University Course: Water Treatment for Safe Drinking Water
VerifiedAdded on 2023/06/05
|9
|2300
|208
Report
AI Summary
This report addresses the critical need for water treatment processes to ensure safe drinking water for a community, considering varying water quality characteristics during drought and flooding conditions. The paper examines key water quality parameters such as total dissolved solids (TDS), color, dissolved organic carbon (DOC), and turbidity, comparing them against Australian water quality guidelines. The report proposes specific treatment methods based on the identified water characteristics. For high TDS levels, reverse osmosis is recommended, detailing the process and its components. Water discoloration is addressed using potassium permanganate to oxidize organic matter. Magnetic ion exchange (MIEX) is proposed for DOC removal, while turbidity is treated with sand and cartridge filters. The report emphasizes the importance of these treatments in providing accessible and safe drinking water throughout the year.

Name of the University
Course Name
Course Code
Proposed treatment process for the two water types to provide safe drinking water for the
community
Author’s Name
Student ID
Name of supervisor
Date of submission
Course Name
Course Code
Proposed treatment process for the two water types to provide safe drinking water for the
community
Author’s Name
Student ID
Name of supervisor
Date of submission
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Abstract
This paper specifically addresses the treatment processes of water in instances where the
total dissolved solid, turbidity, dissolved organic carbon and turbidity levels were found to be
undesirable for drinking water. All references of these parameters are compared to the Australian
water quality guidelines hence determining the need for the water treatment so that safe water for
drinking is made available to consumers. Two contexts are applicable, water quality
characteristics during drought and during flooding. Water quality characteristics to be found
unsuitable are recommended for treatment before its use. The treatment process is as discussed in
the following section based on the water characteristics determined.
This paper specifically addresses the treatment processes of water in instances where the
total dissolved solid, turbidity, dissolved organic carbon and turbidity levels were found to be
undesirable for drinking water. All references of these parameters are compared to the Australian
water quality guidelines hence determining the need for the water treatment so that safe water for
drinking is made available to consumers. Two contexts are applicable, water quality
characteristics during drought and during flooding. Water quality characteristics to be found
unsuitable are recommended for treatment before its use. The treatment process is as discussed in
the following section based on the water characteristics determined.

1. Introduction
Secure and safe water supply for drinking water is essential for ensured and guaranteed
public health. This can be aided by ensuring that the water quality for drinking is significantly
adhered to as stated in the Australian drinking water guidelines. This is attainable through water
treatment process and procedures to rid of any dangerous levels of pollutants (Anon Australian
Drinking Water Guidelines, 2011). For instance, in this case there has been notable observation
on the variations on levels of water characteristics such as amount of total dissolved solids
(TDS), color, dissolved organic carbon (DOC), and turbidity levels hence a cause of concern
since these levels fluctuate to unsafe levels hence compromising quality of drinking water for the
community. The variations in these water characteristics have been identified to vary seasonally,
that is during dry (drought conditions) and rainy (flooding) seasons (McNamara, 2012). The
cause of these variation in water characteristics therefore can be identified to be majorly as a
result of changing source water conditions such as flooding. In this regard therefore, this paper
focuses to propose water treatment processes for the river during drought as well as during
flooding to ensure supply of safe drinking water to the community in both seasons.
Total dissolved solids TDS is defined as the level of dissolved particles in water
including suspended particles not passing the filter system. In a river TDS include both organic
(e.g. planktons, silt, sewage, plant leaves and floating vegetation etc.) and inorganic materials
(rocks and compounds of iron, Sulphur calcium and other mineral constituents). Water color
measure is obtained by use of an arbitrary standard scale that measures color intensity in water
samples. Dissolved organic carbon DOC is defined as the sum amounts of organic carbon
compounds passing the 45 micrometer filter. DOC influences water characteristics such as color,
pollutants degradation, and depth of the photic zone as well as PH levels through its influence on
the geochemical processes in water. Decomposition of plant and/ or mineral particles is the
primary source of organic carbon in water. Turbidity on other hand measures relate water clarity
and is influenced by factors such as soluble colored organic compounds, planktons, silt, clay etc.
(Gray, 2008).
2.0 Proposed treatment process for the river during both the seasons is discussed in the
following section:
2.1 Total dissolved solids (TDS).
Secure and safe water supply for drinking water is essential for ensured and guaranteed
public health. This can be aided by ensuring that the water quality for drinking is significantly
adhered to as stated in the Australian drinking water guidelines. This is attainable through water
treatment process and procedures to rid of any dangerous levels of pollutants (Anon Australian
Drinking Water Guidelines, 2011). For instance, in this case there has been notable observation
on the variations on levels of water characteristics such as amount of total dissolved solids
(TDS), color, dissolved organic carbon (DOC), and turbidity levels hence a cause of concern
since these levels fluctuate to unsafe levels hence compromising quality of drinking water for the
community. The variations in these water characteristics have been identified to vary seasonally,
that is during dry (drought conditions) and rainy (flooding) seasons (McNamara, 2012). The
cause of these variation in water characteristics therefore can be identified to be majorly as a
result of changing source water conditions such as flooding. In this regard therefore, this paper
focuses to propose water treatment processes for the river during drought as well as during
flooding to ensure supply of safe drinking water to the community in both seasons.
Total dissolved solids TDS is defined as the level of dissolved particles in water
including suspended particles not passing the filter system. In a river TDS include both organic
(e.g. planktons, silt, sewage, plant leaves and floating vegetation etc.) and inorganic materials
(rocks and compounds of iron, Sulphur calcium and other mineral constituents). Water color
measure is obtained by use of an arbitrary standard scale that measures color intensity in water
samples. Dissolved organic carbon DOC is defined as the sum amounts of organic carbon
compounds passing the 45 micrometer filter. DOC influences water characteristics such as color,
pollutants degradation, and depth of the photic zone as well as PH levels through its influence on
the geochemical processes in water. Decomposition of plant and/ or mineral particles is the
primary source of organic carbon in water. Turbidity on other hand measures relate water clarity
and is influenced by factors such as soluble colored organic compounds, planktons, silt, clay etc.
(Gray, 2008).
2.0 Proposed treatment process for the river during both the seasons is discussed in the
following section:
2.1 Total dissolved solids (TDS).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
The concentration of total dissolved solids at the source during drought condition was
found to be greater than 1500 mg/ Litre and less than 150 mg/L during flooding. Based on TDS
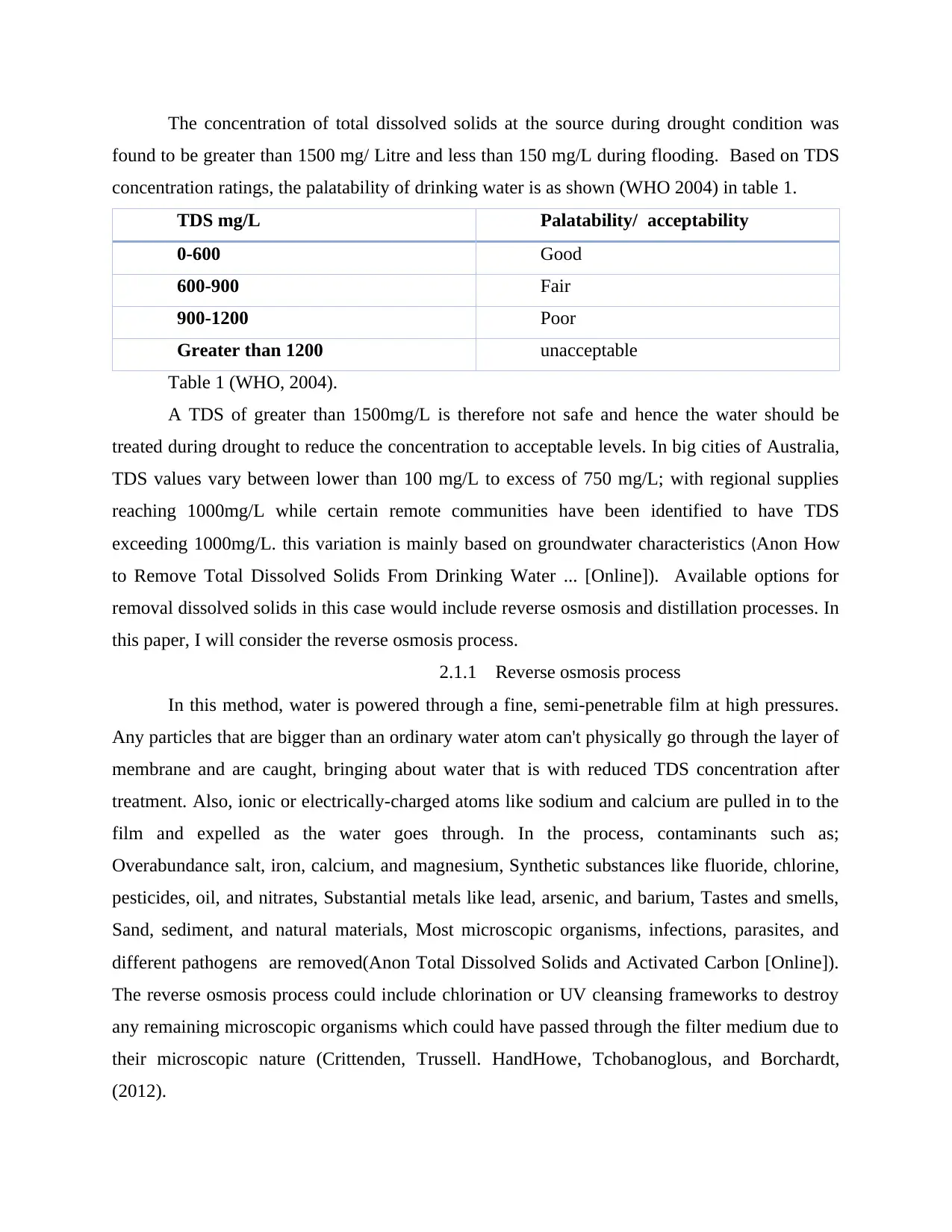
concentration ratings, the palatability of drinking water is as shown (WHO 2004) in table 1.
TDS mg/L Palatability/ acceptability
0-600 Good
600-900 Fair
900-1200 Poor
Greater than 1200 unacceptable
Table 1 (WHO, 2004).
A TDS of greater than 1500mg/L is therefore not safe and hence the water should be
treated during drought to reduce the concentration to acceptable levels. In big cities of Australia,
TDS values vary between lower than 100 mg/L to excess of 750 mg/L; with regional supplies
reaching 1000mg/L while certain remote communities have been identified to have TDS
exceeding 1000mg/L. this variation is mainly based on groundwater characteristics (Anon How
to Remove Total Dissolved Solids From Drinking Water ... [Online]). Available options for
removal dissolved solids in this case would include reverse osmosis and distillation processes. In
this paper, I will consider the reverse osmosis process.
2.1.1 Reverse osmosis process
In this method, water is powered through a fine, semi-penetrable film at high pressures.
Any particles that are bigger than an ordinary water atom can't physically go through the layer of
membrane and are caught, bringing about water that is with reduced TDS concentration after
treatment. Also, ionic or electrically-charged atoms like sodium and calcium are pulled in to the
film and expelled as the water goes through. In the process, contaminants such as;
Overabundance salt, iron, calcium, and magnesium, Synthetic substances like fluoride, chlorine,
pesticides, oil, and nitrates, Substantial metals like lead, arsenic, and barium, Tastes and smells,
Sand, sediment, and natural materials, Most microscopic organisms, infections, parasites, and
different pathogens are removed(Anon Total Dissolved Solids and Activated Carbon [Online]).
The reverse osmosis process could include chlorination or UV cleansing frameworks to destroy
any remaining microscopic organisms which could have passed through the filter medium due to
their microscopic nature (Crittenden, Trussell. HandHowe, Tchobanoglous, and Borchardt,
(2012).
found to be greater than 1500 mg/ Litre and less than 150 mg/L during flooding. Based on TDS
concentration ratings, the palatability of drinking water is as shown (WHO 2004) in table 1.
TDS mg/L Palatability/ acceptability
0-600 Good
600-900 Fair
900-1200 Poor
Greater than 1200 unacceptable
Table 1 (WHO, 2004).
A TDS of greater than 1500mg/L is therefore not safe and hence the water should be
treated during drought to reduce the concentration to acceptable levels. In big cities of Australia,
TDS values vary between lower than 100 mg/L to excess of 750 mg/L; with regional supplies
reaching 1000mg/L while certain remote communities have been identified to have TDS
exceeding 1000mg/L. this variation is mainly based on groundwater characteristics (Anon How
to Remove Total Dissolved Solids From Drinking Water ... [Online]). Available options for
removal dissolved solids in this case would include reverse osmosis and distillation processes. In
this paper, I will consider the reverse osmosis process.
2.1.1 Reverse osmosis process
In this method, water is powered through a fine, semi-penetrable film at high pressures.
Any particles that are bigger than an ordinary water atom can't physically go through the layer of
membrane and are caught, bringing about water that is with reduced TDS concentration after
treatment. Also, ionic or electrically-charged atoms like sodium and calcium are pulled in to the
film and expelled as the water goes through. In the process, contaminants such as;
Overabundance salt, iron, calcium, and magnesium, Synthetic substances like fluoride, chlorine,
pesticides, oil, and nitrates, Substantial metals like lead, arsenic, and barium, Tastes and smells,
Sand, sediment, and natural materials, Most microscopic organisms, infections, parasites, and
different pathogens are removed(Anon Total Dissolved Solids and Activated Carbon [Online]).
The reverse osmosis process could include chlorination or UV cleansing frameworks to destroy
any remaining microscopic organisms which could have passed through the filter medium due to
their microscopic nature (Crittenden, Trussell. HandHowe, Tchobanoglous, and Borchardt,
(2012).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
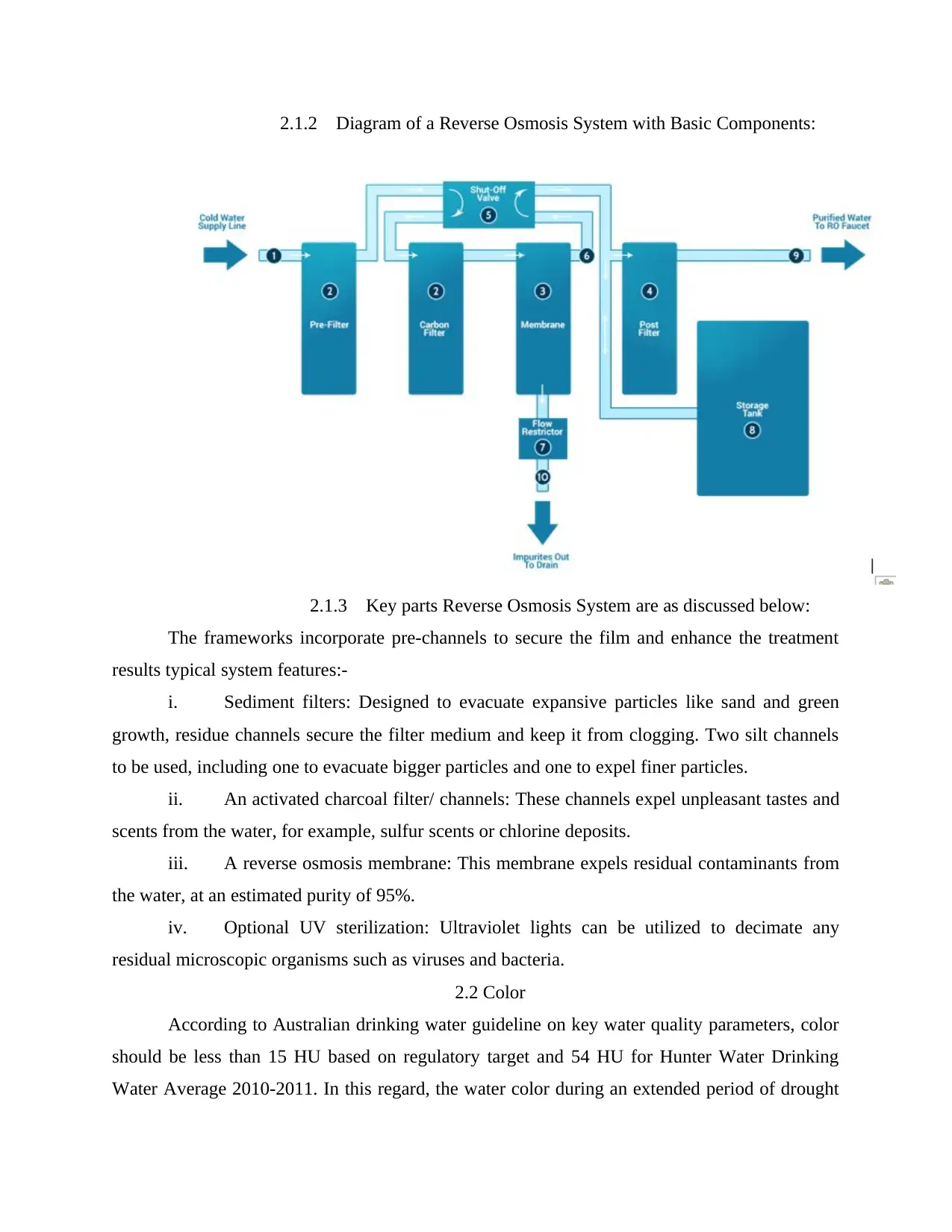
2.1.2 Diagram of a Reverse Osmosis System with Basic Components:
2.1.3 Key parts Reverse Osmosis System are as discussed below:
The frameworks incorporate pre-channels to secure the film and enhance the treatment
results typical system features:-
i. Sediment filters: Designed to evacuate expansive particles like sand and green
growth, residue channels secure the filter medium and keep it from clogging. Two silt channels
to be used, including one to evacuate bigger particles and one to expel finer particles.
ii. An activated charcoal filter/ channels: These channels expel unpleasant tastes and
scents from the water, for example, sulfur scents or chlorine deposits.
iii. A reverse osmosis membrane: This membrane expels residual contaminants from
the water, at an estimated purity of 95%.
iv. Optional UV sterilization: Ultraviolet lights can be utilized to decimate any
residual microscopic organisms such as viruses and bacteria.
2.2 Color
According to Australian drinking water guideline on key water quality parameters, color
should be less than 15 HU based on regulatory target and 54 HU for Hunter Water Drinking
Water Average 2010-2011. In this regard, the water color during an extended period of drought
2.1.3 Key parts Reverse Osmosis System are as discussed below:
The frameworks incorporate pre-channels to secure the film and enhance the treatment
results typical system features:-
i. Sediment filters: Designed to evacuate expansive particles like sand and green
growth, residue channels secure the filter medium and keep it from clogging. Two silt channels
to be used, including one to evacuate bigger particles and one to expel finer particles.
ii. An activated charcoal filter/ channels: These channels expel unpleasant tastes and
scents from the water, for example, sulfur scents or chlorine deposits.
iii. A reverse osmosis membrane: This membrane expels residual contaminants from
the water, at an estimated purity of 95%.
iv. Optional UV sterilization: Ultraviolet lights can be utilized to decimate any
residual microscopic organisms such as viruses and bacteria.
2.2 Color
According to Australian drinking water guideline on key water quality parameters, color
should be less than 15 HU based on regulatory target and 54 HU for Hunter Water Drinking
Water Average 2010-2011. In this regard, the water color during an extended period of drought
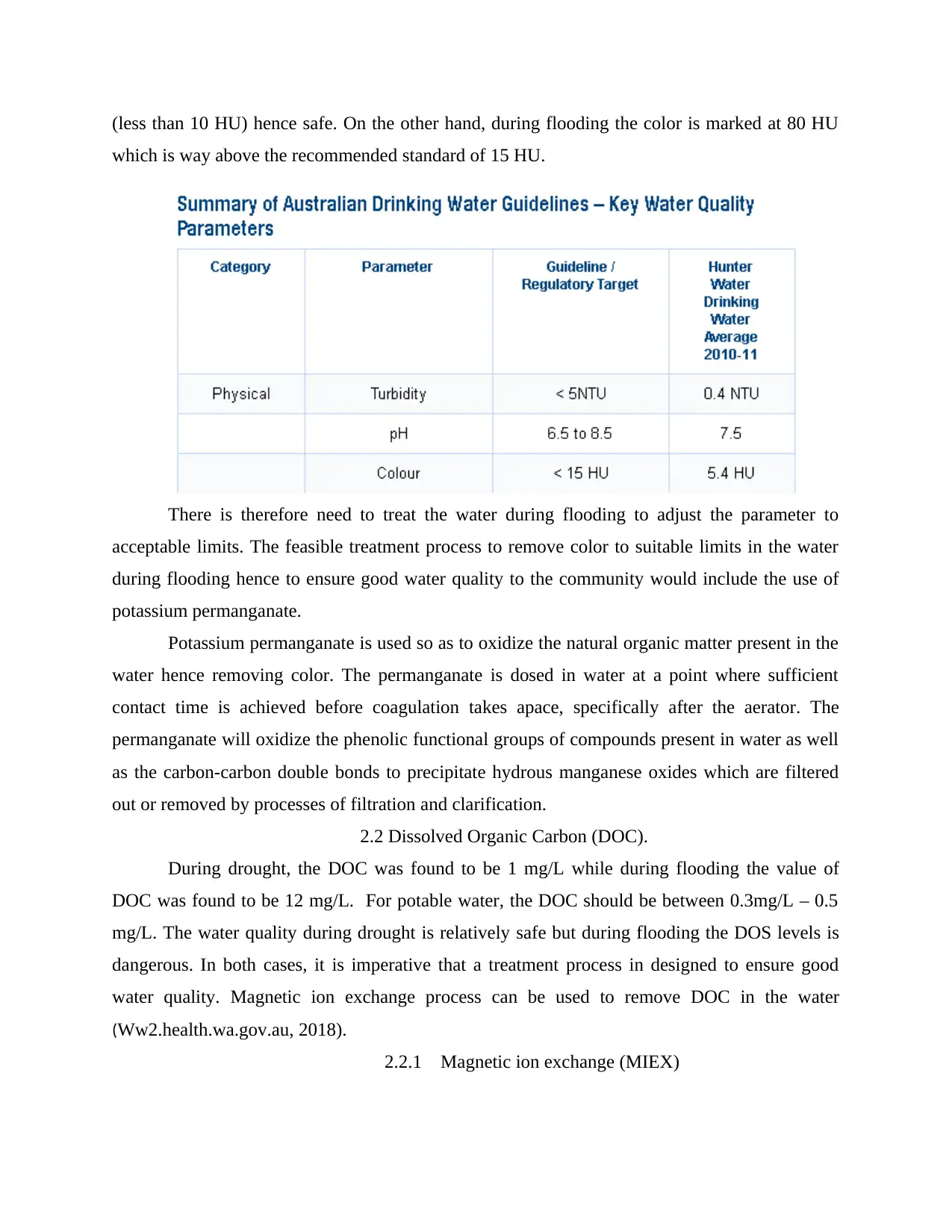
(less than 10 HU) hence safe. On the other hand, during flooding the color is marked at 80 HU
which is way above the recommended standard of 15 HU.
There is therefore need to treat the water during flooding to adjust the parameter to
acceptable limits. The feasible treatment process to remove color to suitable limits in the water
during flooding hence to ensure good water quality to the community would include the use of
potassium permanganate.
Potassium permanganate is used so as to oxidize the natural organic matter present in the
water hence removing color. The permanganate is dosed in water at a point where sufficient
contact time is achieved before coagulation takes apace, specifically after the aerator. The
permanganate will oxidize the phenolic functional groups of compounds present in water as well
as the carbon-carbon double bonds to precipitate hydrous manganese oxides which are filtered
out or removed by processes of filtration and clarification.
2.2 Dissolved Organic Carbon (DOC).
During drought, the DOC was found to be 1 mg/L while during flooding the value of
DOC was found to be 12 mg/L. For potable water, the DOC should be between 0.3mg/L – 0.5
mg/L. The water quality during drought is relatively safe but during flooding the DOS levels is
dangerous. In both cases, it is imperative that a treatment process in designed to ensure good
water quality. Magnetic ion exchange process can be used to remove DOC in the water
(Ww2.health.wa.gov.au, 2018).
2.2.1 Magnetic ion exchange (MIEX)
which is way above the recommended standard of 15 HU.
There is therefore need to treat the water during flooding to adjust the parameter to
acceptable limits. The feasible treatment process to remove color to suitable limits in the water
during flooding hence to ensure good water quality to the community would include the use of
potassium permanganate.
Potassium permanganate is used so as to oxidize the natural organic matter present in the
water hence removing color. The permanganate is dosed in water at a point where sufficient
contact time is achieved before coagulation takes apace, specifically after the aerator. The
permanganate will oxidize the phenolic functional groups of compounds present in water as well
as the carbon-carbon double bonds to precipitate hydrous manganese oxides which are filtered
out or removed by processes of filtration and clarification.
2.2 Dissolved Organic Carbon (DOC).
During drought, the DOC was found to be 1 mg/L while during flooding the value of
DOC was found to be 12 mg/L. For potable water, the DOC should be between 0.3mg/L – 0.5
mg/L. The water quality during drought is relatively safe but during flooding the DOS levels is
dangerous. In both cases, it is imperative that a treatment process in designed to ensure good
water quality. Magnetic ion exchange process can be used to remove DOC in the water
(Ww2.health.wa.gov.au, 2018).
2.2.1 Magnetic ion exchange (MIEX)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

MIEX resin is an attractively upgraded anion trade tar that comprises of a macro porous
polyacrylic dab scattered with attractive iron oxide particles. The resin is functionalized with
quaternary ammonium (i.e., trimethylamine utilitarian gatherings) and commonly utilizes
chloride as the versatile counter ion. The molecule size of MIEX resin is roughly 200 which is 2–
5 times tinier than customary anion exchange resins. On account of the minute molecule measure
and attractive segment, MIEX resin is utilized in a completely mixed flow reactor (CMFR) with
resin reuse and halfway resin recovery. MIEX treatment is normally utilized toward the start of a
treatment in light of the fact that turbidity does not antagonistically affect the procedure
Martínezur, Uche, Rubio, and Carrasquer, 2010). This takes into account high decreases in DOC,
which diminishes resulting compound necessities (e.g., coagulants), and enhances the execution
of downstream procedures (e.g., membranes).
2.3 Turbidity
During drought the turbidity in the river is reported to be less than 5 NTU while during
flooding the value shoots to 75 NTU. In regard to aesthetic considerations, drinking water turbity
level should not exceed 5 NTU. Therefore, during drought the turbidity level of the water is
within acceptable limits for drinking water hence suitable for community use. However, during
flooding turbity levels reach dangerous levels hence there is need to treat the water before it is fit
for consumption (Scher, 2009). Turbidity in water can be dealt with utilizing sand and cartridge
channels/ filters. A sand channel comprises of a tank with fine sand over a layer of gravel. The
sand successfully do away with everything except the fine suspended particles. These channels
must be discharged occasionally to expel the gathered residue from the channel. Cartridge
channels are convincing at expelling greatly fine particles, however they are intended to be
supplanted when they wind up blocked (Radich, 2014).Sand filters are used to expel the greater
part of the suspended particles, and a cartridge filter deals with the fine particles left behind. In
any case, if the turbidity is generally low, a cartridge filer alone would be sufficient without sand
filters being used as well.
In summary, in instances where water quality is found undesirable due to unsuitable
levels of TDS, reverse osmosis process is recommended. In case of poor water coloration,
potassium permanganate is used to settle out oxides of the constituent compounds in the unfit
water. Magnetic ion exchange method is to be used to correct unsuitable Dissolved Organic
Carbon DOC concentrations while turbidity in the water is corrected by means of cartridge and
polyacrylic dab scattered with attractive iron oxide particles. The resin is functionalized with
quaternary ammonium (i.e., trimethylamine utilitarian gatherings) and commonly utilizes
chloride as the versatile counter ion. The molecule size of MIEX resin is roughly 200 which is 2–
5 times tinier than customary anion exchange resins. On account of the minute molecule measure
and attractive segment, MIEX resin is utilized in a completely mixed flow reactor (CMFR) with
resin reuse and halfway resin recovery. MIEX treatment is normally utilized toward the start of a
treatment in light of the fact that turbidity does not antagonistically affect the procedure
Martínezur, Uche, Rubio, and Carrasquer, 2010). This takes into account high decreases in DOC,
which diminishes resulting compound necessities (e.g., coagulants), and enhances the execution
of downstream procedures (e.g., membranes).
2.3 Turbidity
During drought the turbidity in the river is reported to be less than 5 NTU while during
flooding the value shoots to 75 NTU. In regard to aesthetic considerations, drinking water turbity
level should not exceed 5 NTU. Therefore, during drought the turbidity level of the water is
within acceptable limits for drinking water hence suitable for community use. However, during
flooding turbity levels reach dangerous levels hence there is need to treat the water before it is fit
for consumption (Scher, 2009). Turbidity in water can be dealt with utilizing sand and cartridge
channels/ filters. A sand channel comprises of a tank with fine sand over a layer of gravel. The
sand successfully do away with everything except the fine suspended particles. These channels
must be discharged occasionally to expel the gathered residue from the channel. Cartridge
channels are convincing at expelling greatly fine particles, however they are intended to be
supplanted when they wind up blocked (Radich, 2014).Sand filters are used to expel the greater
part of the suspended particles, and a cartridge filter deals with the fine particles left behind. In
any case, if the turbidity is generally low, a cartridge filer alone would be sufficient without sand
filters being used as well.
In summary, in instances where water quality is found undesirable due to unsuitable
levels of TDS, reverse osmosis process is recommended. In case of poor water coloration,
potassium permanganate is used to settle out oxides of the constituent compounds in the unfit
water. Magnetic ion exchange method is to be used to correct unsuitable Dissolved Organic
Carbon DOC concentrations while turbidity in the water is corrected by means of cartridge and
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

sand filters. In doing so, the community being served by the river would be able to access good
quality drinking in both the dry seasons (extended drought) and during rainy (flooding) seasons.
quality drinking in both the dry seasons (extended drought) and during rainy (flooding) seasons.

References
Anon Australian Drinking Water Guidelines (2011) - Updated ... [Online]. Available at:
https://www.nhmrc.gov.au/guidelines/publications/eh52 [Accessed: 13 October 2018].
Anon How to Remove Total Dissolved Solids From Drinking Water ... [Online]. Available at:
https://sciencing.com/remove-dissolved-solids-drinking-water-8164483.html [Accessed:
13 October 2018].
Anon Total Dissolved Solids and Activated Carbon [Online]. Available at:
http://www.unhas.ac.id/hasbi/TOT-Atm-eSpr/eSpring TTT/background/Total Dissolved
Solids and Activated Carbon.pdf [Accessed: 13 October 2018].
Crittenden, J.C., Trussell, R.R., Hand, D.W., Howe, K.J., Tchobanoglous, G. and Borchardt, J.H.
(2012). MWHs Water Treatment: Principles and Design. Hoboken, NJ: John Wiley &
Sons, Inc.
Gray, N.F. (2008). Drinking Water Quality: Problems and Solutions. Cambridge: Cambridge
University Press.
Martínez, A., Uche, J., Rubio, C. and Carrasquer, B. (2010). Exergy cost of water supply and
water treatment technologies. Desalination and Water Treatment 24:123–131.
Mcnamara, L. (2012). Water quality modelling for decision-making: the drinking-water
watersheds of Sydney, Australia. From landscape research to landscape planning
Wageningen UR Frontis Series:133–142.
Radich, S. (2014). The Water Treatment. Nelson, New Zealand: Copy Press Books.
Scher, D. (2009). Drinking Water Quality: Community Water Data & Measures 1999-2007. St.
Paul, MN: Minnesota Dept. of Health.
Ww2.health.wa.gov.au. (2018). Drinking water guidelines and standards. [online] Available at:
http://ww2.health.wa.gov.au/Articles/A_E/Drinking-water-guidelines-and-standards
[Accessed 13 Oct. 2018].
Anon Australian Drinking Water Guidelines (2011) - Updated ... [Online]. Available at:
https://www.nhmrc.gov.au/guidelines/publications/eh52 [Accessed: 13 October 2018].
Anon How to Remove Total Dissolved Solids From Drinking Water ... [Online]. Available at:
https://sciencing.com/remove-dissolved-solids-drinking-water-8164483.html [Accessed:
13 October 2018].
Anon Total Dissolved Solids and Activated Carbon [Online]. Available at:
http://www.unhas.ac.id/hasbi/TOT-Atm-eSpr/eSpring TTT/background/Total Dissolved
Solids and Activated Carbon.pdf [Accessed: 13 October 2018].
Crittenden, J.C., Trussell, R.R., Hand, D.W., Howe, K.J., Tchobanoglous, G. and Borchardt, J.H.
(2012). MWHs Water Treatment: Principles and Design. Hoboken, NJ: John Wiley &
Sons, Inc.
Gray, N.F. (2008). Drinking Water Quality: Problems and Solutions. Cambridge: Cambridge
University Press.
Martínez, A., Uche, J., Rubio, C. and Carrasquer, B. (2010). Exergy cost of water supply and
water treatment technologies. Desalination and Water Treatment 24:123–131.
Mcnamara, L. (2012). Water quality modelling for decision-making: the drinking-water
watersheds of Sydney, Australia. From landscape research to landscape planning
Wageningen UR Frontis Series:133–142.
Radich, S. (2014). The Water Treatment. Nelson, New Zealand: Copy Press Books.
Scher, D. (2009). Drinking Water Quality: Community Water Data & Measures 1999-2007. St.
Paul, MN: Minnesota Dept. of Health.
Ww2.health.wa.gov.au. (2018). Drinking water guidelines and standards. [online] Available at:
http://ww2.health.wa.gov.au/Articles/A_E/Drinking-water-guidelines-and-standards
[Accessed 13 Oct. 2018].
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

