Comprehensive Lab Report: Water Treatment and Quality Analysis
VerifiedAdded on 2020/04/01
|7
|923
|457
Report
AI Summary
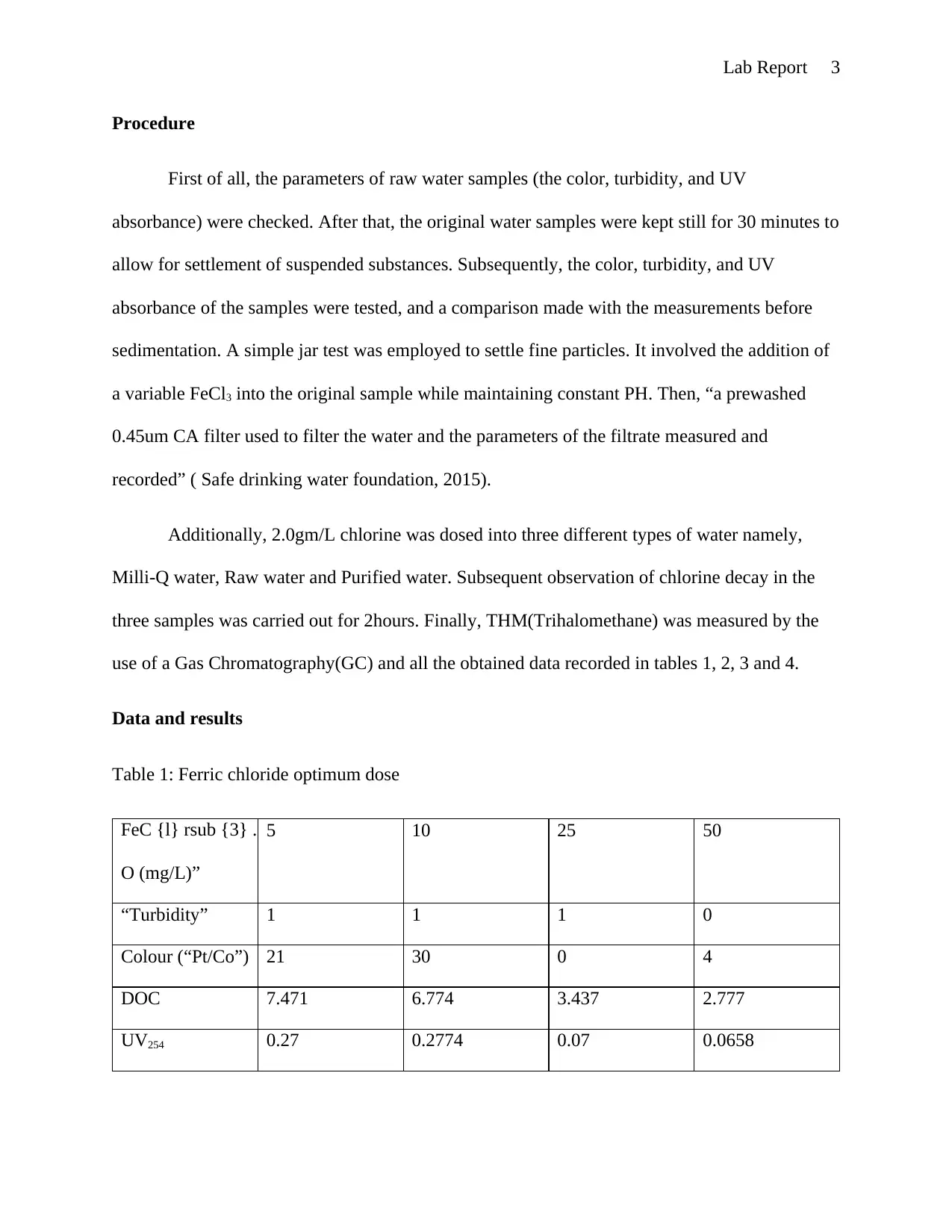
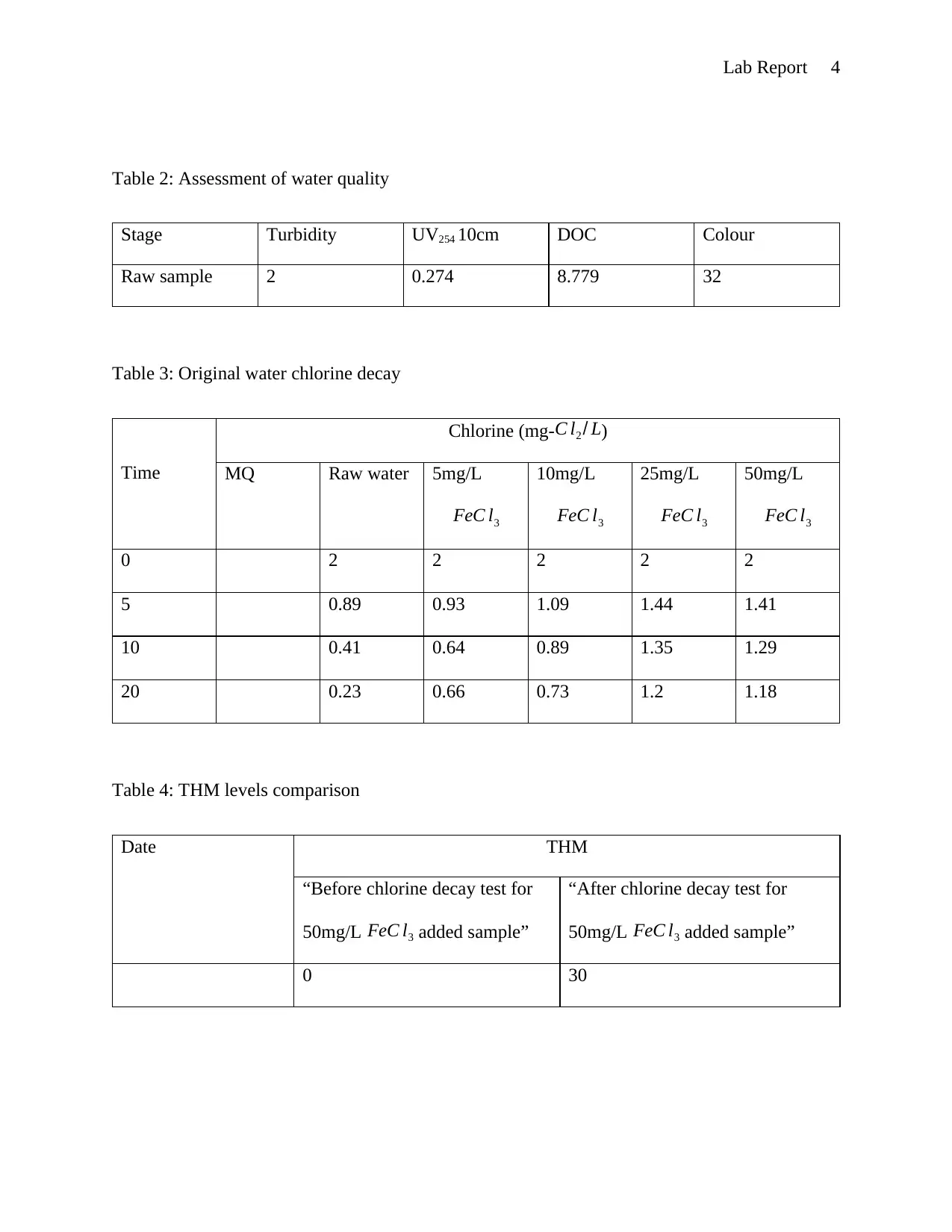
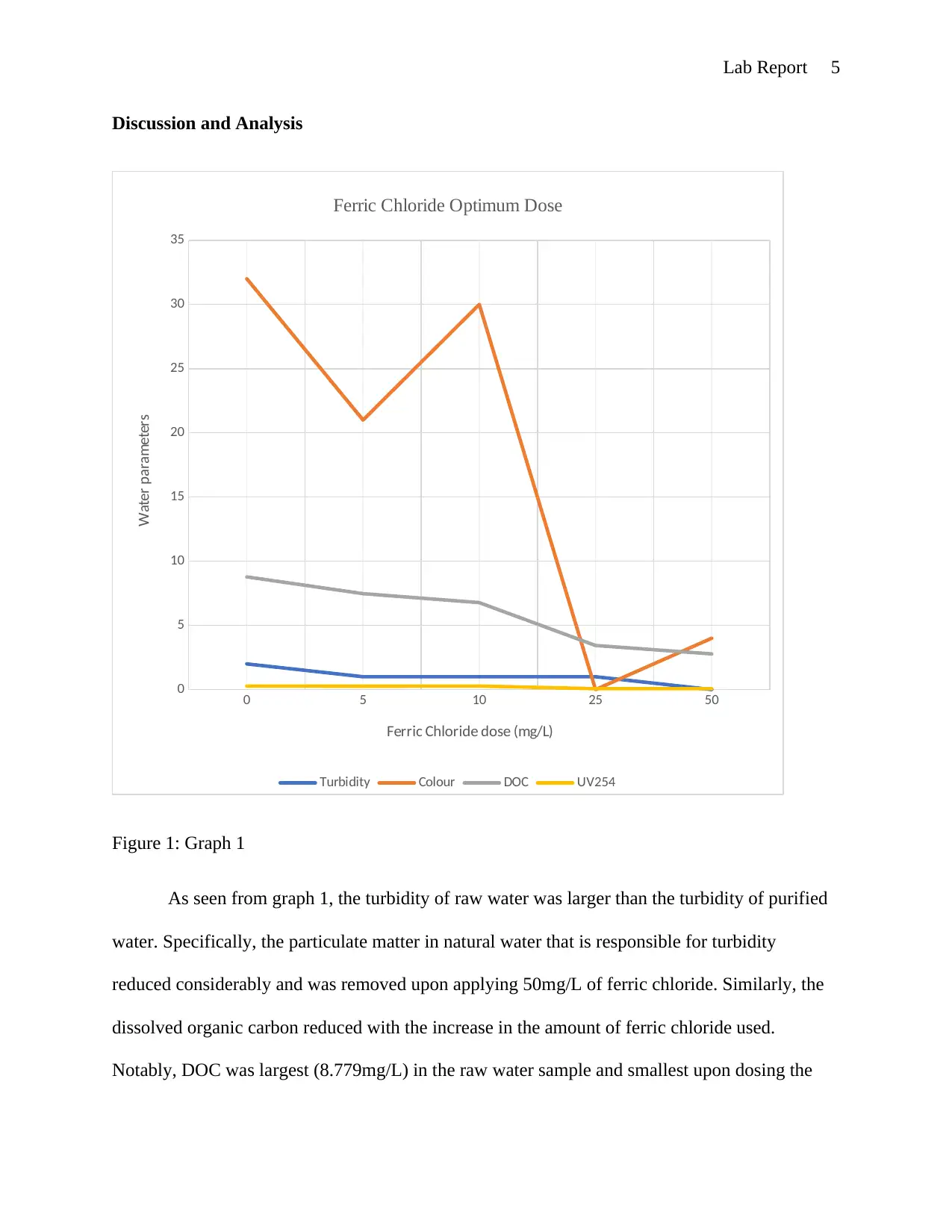
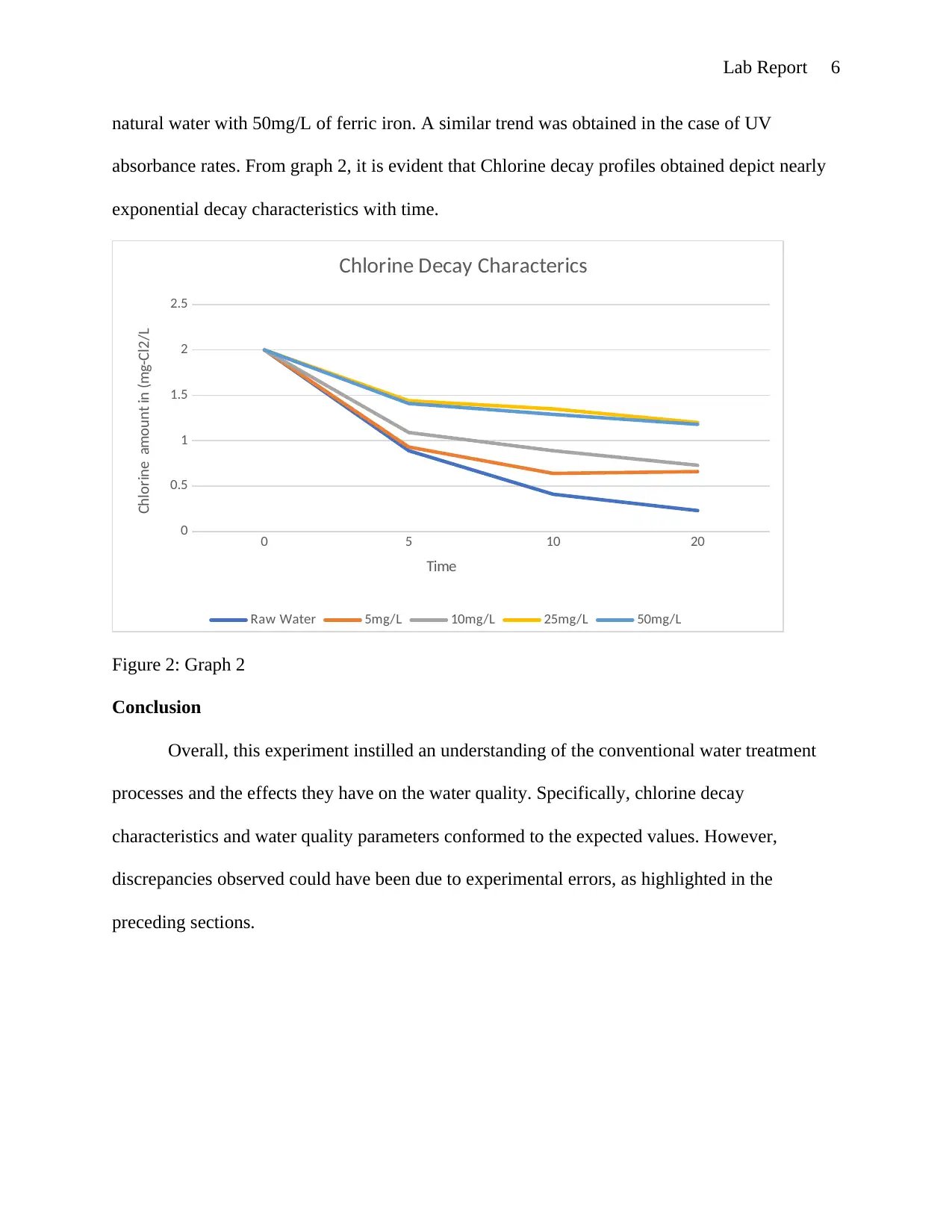
This lab report details an experiment on conventional water treatment processes and their effects on water quality. The objectives included understanding the measurement of various water parameters like turbidity, color, and UV absorbance, and demonstrating the impact of chlorine on disinfection by-products formation. The experiment involved measuring these parameters in raw water samples, after sedimentation, and after treatment with ferric chloride. Chlorine decay was observed in Milli-Q, raw, and purified water samples. The results, including the optimal dose of ferric chloride, water quality assessments at different stages, chlorine decay profiles, and THM levels, are presented in tables and graphs. The discussion analyzes the data, highlighting the reduction in turbidity and DOC with ferric chloride treatment, and the exponential chlorine decay. The report concludes with an understanding of water treatment processes and the effects on water quality, with discrepancies potentially due to experimental errors. The report references relevant literature on water treatment processes.
1 out of 7







![[object Object]](/_next/static/media/star-bottom.7253800d.svg)