
obtained which cause error in results. The receiver used was Erlenmeyer flask and the
measuring device for weight. Calibration has been done the same manner as the
pipette. The only difference was the trials had to stop if there was a ±0.02mL agreement
on the corrected volume between trials. In this experiment the calibration stopped at 2 nd
trial because it already had -0.0176mL difference. The true volume obtained was lower
than the apparent volume which is 5mL.
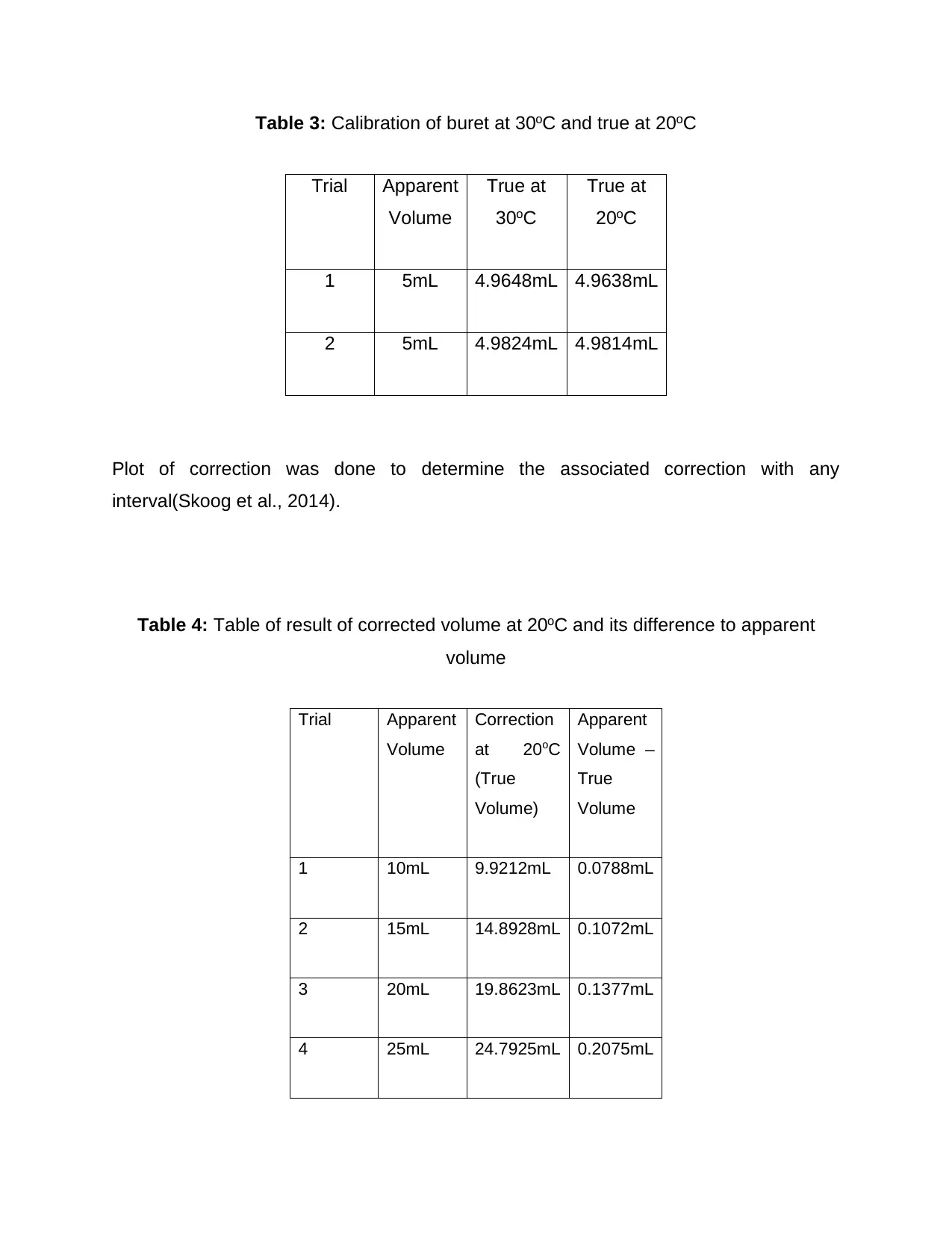
Table 3: Calibration of buret at 30oC and true at 20oC
Trial Apparent
Volume
True at
30oC
True at
20oC
1 5mL 4.9648mL 4.9638mL
2 5mL 4.9824mL 4.9814mL
Plot of correction was done to determine the associated correction with any
interval(Skoog et al., 2014).
Table 4: Table of result of corrected volume at 20oC and its difference to apparent
volume
Trial Apparent
Volume
Correction
at 20oC
(True
Volume)
Apparent
Volume –
True
Volume
1 10mL 9.9212mL 0.0788mL
2 15mL 14.8928mL 0.1072mL
3 20mL 19.8623mL 0.1377mL
4 25mL 24.7925mL 0.2075mL
Trial Apparent
Volume
True at
30oC
True at
20oC
1 5mL 4.9648mL 4.9638mL
2 5mL 4.9824mL 4.9814mL
Plot of correction was done to determine the associated correction with any
interval(Skoog et al., 2014).
Table 4: Table of result of corrected volume at 20oC and its difference to apparent
volume
Trial Apparent
Volume
Correction
at 20oC
(True
Volume)
Apparent
Volume –
True
Volume
1 10mL 9.9212mL 0.0788mL
2 15mL 14.8928mL 0.1072mL
3 20mL 19.8623mL 0.1377mL
4 25mL 24.7925mL 0.2075mL
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

The volumetric flask was used to contain with capacities ranging from 5mL to 5L (Skoog
et al., 2014). In this experiment, 250mL of volumetric flask was used. The measuring
device for weight was the top loading balance. It is because the volumetric flask can’t fit
inside the analytical balance. The obtained weight of volumetric flask was 89.99g. The
weight of volumetric flask and water was 338.41g. The weight of the water was obtained
by getting the difference between the weight of flask + water and weight of flask which is
248.42g. The computed volume at 30 oC was 249.76mL and at corrected to 20 oC was
249.71mL. This means that the apparent volume of the flask was greater than the true
volume.
4.0 Conclusion
The calibrated volumetric glass wares used were efficiently verified by computing its
true volume. Moreover, proper skills in using volumetric glass wares were effectively
exhibited in this experiment.
References
Hargis, L. (1988). Analytical Chemistry Principle and Technique. Laboratory Operations
and Practices, Prentice-Hall Inc., Republic of Singapore, pg. 551-556,
Harris, Daniel C. (2007). Quantitative Chemical Analysis Seventh Edition. Calibration of
Volumetric Glasswares, W.H Freeman and Company, United Sates of America, pg. 22-
33.
Harvey, David (2000). Modern Analytical Chemistry. Calibration, Standardization, and
Blank Corrections, McGraw-Hill Companies, Inc., United States of America, pg. 47 and
105.
et al., 2014). In this experiment, 250mL of volumetric flask was used. The measuring
device for weight was the top loading balance. It is because the volumetric flask can’t fit
inside the analytical balance. The obtained weight of volumetric flask was 89.99g. The
weight of volumetric flask and water was 338.41g. The weight of the water was obtained
by getting the difference between the weight of flask + water and weight of flask which is
248.42g. The computed volume at 30 oC was 249.76mL and at corrected to 20 oC was
249.71mL. This means that the apparent volume of the flask was greater than the true
volume.
4.0 Conclusion
The calibrated volumetric glass wares used were efficiently verified by computing its
true volume. Moreover, proper skills in using volumetric glass wares were effectively
exhibited in this experiment.
References
Hargis, L. (1988). Analytical Chemistry Principle and Technique. Laboratory Operations
and Practices, Prentice-Hall Inc., Republic of Singapore, pg. 551-556,
Harris, Daniel C. (2007). Quantitative Chemical Analysis Seventh Edition. Calibration of
Volumetric Glasswares, W.H Freeman and Company, United Sates of America, pg. 22-
33.
Harvey, David (2000). Modern Analytical Chemistry. Calibration, Standardization, and
Blank Corrections, McGraw-Hill Companies, Inc., United States of America, pg. 47 and
105.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Skoog, D.; West D.; Holler, FJ; Crouch S. (2014) Fundamentals of Analytical Chemistry
pg. 32-43.
UNIDO (2006). Role of Measurement and Calibration.
http://www.unido.org/fileadmin/user_media/Publications/Pub_free/Role_of_measureme
nt_and_calibration.pdf. November 28, 2014
Waser, Jϋrg (1964). Quantitative Chemistry. Calibration of Volumetric Equipment,
United States of America, pg. 100-107.
pg. 32-43.
UNIDO (2006). Role of Measurement and Calibration.
http://www.unido.org/fileadmin/user_media/Publications/Pub_free/Role_of_measureme
nt_and_calibration.pdf. November 28, 2014
Waser, Jϋrg (1964). Quantitative Chemistry. Calibration of Volumetric Equipment,
United States of America, pg. 100-107.
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




