Protocol for Water System Validation in a Pilot Plant: Analysis
VerifiedAdded on 2021/09/14
|14
|2778
|183
Project
AI Summary
This document presents a detailed validation protocol for a water purification system within a pilot plant setting. It outlines the aim of the protocol to ensure the water system functions as expected, meeting the required standards and consistently producing water of the desired quality. The scope encompasses the system's components, including pre-treatment, generation of purified water via deionization and reverse osmosis, sterilization, and storage. The document provides an overview of the process, defines roles and responsibilities, and explains the rationale behind the system validation, covering Installation Qualification (IQ), Operational Qualification (OQ), and the four validation phases. It details sampling procedures, testing methods, and acceptable criteria, along with log verification entries and preconditions. The protocol also includes operational procedures, a discussion of portable water purification, and conclusions. The appendices include checklists, sampling plans and tables for log entries, and references. This comprehensive guide ensures that the water purification system operates consistently and effectively.

Protocol for the Water System Validation in the Pilot Plant
Name of the Student
Name of the University
Author’s Note:
Name of the Student
Name of the University
Author’s Note:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
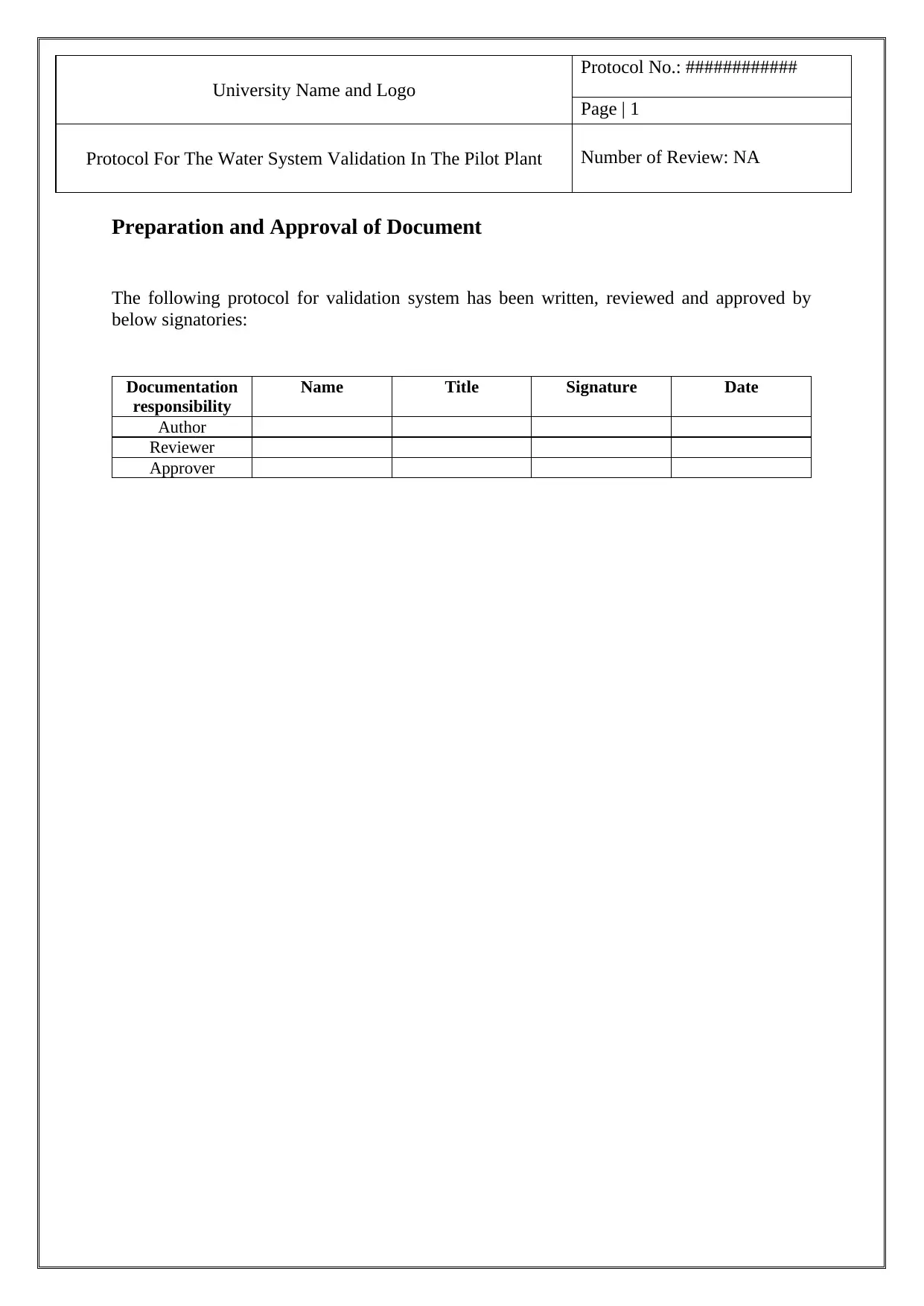
University Name and Logo
Protocol No.: ############
Page | 1
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
Preparation and Approval of Document
The following protocol for validation system has been written, reviewed and approved by
below signatories:
Documentation
responsibility
Name Title Signature Date
Author
Reviewer
Approver
Protocol No.: ############
Page | 1
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
Preparation and Approval of Document
The following protocol for validation system has been written, reviewed and approved by
below signatories:
Documentation
responsibility
Name Title Signature Date
Author
Reviewer
Approver

University Name and Logo
Protocol No.: ############
Page | 2
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
Validation protocol for water purifying system
1.0 Aim..................................................................................................................................3
2.0 Scope...............................................................................................................................3
2.1 System component incorporated in this validating protocol............................................3
2.2 Overview of process and system description...................................................................3
3.0 Roles and Responsibilities...................................................................................................5
4.0 Rationale for system validation............................................................................................6
4.1 Validation of system IQ and OQ......................................................................................6
4.2 Rationale of system validation.........................................................................................7
4.3 Execution of validation....................................................................................................8
4.4 Operational protocol.........................................................................................................8
5.0 Sampling..............................................................................................................................8
5.1 Sampling procedures........................................................................................................8
5.2 Point of sample collection................................................................................................8
5.3 Test to be performed........................................................................................................9
5.3.1 Log entry for equipment and instruments calibration...............................................9
5.4 Acceptable criterion for end product of purified water....................................................9
6.0 Log verification entry...........................................................................................................9
7.0 Preconditions for verification.............................................................................................10
8.0 Execution of protocol for purified water system................................................................10
9.0 Portable water purification.................................................................................................10
9.1 Objectives.......................................................................................................................10
9.2 Procedure........................................................................................................................10
10.0 Conclusions and Summary...............................................................................................11
11.0 Protocol deviation form....................................................................................................11
12.0 Log entry for deviation.....................................................................................................11
13.0 Attachments......................................................................................................................11
14.0 References........................................................................................................................12
Protocol No.: ############
Page | 2
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
Validation protocol for water purifying system
1.0 Aim..................................................................................................................................3
2.0 Scope...............................................................................................................................3
2.1 System component incorporated in this validating protocol............................................3
2.2 Overview of process and system description...................................................................3
3.0 Roles and Responsibilities...................................................................................................5
4.0 Rationale for system validation............................................................................................6
4.1 Validation of system IQ and OQ......................................................................................6
4.2 Rationale of system validation.........................................................................................7
4.3 Execution of validation....................................................................................................8
4.4 Operational protocol.........................................................................................................8
5.0 Sampling..............................................................................................................................8
5.1 Sampling procedures........................................................................................................8
5.2 Point of sample collection................................................................................................8
5.3 Test to be performed........................................................................................................9
5.3.1 Log entry for equipment and instruments calibration...............................................9
5.4 Acceptable criterion for end product of purified water....................................................9
6.0 Log verification entry...........................................................................................................9
7.0 Preconditions for verification.............................................................................................10
8.0 Execution of protocol for purified water system................................................................10
9.0 Portable water purification.................................................................................................10
9.1 Objectives.......................................................................................................................10
9.2 Procedure........................................................................................................................10
10.0 Conclusions and Summary...............................................................................................11
11.0 Protocol deviation form....................................................................................................11
12.0 Log entry for deviation.....................................................................................................11
13.0 Attachments......................................................................................................................11
14.0 References........................................................................................................................12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

University Name and Logo
Protocol No.: ############
Page | 3
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
1.0 Aim
The aim of this validation protocol to ensure in documented format that water purification
system works as expected. The primary objective of this protocol to authenticate the purified
water system as specified after the completion of successful Installation Qualification (IQ),
Design Qualification (DQ), and Operational Qualification (OQ). This purified water system
validation protocol will determine the testing procedures and acceptable criteria should be
used while validating Phase 1, Phase 2, Phase 3 and Phase 4. The intention behind this
protocol is to certify that the water purification system will work consistently as intended.
2.0 Scope
2.1 System component incorporated in this validating protocol
This validation protocol will determine the scope, methodology, documentation, and
references that should be used for the validation of the purified water system. Associated
tools for system validation is situated in the pilot plant. The specific components of the
equipment that will be mentioned in this protocol are as follows:
This protocol will determine the testing required for validation, instruction steps, and the
operating procedures for testing and implementation of this protocol. Data will be collected
and documented as mentioned into the protocol and each sets of data will be used to
determine whether each system component is working as intended.
A brief report will condense the collected raw data which will be analysed to draw
conclusions about the success implementation of validation process.
2.2 Overview of process and system description
The objective of this validation system is to provide purified water as per the standards
required by Pharmocopeia.
This process incorporated with different treatment process which area Pre- treatment,
Generation of purified water by deionisation and Reverse Osmosis (RO), sterilisation by UV
method, Ultrafiltration and finally Storage and Recirculation.
Overview of the process is provided in the table below:
Pre- treatment steps:
Portable supply of water
Particle filter (size 55 micron)
Water softening treatment
Particle filter (size 10 micron)
Steps of purified water generation:
RO system
Protocol No.: ############
Page | 3
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
1.0 Aim
The aim of this validation protocol to ensure in documented format that water purification
system works as expected. The primary objective of this protocol to authenticate the purified
water system as specified after the completion of successful Installation Qualification (IQ),
Design Qualification (DQ), and Operational Qualification (OQ). This purified water system
validation protocol will determine the testing procedures and acceptable criteria should be
used while validating Phase 1, Phase 2, Phase 3 and Phase 4. The intention behind this
protocol is to certify that the water purification system will work consistently as intended.
2.0 Scope
2.1 System component incorporated in this validating protocol
This validation protocol will determine the scope, methodology, documentation, and
references that should be used for the validation of the purified water system. Associated
tools for system validation is situated in the pilot plant. The specific components of the
equipment that will be mentioned in this protocol are as follows:
This protocol will determine the testing required for validation, instruction steps, and the
operating procedures for testing and implementation of this protocol. Data will be collected
and documented as mentioned into the protocol and each sets of data will be used to
determine whether each system component is working as intended.
A brief report will condense the collected raw data which will be analysed to draw
conclusions about the success implementation of validation process.
2.2 Overview of process and system description
The objective of this validation system is to provide purified water as per the standards
required by Pharmocopeia.
This process incorporated with different treatment process which area Pre- treatment,
Generation of purified water by deionisation and Reverse Osmosis (RO), sterilisation by UV
method, Ultrafiltration and finally Storage and Recirculation.
Overview of the process is provided in the table below:
Pre- treatment steps:
Portable supply of water
Particle filter (size 55 micron)
Water softening treatment
Particle filter (size 10 micron)
Steps of purified water generation:
RO system
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
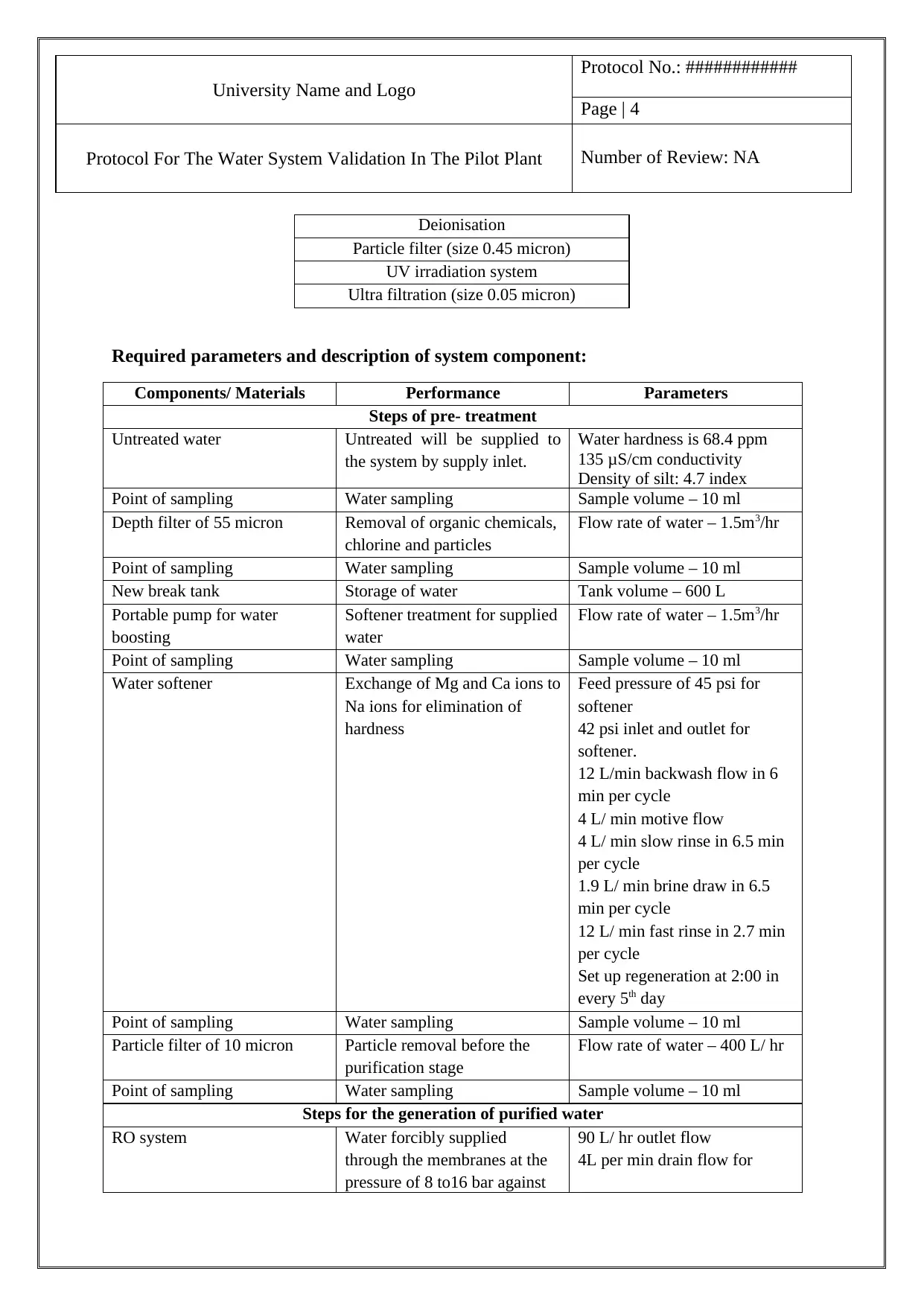
University Name and Logo
Protocol No.: ############
Page | 4
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
Deionisation
Particle filter (size 0.45 micron)
UV irradiation system
Ultra filtration (size 0.05 micron)
Required parameters and description of system component:
Components/ Materials Performance Parameters
Steps of pre- treatment
Untreated water Untreated will be supplied to
the system by supply inlet.
Water hardness is 68.4 ppm
135 μS/cm conductivity
Density of silt: 4.7 index
Point of sampling Water sampling Sample volume – 10 ml
Depth filter of 55 micron Removal of organic chemicals,
chlorine and particles
Flow rate of water – 1.5m3/hr
Point of sampling Water sampling Sample volume – 10 ml
New break tank Storage of water Tank volume – 600 L
Portable pump for water
boosting
Softener treatment for supplied
water
Flow rate of water – 1.5m3/hr
Point of sampling Water sampling Sample volume – 10 ml
Water softener Exchange of Mg and Ca ions to
Na ions for elimination of
hardness
Feed pressure of 45 psi for
softener
42 psi inlet and outlet for
softener.
12 L/min backwash flow in 6
min per cycle
4 L/ min motive flow
4 L/ min slow rinse in 6.5 min
per cycle
1.9 L/ min brine draw in 6.5
min per cycle
12 L/ min fast rinse in 2.7 min
per cycle
Set up regeneration at 2:00 in
every 5th day
Point of sampling Water sampling Sample volume – 10 ml
Particle filter of 10 micron Particle removal before the
purification stage
Flow rate of water – 400 L/ hr
Point of sampling Water sampling Sample volume – 10 ml
Steps for the generation of purified water
RO system Water forcibly supplied
through the membranes at the
pressure of 8 to16 bar against
90 L/ hr outlet flow
4L per min drain flow for
Protocol No.: ############
Page | 4
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
Deionisation
Particle filter (size 0.45 micron)
UV irradiation system
Ultra filtration (size 0.05 micron)
Required parameters and description of system component:
Components/ Materials Performance Parameters
Steps of pre- treatment
Untreated water Untreated will be supplied to
the system by supply inlet.
Water hardness is 68.4 ppm
135 μS/cm conductivity
Density of silt: 4.7 index
Point of sampling Water sampling Sample volume – 10 ml
Depth filter of 55 micron Removal of organic chemicals,
chlorine and particles
Flow rate of water – 1.5m3/hr
Point of sampling Water sampling Sample volume – 10 ml
New break tank Storage of water Tank volume – 600 L
Portable pump for water
boosting
Softener treatment for supplied
water
Flow rate of water – 1.5m3/hr
Point of sampling Water sampling Sample volume – 10 ml
Water softener Exchange of Mg and Ca ions to
Na ions for elimination of
hardness
Feed pressure of 45 psi for
softener
42 psi inlet and outlet for
softener.
12 L/min backwash flow in 6
min per cycle
4 L/ min motive flow
4 L/ min slow rinse in 6.5 min
per cycle
1.9 L/ min brine draw in 6.5
min per cycle
12 L/ min fast rinse in 2.7 min
per cycle
Set up regeneration at 2:00 in
every 5th day
Point of sampling Water sampling Sample volume – 10 ml
Particle filter of 10 micron Particle removal before the
purification stage
Flow rate of water – 400 L/ hr
Point of sampling Water sampling Sample volume – 10 ml
Steps for the generation of purified water
RO system Water forcibly supplied
through the membranes at the
pressure of 8 to16 bar against
90 L/ hr outlet flow
4L per min drain flow for
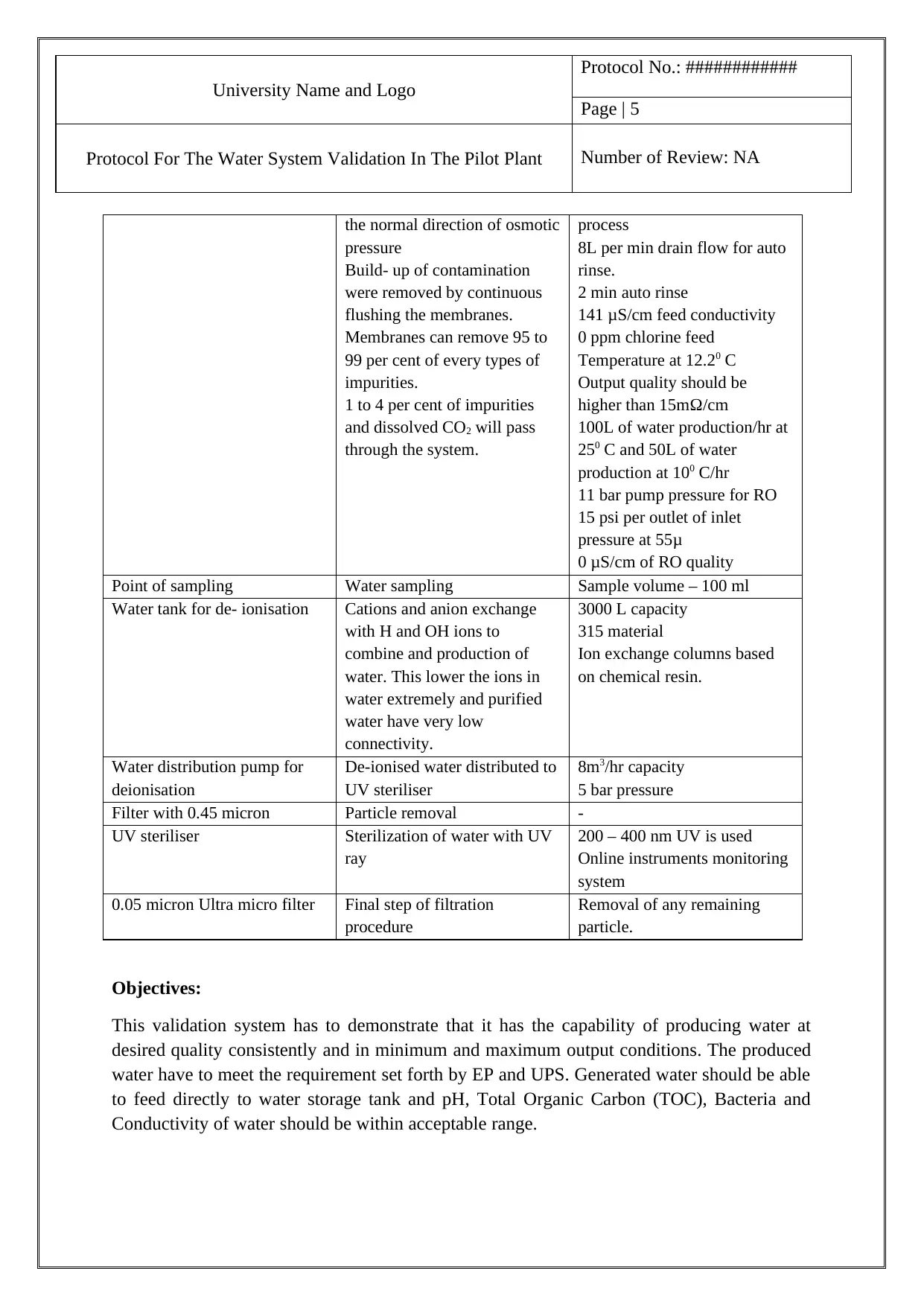
University Name and Logo
Protocol No.: ############
Page | 5
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
the normal direction of osmotic
pressure
Build- up of contamination
were removed by continuous
flushing the membranes.
Membranes can remove 95 to
99 per cent of every types of
impurities.
1 to 4 per cent of impurities
and dissolved CO2 will pass
through the system.
process
8L per min drain flow for auto
rinse.
2 min auto rinse
141 μS/cm feed conductivity
0 ppm chlorine feed
Temperature at 12.20 C
Output quality should be
higher than 15mΩ/cm
100L of water production/hr at
250 C and 50L of water
production at 100 C/hr
11 bar pump pressure for RO
15 psi per outlet of inlet
pressure at 55μ
0 μS/cm of RO quality
Point of sampling Water sampling Sample volume – 100 ml
Water tank for de- ionisation Cations and anion exchange
with H and OH ions to
combine and production of
water. This lower the ions in
water extremely and purified
water have very low
connectivity.
3000 L capacity
315 material
Ion exchange columns based
on chemical resin.
Water distribution pump for
deionisation
De-ionised water distributed to
UV steriliser
8m3/hr capacity
5 bar pressure
Filter with 0.45 micron Particle removal -
UV steriliser Sterilization of water with UV
ray
200 – 400 nm UV is used
Online instruments monitoring
system
0.05 micron Ultra micro filter Final step of filtration
procedure
Removal of any remaining
particle.
Objectives:
This validation system has to demonstrate that it has the capability of producing water at
desired quality consistently and in minimum and maximum output conditions. The produced
water have to meet the requirement set forth by EP and UPS. Generated water should be able
to feed directly to water storage tank and pH, Total Organic Carbon (TOC), Bacteria and
Conductivity of water should be within acceptable range.
Protocol No.: ############
Page | 5
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
the normal direction of osmotic
pressure
Build- up of contamination
were removed by continuous
flushing the membranes.
Membranes can remove 95 to
99 per cent of every types of
impurities.
1 to 4 per cent of impurities
and dissolved CO2 will pass
through the system.
process
8L per min drain flow for auto
rinse.
2 min auto rinse
141 μS/cm feed conductivity
0 ppm chlorine feed
Temperature at 12.20 C
Output quality should be
higher than 15mΩ/cm
100L of water production/hr at
250 C and 50L of water
production at 100 C/hr
11 bar pump pressure for RO
15 psi per outlet of inlet
pressure at 55μ
0 μS/cm of RO quality
Point of sampling Water sampling Sample volume – 100 ml
Water tank for de- ionisation Cations and anion exchange
with H and OH ions to
combine and production of
water. This lower the ions in
water extremely and purified
water have very low
connectivity.
3000 L capacity
315 material
Ion exchange columns based
on chemical resin.
Water distribution pump for
deionisation
De-ionised water distributed to
UV steriliser
8m3/hr capacity
5 bar pressure
Filter with 0.45 micron Particle removal -
UV steriliser Sterilization of water with UV
ray
200 – 400 nm UV is used
Online instruments monitoring
system
0.05 micron Ultra micro filter Final step of filtration
procedure
Removal of any remaining
particle.
Objectives:
This validation system has to demonstrate that it has the capability of producing water at
desired quality consistently and in minimum and maximum output conditions. The produced
water have to meet the requirement set forth by EP and UPS. Generated water should be able
to feed directly to water storage tank and pH, Total Organic Carbon (TOC), Bacteria and
Conductivity of water should be within acceptable range.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
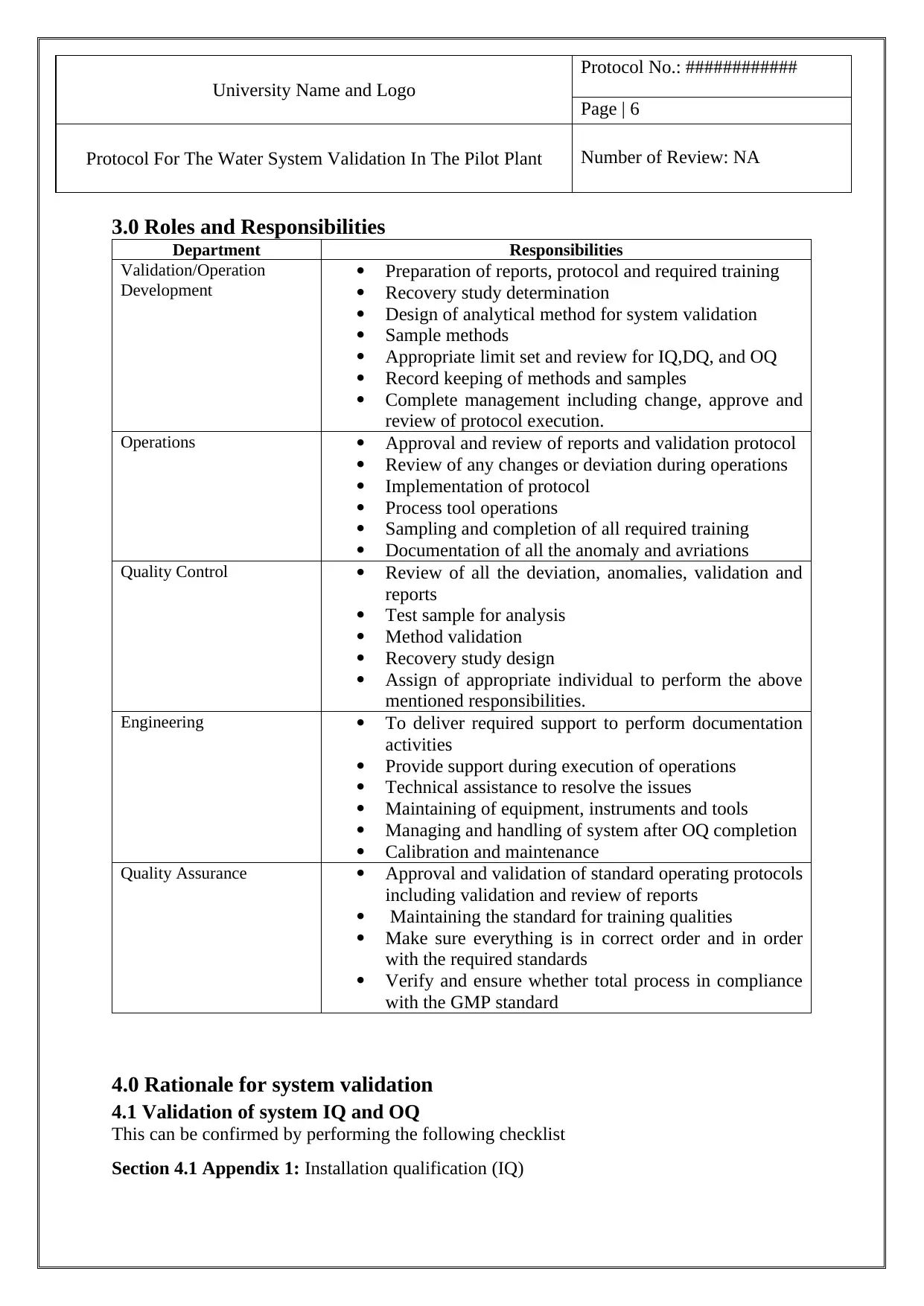
University Name and Logo
Protocol No.: ############
Page | 6
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
3.0 Roles and Responsibilities
Department Responsibilities
Validation/Operation
Development
Preparation of reports, protocol and required training
Recovery study determination
Design of analytical method for system validation
Sample methods
Appropriate limit set and review for IQ,DQ, and OQ
Record keeping of methods and samples
Complete management including change, approve and
review of protocol execution.
Operations Approval and review of reports and validation protocol
Review of any changes or deviation during operations
Implementation of protocol
Process tool operations
Sampling and completion of all required training
Documentation of all the anomaly and avriations
Quality Control Review of all the deviation, anomalies, validation and
reports
Test sample for analysis
Method validation
Recovery study design
Assign of appropriate individual to perform the above
mentioned responsibilities.
Engineering To deliver required support to perform documentation
activities
Provide support during execution of operations
Technical assistance to resolve the issues
Maintaining of equipment, instruments and tools
Managing and handling of system after OQ completion
Calibration and maintenance
Quality Assurance Approval and validation of standard operating protocols
including validation and review of reports
Maintaining the standard for training qualities
Make sure everything is in correct order and in order
with the required standards
Verify and ensure whether total process in compliance
with the GMP standard
4.0 Rationale for system validation
4.1 Validation of system IQ and OQ
This can be confirmed by performing the following checklist
Section 4.1 Appendix 1: Installation qualification (IQ)
Protocol No.: ############
Page | 6
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
3.0 Roles and Responsibilities
Department Responsibilities
Validation/Operation
Development
Preparation of reports, protocol and required training
Recovery study determination
Design of analytical method for system validation
Sample methods
Appropriate limit set and review for IQ,DQ, and OQ
Record keeping of methods and samples
Complete management including change, approve and
review of protocol execution.
Operations Approval and review of reports and validation protocol
Review of any changes or deviation during operations
Implementation of protocol
Process tool operations
Sampling and completion of all required training
Documentation of all the anomaly and avriations
Quality Control Review of all the deviation, anomalies, validation and
reports
Test sample for analysis
Method validation
Recovery study design
Assign of appropriate individual to perform the above
mentioned responsibilities.
Engineering To deliver required support to perform documentation
activities
Provide support during execution of operations
Technical assistance to resolve the issues
Maintaining of equipment, instruments and tools
Managing and handling of system after OQ completion
Calibration and maintenance
Quality Assurance Approval and validation of standard operating protocols
including validation and review of reports
Maintaining the standard for training qualities
Make sure everything is in correct order and in order
with the required standards
Verify and ensure whether total process in compliance
with the GMP standard
4.0 Rationale for system validation
4.1 Validation of system IQ and OQ
This can be confirmed by performing the following checklist
Section 4.1 Appendix 1: Installation qualification (IQ)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
University Name and Logo
Protocol No.: ############
Page | 7
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
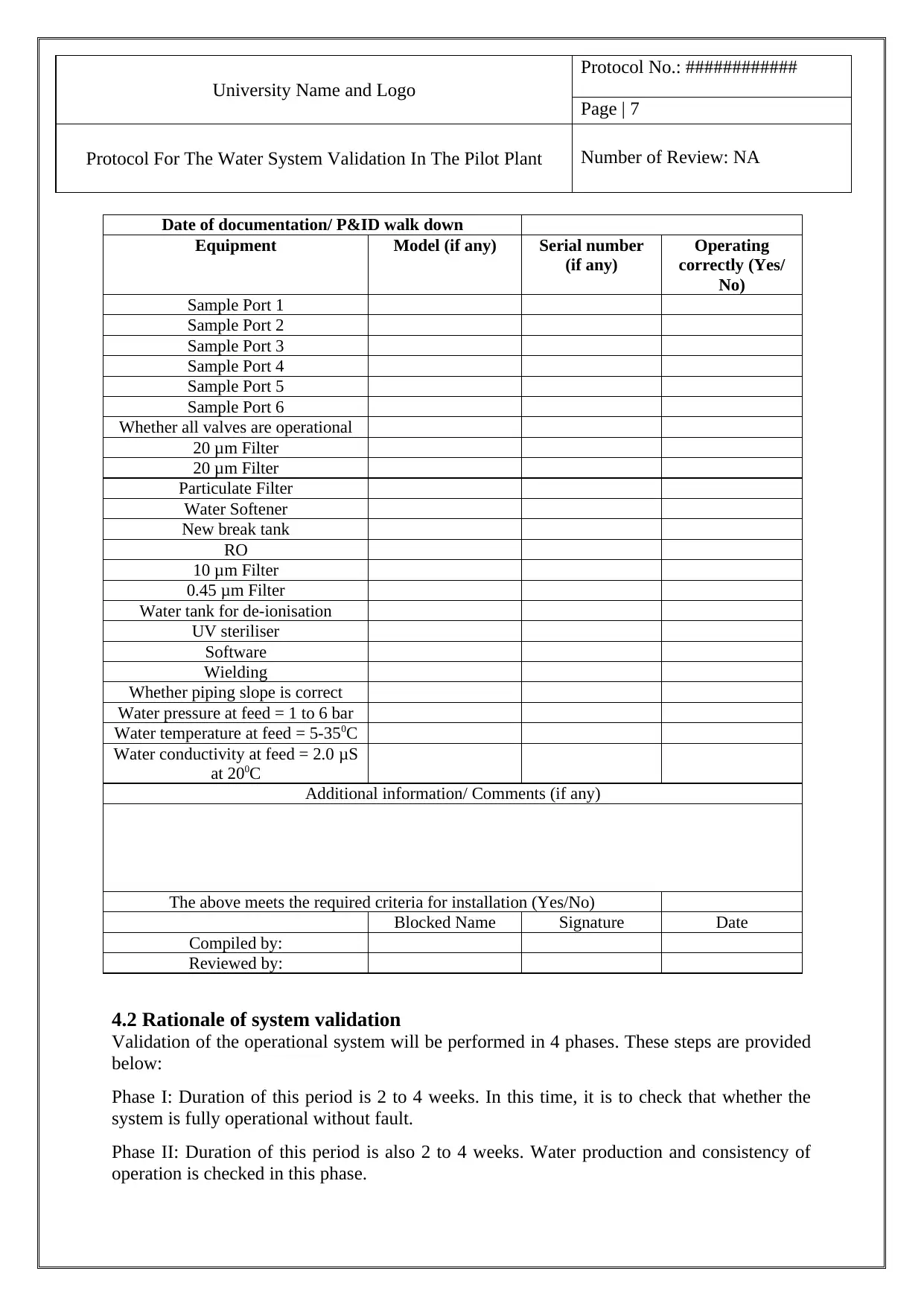
Date of documentation/ P&ID walk down
Equipment Model (if any) Serial number
(if any)
Operating
correctly (Yes/
No)
Sample Port 1
Sample Port 2
Sample Port 3
Sample Port 4
Sample Port 5
Sample Port 6
Whether all valves are operational
20 μm Filter
20 μm Filter
Particulate Filter
Water Softener
New break tank
RO
10 μm Filter
0.45 μm Filter
Water tank for de-ionisation
UV steriliser
Software
Wielding
Whether piping slope is correct
Water pressure at feed = 1 to 6 bar
Water temperature at feed = 5-350C
Water conductivity at feed = 2.0 μS
at 200C
Additional information/ Comments (if any)
The above meets the required criteria for installation (Yes/No)
Blocked Name Signature Date
Compiled by:
Reviewed by:
4.2 Rationale of system validation
Validation of the operational system will be performed in 4 phases. These steps are provided
below:
Phase I: Duration of this period is 2 to 4 weeks. In this time, it is to check that whether the
system is fully operational without fault.
Phase II: Duration of this period is also 2 to 4 weeks. Water production and consistency of
operation is checked in this phase.
Protocol No.: ############
Page | 7
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
Date of documentation/ P&ID walk down
Equipment Model (if any) Serial number
(if any)
Operating
correctly (Yes/
No)
Sample Port 1
Sample Port 2
Sample Port 3
Sample Port 4
Sample Port 5
Sample Port 6
Whether all valves are operational
20 μm Filter
20 μm Filter
Particulate Filter
Water Softener
New break tank
RO
10 μm Filter
0.45 μm Filter
Water tank for de-ionisation
UV steriliser
Software
Wielding
Whether piping slope is correct
Water pressure at feed = 1 to 6 bar
Water temperature at feed = 5-350C
Water conductivity at feed = 2.0 μS
at 200C
Additional information/ Comments (if any)
The above meets the required criteria for installation (Yes/No)
Blocked Name Signature Date
Compiled by:
Reviewed by:
4.2 Rationale of system validation
Validation of the operational system will be performed in 4 phases. These steps are provided
below:
Phase I: Duration of this period is 2 to 4 weeks. In this time, it is to check that whether the
system is fully operational without fault.
Phase II: Duration of this period is also 2 to 4 weeks. Water production and consistency of
operation is checked in this phase.
University Name and Logo
Protocol No.: ############
Page | 8
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
Phase III: Duration of this step is for 1 year after the initial success of Phase I. This step
checks the reliability for extended period.
Phase IV: In this phase, continuous monitoring of all the process is performed. Additionally,
this step verifies that all the process parameters are within acceptable range.
4.3 Execution of validation
Validation of the water system protocol will performed according to this protocol and
validation report will be generated as per Table 10.
4.4 Operational protocol
Standard operational procedures will be adopted during the execution of this protocol for
operation of water purified system.
5.0 Sampling
Sampling will be performed according to the sampling point mentioned in Table 10.
5.1 Sampling procedures
Aseptic technique will be adopted during sampling process to avoid any
contamination
All sampling points in the system will be used for sample collection
70 per cent ethanol will be used to sterilized the sampling point before sample
collection
10 ml sample to be taken and sealed before pre- treatment process.
100 ml sample will be collected in sterile container after pre-treatment zone.
All sampling container will be labelled appropriately and to be kept in dark at 40C.
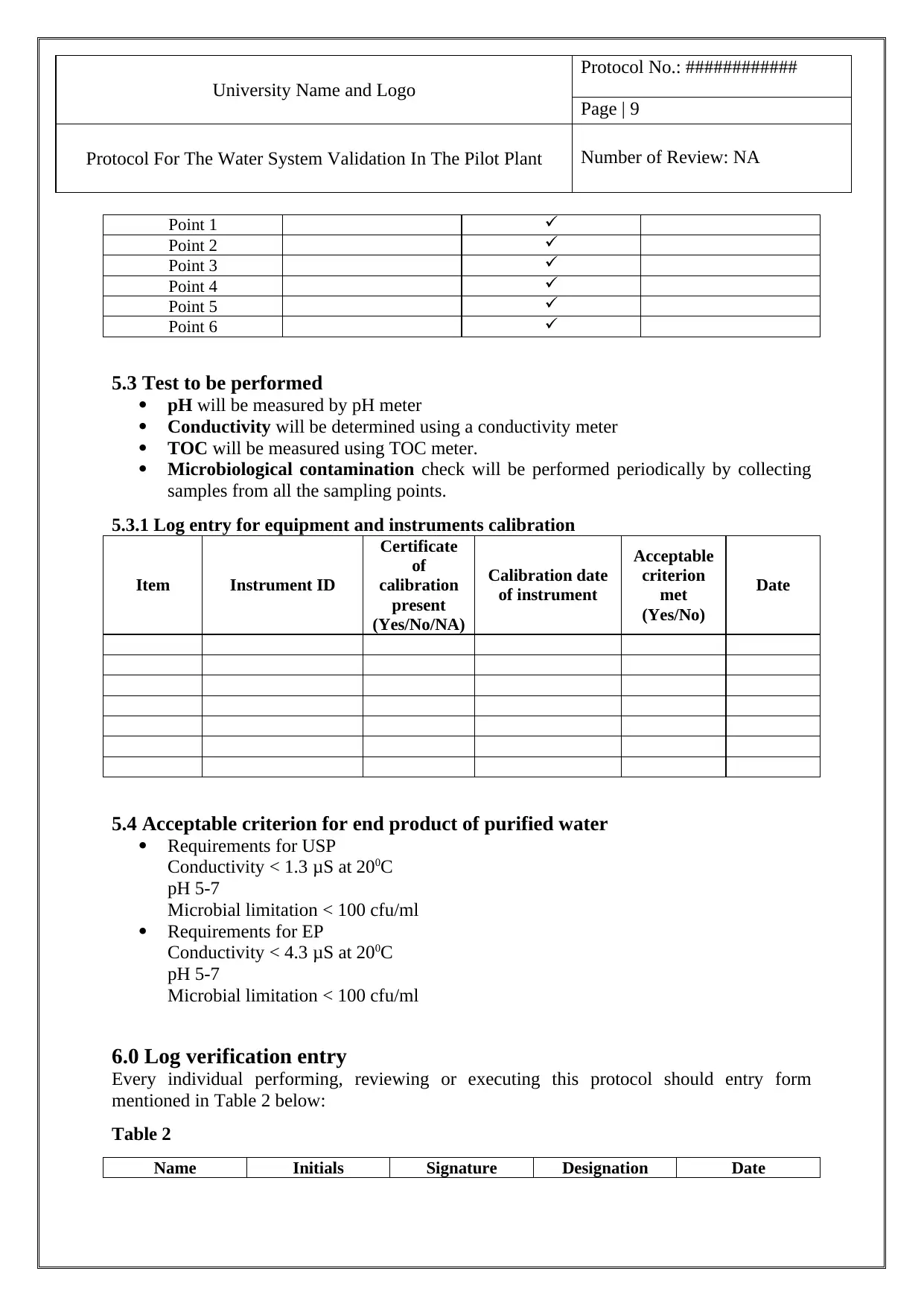
5.2 Point of sample collection
Point 1
Point 2
Point 3
Point 4
Point 5
Point 6
Sampling Plan:
Phase 1 and 2
( 2 to 4 weeks) Periods
Point of sampling Daily Weekly Monthly
Point 1
Point 2
Point 3
Point 4
Point 5
Point 6
Phase 3 (1 year)
Point of sampling Daily Weekly Monthly
Protocol No.: ############
Page | 8
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
Phase III: Duration of this step is for 1 year after the initial success of Phase I. This step
checks the reliability for extended period.
Phase IV: In this phase, continuous monitoring of all the process is performed. Additionally,
this step verifies that all the process parameters are within acceptable range.
4.3 Execution of validation
Validation of the water system protocol will performed according to this protocol and
validation report will be generated as per Table 10.
4.4 Operational protocol
Standard operational procedures will be adopted during the execution of this protocol for
operation of water purified system.
5.0 Sampling
Sampling will be performed according to the sampling point mentioned in Table 10.
5.1 Sampling procedures
Aseptic technique will be adopted during sampling process to avoid any
contamination
All sampling points in the system will be used for sample collection
70 per cent ethanol will be used to sterilized the sampling point before sample
collection
10 ml sample to be taken and sealed before pre- treatment process.
100 ml sample will be collected in sterile container after pre-treatment zone.
All sampling container will be labelled appropriately and to be kept in dark at 40C.
5.2 Point of sample collection
Point 1
Point 2
Point 3
Point 4
Point 5
Point 6
Sampling Plan:
Phase 1 and 2
( 2 to 4 weeks) Periods
Point of sampling Daily Weekly Monthly
Point 1
Point 2
Point 3
Point 4
Point 5
Point 6
Phase 3 (1 year)
Point of sampling Daily Weekly Monthly
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
University Name and Logo
Protocol No.: ############
Page | 9
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
Point 1
Point 2
Point 3
Point 4
Point 5
Point 6
5.3 Test to be performed
pH will be measured by pH meter
Conductivity will be determined using a conductivity meter
TOC will be measured using TOC meter.
Microbiological contamination check will be performed periodically by collecting
samples from all the sampling points.
5.3.1 Log entry for equipment and instruments calibration
Item Instrument ID
Certificate
of
calibration
present
(Yes/No/NA)
Calibration date
of instrument
Acceptable
criterion
met
(Yes/No)
Date
5.4 Acceptable criterion for end product of purified water
Requirements for USP
Conductivity < 1.3 μS at 200C
pH 5-7
Microbial limitation < 100 cfu/ml
Requirements for EP
Conductivity < 4.3 μS at 200C
pH 5-7
Microbial limitation < 100 cfu/ml
6.0 Log verification entry
Every individual performing, reviewing or executing this protocol should entry form
mentioned in Table 2 below:
Table 2
Name Initials Signature Designation Date
Protocol No.: ############
Page | 9
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
Point 1
Point 2
Point 3
Point 4
Point 5
Point 6
5.3 Test to be performed
pH will be measured by pH meter
Conductivity will be determined using a conductivity meter
TOC will be measured using TOC meter.
Microbiological contamination check will be performed periodically by collecting
samples from all the sampling points.
5.3.1 Log entry for equipment and instruments calibration
Item Instrument ID
Certificate
of
calibration
present
(Yes/No/NA)
Calibration date
of instrument
Acceptable
criterion
met
(Yes/No)
Date
5.4 Acceptable criterion for end product of purified water
Requirements for USP
Conductivity < 1.3 μS at 200C
pH 5-7
Microbial limitation < 100 cfu/ml
Requirements for EP
Conductivity < 4.3 μS at 200C
pH 5-7
Microbial limitation < 100 cfu/ml
6.0 Log verification entry
Every individual performing, reviewing or executing this protocol should entry form
mentioned in Table 2 below:
Table 2
Name Initials Signature Designation Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

University Name and Logo
Protocol No.: ############
Page | 10
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
Comments:
Reviewed by: Date:
7.0 Preconditions for verification
All the preconditions should be verified and signed by the respective authorities before the
start of the validation run. Additionally, it has to ensure that IQ, DQ and OQ have been
completed successfully along with required documentation.
8.0 Execution of protocol for purified water system
Phase I
Phase II
Phase III
9.0 Portable water purification
9.1 Objectives
The goal of this step is to execute validation system to validate overall water purification
system which includes Phase I, Phase II, and Phase III.
9.2 Procedure
To be performed according to the Table 7 below:
Table 7
Phase 1 to 3
1. Personnel responsible for executing the protocol should fill up the Table 2 first.
2. All the responsible personnel have the required qualification and training.
3. Verification should be done according to the Table 1 and calibration verification according to the
Table 3
4. Verification of every component OQ have been successfully documented and completed.
5. Records must be kept for all the procedures and execution according to the Table 4.
6. Each equipment should be prepared according to the operating instructions
7. Make certain all the components, materials and filters are documented and accounted.
8. Purification system should be start as per the instructions.
9. Each phase of sampling validation should be performed according to the Table 5.
10. Samples to be tested according to the QC’s SOP.
Protocol No.: ############
Page | 10
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
Comments:
Reviewed by: Date:
7.0 Preconditions for verification
All the preconditions should be verified and signed by the respective authorities before the
start of the validation run. Additionally, it has to ensure that IQ, DQ and OQ have been
completed successfully along with required documentation.
8.0 Execution of protocol for purified water system
Phase I
Phase II
Phase III
9.0 Portable water purification
9.1 Objectives
The goal of this step is to execute validation system to validate overall water purification
system which includes Phase I, Phase II, and Phase III.
9.2 Procedure
To be performed according to the Table 7 below:
Table 7
Phase 1 to 3
1. Personnel responsible for executing the protocol should fill up the Table 2 first.
2. All the responsible personnel have the required qualification and training.
3. Verification should be done according to the Table 1 and calibration verification according to the
Table 3
4. Verification of every component OQ have been successfully documented and completed.
5. Records must be kept for all the procedures and execution according to the Table 4.
6. Each equipment should be prepared according to the operating instructions
7. Make certain all the components, materials and filters are documented and accounted.
8. Purification system should be start as per the instructions.
9. Each phase of sampling validation should be performed according to the Table 5.
10. Samples to be tested according to the QC’s SOP.
University Name and Logo
Protocol No.: ############
Page | 11
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
11. Records should be kept in the print out attached to the protocol.
12. Results have to be compared with the standard criterion and PASS or FAIL remark has to be
entered in the Table 6.
13. All deviation and failures should be recorded in the Table 6.
14. 1 to 3 steps are to be repeated until the Phases 1 to 3 are to be completed.
15. Phase 4 can only start after the successful completion of Phase I to III
16. Phase IV is to be performed routinely to check the consistency and to assess the compliance of
final quality of produced water.
10.0 Conclusions and Summary
Ensure that all the procedures have been carried out according to the protocol instruction and
completed with the assurance of quality that final water produced have been met the
predetermined standard criterion.
11.0 Protocol deviation form
Deviations will be resolved as per the validation deviation instruction and will be recorded in
validation report. All the deviation should be addressed before the approval of validation
report.
12.0 Log entry for deviation
Description of log entry for deviation is mentioned in the Table 8 below:
Table 8
Deviation No. Category of
deviation
Section and page
reference
Details Date
Comments:
Reviewed by: Date:
13.0 Attachments
At the time of the protocol execution, signed detailed record should be kept for each
attachment according to the Table 9 mentioned below:
Table 9
Attachment Step Particulars Signature Date
Protocol No.: ############
Page | 11
Protocol For The Water System Validation In The Pilot Plant Number of Review: NA
11. Records should be kept in the print out attached to the protocol.
12. Results have to be compared with the standard criterion and PASS or FAIL remark has to be
entered in the Table 6.
13. All deviation and failures should be recorded in the Table 6.
14. 1 to 3 steps are to be repeated until the Phases 1 to 3 are to be completed.
15. Phase 4 can only start after the successful completion of Phase I to III
16. Phase IV is to be performed routinely to check the consistency and to assess the compliance of
final quality of produced water.
10.0 Conclusions and Summary
Ensure that all the procedures have been carried out according to the protocol instruction and
completed with the assurance of quality that final water produced have been met the
predetermined standard criterion.
11.0 Protocol deviation form
Deviations will be resolved as per the validation deviation instruction and will be recorded in
validation report. All the deviation should be addressed before the approval of validation
report.
12.0 Log entry for deviation
Description of log entry for deviation is mentioned in the Table 8 below:
Table 8
Deviation No. Category of
deviation
Section and page
reference
Details Date
Comments:
Reviewed by: Date:
13.0 Attachments
At the time of the protocol execution, signed detailed record should be kept for each
attachment according to the Table 9 mentioned below:
Table 9
Attachment Step Particulars Signature Date
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 14
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
