Feasibility Study: Medical Wearable for Haemoglobin Patients
VerifiedAdded on 2020/04/01
|19
|2792
|49
Report
AI Summary
This report presents a feasibility study for a wearable medical device designed for patients with hemoglobin disorders, such as thalassaemia and sickle cell disease. The study encompasses a comprehensive analysis, including the development of a minimum viable product (MVP), a detailed business model canvas outlining key partnerships, activities, value propositions, customer segments, relationships, channels, resources, cost structures, and revenue streams. Furthermore, a value proposition canvas is utilized to align customer needs with the device's benefits. The report provides initial financial estimates, market segmentation, and revenue projections over a three-year period, along with profit and loss statements. It also explores funding requirements and required resources, ultimately concluding that the project is viable and beneficial for patients. The report highlights the potential of the wearable technology to improve health management and reduce hospitalizations.

Running head: FEASIBILITY – MEDICAL WEARABLE 1
Feasibility Study -Wearable Technology for Patients with Haemoglobin Disorders
Name:
Institution:
Affiliation:
Feasibility Study -Wearable Technology for Patients with Haemoglobin Disorders
Name:
Institution:
Affiliation:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

FEASIBILITY - MEDICAL WEARABLE 2
1.0 EXECUTIVE SUMMARY
The main aim of the project is to come up with a wearable device for people who suffer from
thalassaemia and sickle cell disease, which are the known hemoglobin disorders. These diseases
are chronic and the conditions are managed. This is because bone marrow and stem cell
transplants are not affordable for many people. Most patients end up in hospital once they get
chronic attacks and mostly do not have a way of predicting that they are about to fall sick. The
diseases lead to anaemia hence mostly lead to blood transfusions, yet if detected early can be
managed through certain foods or drugs (Galanello, 2013). The diseases can also lead to other
complications like strokes, very painful episodes, chronic wounds, jaundice and bacterial
infections. The wearable device will be a thin strip wearable on the arm of a patient and will
have small chips attached. It will constantly monitor the condition of a patient and raise an alarm
if there are any changes to the body that may lead a patient into a crisis. This paper is a
feasibility study, which looks at various aspects like the minimum viable product, market
analysis and financial projects, to determine if the project is viable.
1.0 EXECUTIVE SUMMARY
The main aim of the project is to come up with a wearable device for people who suffer from
thalassaemia and sickle cell disease, which are the known hemoglobin disorders. These diseases
are chronic and the conditions are managed. This is because bone marrow and stem cell
transplants are not affordable for many people. Most patients end up in hospital once they get
chronic attacks and mostly do not have a way of predicting that they are about to fall sick. The
diseases lead to anaemia hence mostly lead to blood transfusions, yet if detected early can be
managed through certain foods or drugs (Galanello, 2013). The diseases can also lead to other
complications like strokes, very painful episodes, chronic wounds, jaundice and bacterial
infections. The wearable device will be a thin strip wearable on the arm of a patient and will
have small chips attached. It will constantly monitor the condition of a patient and raise an alarm
if there are any changes to the body that may lead a patient into a crisis. This paper is a
feasibility study, which looks at various aspects like the minimum viable product, market
analysis and financial projects, to determine if the project is viable.

FEASIBILITY - MEDICAL WEARABLE 3
Table of Contents
1.0 EXECUTIVE SUMMARY.....................................................................................................................2
2.0 MINIMUM VIABLE PRODUCT...........................................................................................................5
2.1 Lean Start up Version..........................................................................................................................5
2.1.1 Vision............................................................................................................................................6
2.1.2 Steer..............................................................................................................................................6
2.1.3 Accelerate.....................................................................................................................................6
3.0 BUSINESS MODEL CANVAS..............................................................................................................7
4.0 VALUE PROPOSITION CANVAS........................................................................................................8
4.1 Value Proposition.................................................................................................................................8
4.2 Customer..............................................................................................................................................8
4.3 Brief Discussion on Fit between Customer Profile and Value Proposition.........................................9
5.0 INITIAL FINANCE ESTIMATES..........................................................................................................9
5.1 Initial Market Segment Estimates........................................................................................................9
5.1.1 Market Analysis Summary...........................................................................................................9
5.1.2 Market Segmentation..................................................................................................................10
5.1.3 Market Analysis..........................................................................................................................11
5.2 Initial 1-3 Year Revenue Estimates...................................................................................................11
5.3 Initial 1-3 Year Profit and Loss Statements.......................................................................................12
5.4 Estimated Initial Funding level Request............................................................................................13
5.5 Initial Additional Required Resources...............................................................................................14
6.0 CONCLUSIONS....................................................................................................................................14
7.0 REFERENCES......................................................................................................................................15
8.0 APPENDICES.......................................................................................................................................15
8.1 Statistics on Global Wearable Devices Market.................................................................................15
8.2 Design Principles of Wearable Devices.............................................................................................15
8.3 Global Outlook on Medical Wearable Devices.................................................................................16
8.4 Wearable device with sensor example...............................................................................................16
8.5 Wearable wrist band example for heart rate monitor.........................................................................17
8.6 Wearable technology for hemoglobin disorder patients project milestones......................................17
Table of Contents
1.0 EXECUTIVE SUMMARY.....................................................................................................................2
2.0 MINIMUM VIABLE PRODUCT...........................................................................................................5
2.1 Lean Start up Version..........................................................................................................................5
2.1.1 Vision............................................................................................................................................6
2.1.2 Steer..............................................................................................................................................6
2.1.3 Accelerate.....................................................................................................................................6
3.0 BUSINESS MODEL CANVAS..............................................................................................................7
4.0 VALUE PROPOSITION CANVAS........................................................................................................8
4.1 Value Proposition.................................................................................................................................8
4.2 Customer..............................................................................................................................................8
4.3 Brief Discussion on Fit between Customer Profile and Value Proposition.........................................9
5.0 INITIAL FINANCE ESTIMATES..........................................................................................................9
5.1 Initial Market Segment Estimates........................................................................................................9
5.1.1 Market Analysis Summary...........................................................................................................9
5.1.2 Market Segmentation..................................................................................................................10
5.1.3 Market Analysis..........................................................................................................................11
5.2 Initial 1-3 Year Revenue Estimates...................................................................................................11
5.3 Initial 1-3 Year Profit and Loss Statements.......................................................................................12
5.4 Estimated Initial Funding level Request............................................................................................13
5.5 Initial Additional Required Resources...............................................................................................14
6.0 CONCLUSIONS....................................................................................................................................14
7.0 REFERENCES......................................................................................................................................15
8.0 APPENDICES.......................................................................................................................................15
8.1 Statistics on Global Wearable Devices Market.................................................................................15
8.2 Design Principles of Wearable Devices.............................................................................................15
8.3 Global Outlook on Medical Wearable Devices.................................................................................16
8.4 Wearable device with sensor example...............................................................................................16
8.5 Wearable wrist band example for heart rate monitor.........................................................................17
8.6 Wearable technology for hemoglobin disorder patients project milestones......................................17
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

FEASIBILITY - MEDICAL WEARABLE 4
Wearable Technology for Patients with Haemoglobin Disorders
2.0 MINIMUM VIABLE PRODUCT
The minimum viable product is a version of a new product allowing the team to collect
maximum amount of information about customer needs. The minimum viable product in this
case will be a very slim wrist device made of simple biodegradable material and it will be
connected to a mobile application. The biodegradable material is very affordable hence; it will
ensure viability while at the same time reducing costs (Moogk, 2012). It will detect any kind of
discomfort experienced by patients with hemoglobin disorders through a sensor.
2.1 Lean Start up Version
Lean start-ups are developed in three processes, setting a vision, steering and accelerating. The
same procedure
2.1.1 Vision
Good Life Hematology Centre will come up with a vision of exactly how they want the product
to look like and draw a prototype of the same. They will define what exactly they want the
product to do, which in this case is to monitor vital signs of patients who suffer from hemoglobin
disorders so that they can manage the condition before it further escalates (World Health
Organization, 2013). The company will then do a market survey to learn how such devices
operate and the kind of technology and software that would be required. A prototype will then be
made to experiment whether the idea is viable enough (Blank, 2013). The vision for the company
in this project is, “Re-defining health care for patients with hemoglobin disorders.”
2.1.2 Steer
Once Good Life Hematology Centre has seen how the prototype works, they will then leap into
the project. Here, a few samples will be produced and they will be sampled on patients who
suffer from sickle cell anaemia and thalassaemia to see how they work. Feedback will be taken at
Wearable Technology for Patients with Haemoglobin Disorders
2.0 MINIMUM VIABLE PRODUCT
The minimum viable product is a version of a new product allowing the team to collect
maximum amount of information about customer needs. The minimum viable product in this
case will be a very slim wrist device made of simple biodegradable material and it will be
connected to a mobile application. The biodegradable material is very affordable hence; it will
ensure viability while at the same time reducing costs (Moogk, 2012). It will detect any kind of
discomfort experienced by patients with hemoglobin disorders through a sensor.
2.1 Lean Start up Version
Lean start-ups are developed in three processes, setting a vision, steering and accelerating. The
same procedure
2.1.1 Vision
Good Life Hematology Centre will come up with a vision of exactly how they want the product
to look like and draw a prototype of the same. They will define what exactly they want the
product to do, which in this case is to monitor vital signs of patients who suffer from hemoglobin
disorders so that they can manage the condition before it further escalates (World Health
Organization, 2013). The company will then do a market survey to learn how such devices
operate and the kind of technology and software that would be required. A prototype will then be
made to experiment whether the idea is viable enough (Blank, 2013). The vision for the company
in this project is, “Re-defining health care for patients with hemoglobin disorders.”
2.1.2 Steer
Once Good Life Hematology Centre has seen how the prototype works, they will then leap into
the project. Here, a few samples will be produced and they will be sampled on patients who
suffer from sickle cell anaemia and thalassaemia to see how they work. Feedback will be taken at
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

FEASIBILITY - MEDICAL WEARABLE 5
this point and output measured against the initially set targets for the wearable technology
(Cheng, 2016). Here, the company will note discrepancies and add the necessary features to
enhance the product.
2.1.3 Accelerate
The final step in the lean start up will be to batch, grow, adapt and innovate (Sultan, 2015). Here,
a few batches will be produced and marketed so that the company sees how the market responds
to the new product. Once this is done, there will be an emphasis on marketing and advertising to
grow the wearable technology. This will lead to full adaptation of the product by the market
(Drumright, 2017). The company will then continuously innovate new ways of improving the
product.
this point and output measured against the initially set targets for the wearable technology
(Cheng, 2016). Here, the company will note discrepancies and add the necessary features to
enhance the product.
2.1.3 Accelerate
The final step in the lean start up will be to batch, grow, adapt and innovate (Sultan, 2015). Here,
a few batches will be produced and marketed so that the company sees how the market responds
to the new product. Once this is done, there will be an emphasis on marketing and advertising to
grow the wearable technology. This will lead to full adaptation of the product by the market
(Drumright, 2017). The company will then continuously innovate new ways of improving the
product.
FEASIBILITY - MEDICAL WEARABLE 6
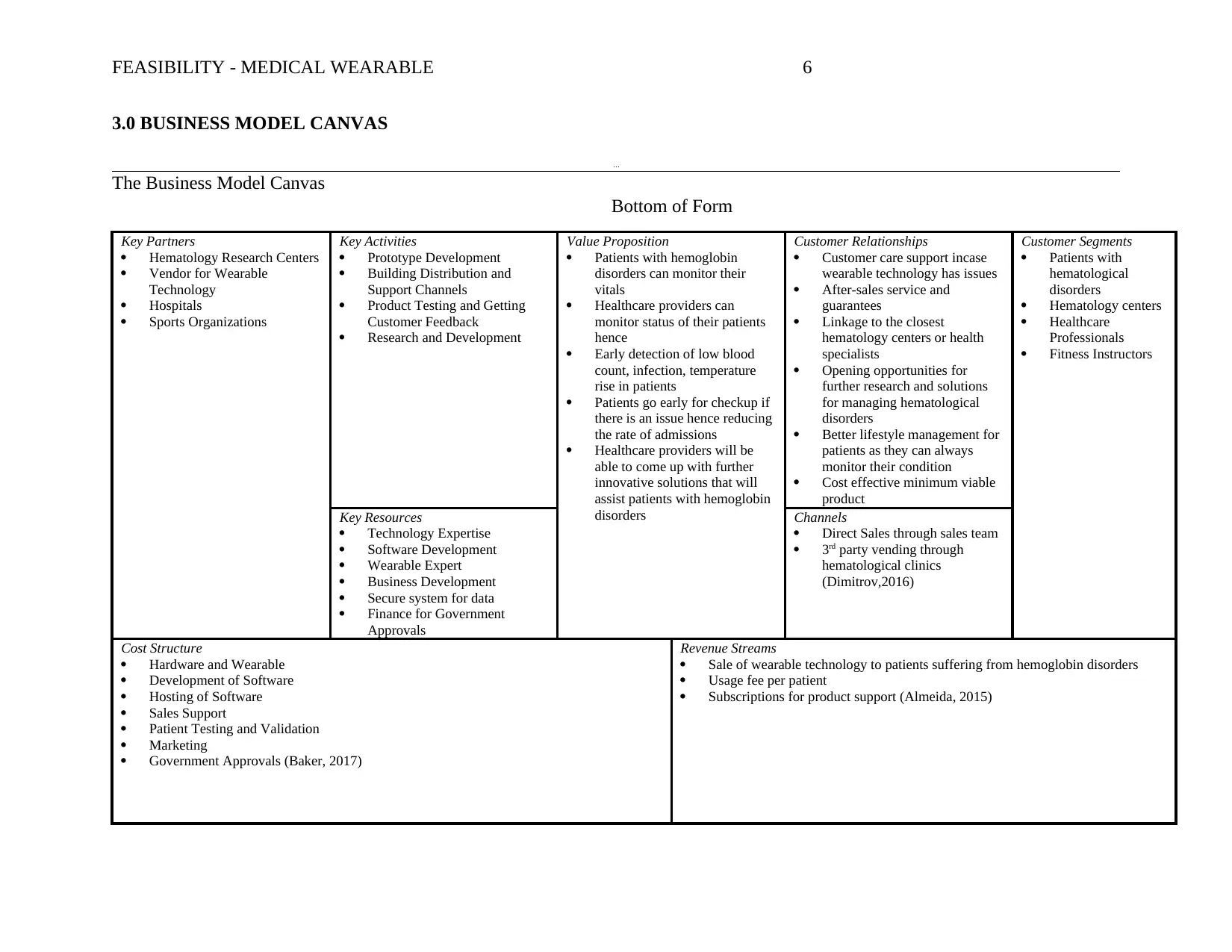
3.0 BUSINESS MODEL CANVAS
The Business Model Canvas
Bottom of Form
Key Partners
Hematology Research Centers
Vendor for Wearable
Technology
Hospitals
Sports Organizations
Key Activities
Prototype Development
Building Distribution and
Support Channels
Product Testing and Getting
Customer Feedback
Research and Development
Value Proposition
Patients with hemoglobin
disorders can monitor their
vitals
Healthcare providers can
monitor status of their patients
hence
Early detection of low blood
count, infection, temperature
rise in patients
Patients go early for checkup if
there is an issue hence reducing
the rate of admissions
Healthcare providers will be
able to come up with further
innovative solutions that will
assist patients with hemoglobin
disorders
Customer Relationships
Customer care support incase
wearable technology has issues
After-sales service and
guarantees
Linkage to the closest
hematology centers or health
specialists
Opening opportunities for
further research and solutions
for managing hematological
disorders
Better lifestyle management for
patients as they can always
monitor their condition
Cost effective minimum viable
product
Customer Segments
Patients with
hematological
disorders
Hematology centers
Healthcare
Professionals
Fitness Instructors
Key Resources
Technology Expertise
Software Development
Wearable Expert
Business Development
Secure system for data
Finance for Government
Approvals
Channels
Direct Sales through sales team
3rd party vending through
hematological clinics
(Dimitrov,2016)
Cost Structure
Hardware and Wearable
Development of Software
Hosting of Software
Sales Support
Patient Testing and Validation
Marketing
Government Approvals (Baker, 2017)
Revenue Streams
Sale of wearable technology to patients suffering from hemoglobin disorders
Usage fee per patient
Subscriptions for product support (Almeida, 2015)
3.0 BUSINESS MODEL CANVAS
The Business Model Canvas
Bottom of Form
Key Partners
Hematology Research Centers
Vendor for Wearable
Technology
Hospitals
Sports Organizations
Key Activities
Prototype Development
Building Distribution and
Support Channels
Product Testing and Getting
Customer Feedback
Research and Development
Value Proposition
Patients with hemoglobin
disorders can monitor their
vitals
Healthcare providers can
monitor status of their patients
hence
Early detection of low blood
count, infection, temperature
rise in patients
Patients go early for checkup if
there is an issue hence reducing
the rate of admissions
Healthcare providers will be
able to come up with further
innovative solutions that will
assist patients with hemoglobin
disorders
Customer Relationships
Customer care support incase
wearable technology has issues
After-sales service and
guarantees
Linkage to the closest
hematology centers or health
specialists
Opening opportunities for
further research and solutions
for managing hematological
disorders
Better lifestyle management for
patients as they can always
monitor their condition
Cost effective minimum viable
product
Customer Segments
Patients with
hematological
disorders
Hematology centers
Healthcare
Professionals
Fitness Instructors
Key Resources
Technology Expertise
Software Development
Wearable Expert
Business Development
Secure system for data
Finance for Government
Approvals
Channels
Direct Sales through sales team
3rd party vending through
hematological clinics
(Dimitrov,2016)
Cost Structure
Hardware and Wearable
Development of Software
Hosting of Software
Sales Support
Patient Testing and Validation
Marketing
Government Approvals (Baker, 2017)
Revenue Streams
Sale of wearable technology to patients suffering from hemoglobin disorders
Usage fee per patient
Subscriptions for product support (Almeida, 2015)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
FEASIBILITY - MEDICAL WEARABLE 7
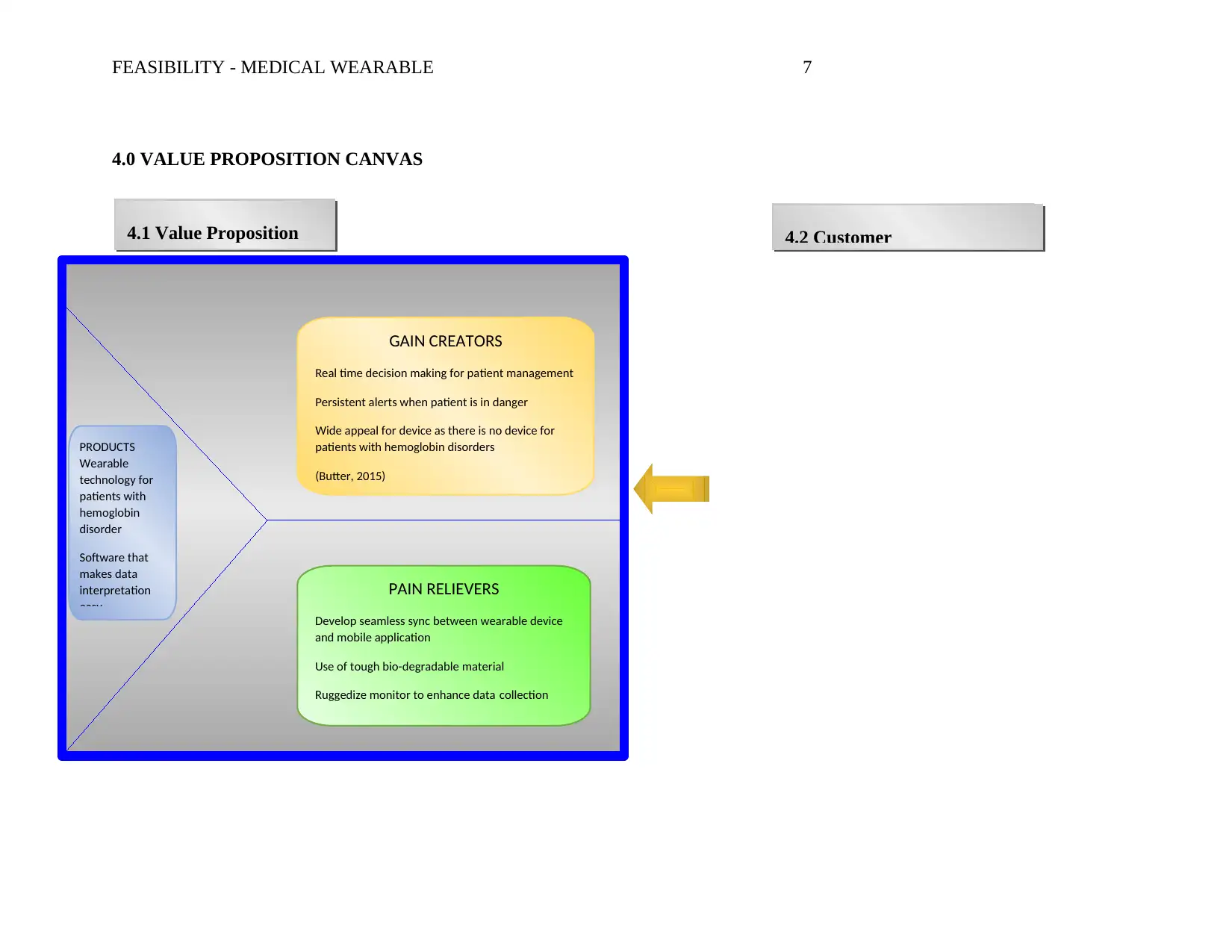
4.0 VALUE PROPOSITION CANVAS
CustomerJobsCollectionandanalysisofdataforhemoglobindisorderpatients(Sprenger,2016)PainsDurabilityofmaterialsusedondeviceSeamlesssynctimebetweendeviceandmobileappEfficientdatacollectionGainsFlexibilityoffundingapprovalfordeviceScheduletestingofpatientswithhemoglobindisordersInstitutionalcredibilityGAIN CREATORS
Real time decision making for patient management
Persistent alerts when patient is in danger
Wide appeal for device as there is no device for
patients with hemoglobin disorders
(Butter, 2015)
PRODUCTS
Wearable
technology for
patients with
hemoglobin
disorder
Software that
makes data
interpretation
easy
PAIN RELIEVERS
Develop seamless sync between wearable device
and mobile application
Use of tough bio-degradable material
Ruggedize monitor to enhance data collection
4.2 Customer4.1 Value Proposition
4.0 VALUE PROPOSITION CANVAS
CustomerJobsCollectionandanalysisofdataforhemoglobindisorderpatients(Sprenger,2016)PainsDurabilityofmaterialsusedondeviceSeamlesssynctimebetweendeviceandmobileappEfficientdatacollectionGainsFlexibilityoffundingapprovalfordeviceScheduletestingofpatientswithhemoglobindisordersInstitutionalcredibilityGAIN CREATORS
Real time decision making for patient management
Persistent alerts when patient is in danger
Wide appeal for device as there is no device for
patients with hemoglobin disorders
(Butter, 2015)
PRODUCTS
Wearable
technology for
patients with
hemoglobin
disorder
Software that
makes data
interpretation
easy
PAIN RELIEVERS
Develop seamless sync between wearable device
and mobile application
Use of tough bio-degradable material
Ruggedize monitor to enhance data collection
4.2 Customer4.1 Value Proposition
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

FEASIBILITY - MEDICAL WEARABLE 8
4.3 Brief Discussion on Fit between Customer Profile and Value Proposition
The value proposition is a description of the benefits customers will receive from the wearable
technology. The gain creators describe how the wearable technology will create customer gains. In this
case, the wearable technology will have enabled alerts that will show when a patient is in distress
(Lewy,2015). It will also lead to quick decision making for patient management. The market is
untapped as this will be a first for hemoglobin disorder patients.
Pain relievers describe how the wearable technology will relieve pain. In this case, the customer
has an issue with time synchronization between device and mobile application and the pain
reliever ensures seamless integration of device and mobile application. The customer is also
concerned about device durability and pain reliever will ensure use of tough biodegradable
materials.
5.0 INITIAL FINANCE ESTIMATES
5.1 Initial Market Segment Estimates
5.1.1 Market Analysis Summary
The wearable technology device is a growing sector and has high growth potential due to
decreasing costs in technology. Hemoglobin disease patients currently do not have a specific
monitoring device for their cases. Research shows that the wearable technology industry will
have grown to revenues of $7.8 billion in the next three years (Page, 2015). The market analysis
will focus on three key groups of customers:
Sickle Cell Anaemia Patients
Thalassaemia Patients
Pregnant women
4.3 Brief Discussion on Fit between Customer Profile and Value Proposition
The value proposition is a description of the benefits customers will receive from the wearable
technology. The gain creators describe how the wearable technology will create customer gains. In this
case, the wearable technology will have enabled alerts that will show when a patient is in distress
(Lewy,2015). It will also lead to quick decision making for patient management. The market is
untapped as this will be a first for hemoglobin disorder patients.
Pain relievers describe how the wearable technology will relieve pain. In this case, the customer
has an issue with time synchronization between device and mobile application and the pain
reliever ensures seamless integration of device and mobile application. The customer is also
concerned about device durability and pain reliever will ensure use of tough biodegradable
materials.
5.0 INITIAL FINANCE ESTIMATES
5.1 Initial Market Segment Estimates
5.1.1 Market Analysis Summary
The wearable technology device is a growing sector and has high growth potential due to
decreasing costs in technology. Hemoglobin disease patients currently do not have a specific
monitoring device for their cases. Research shows that the wearable technology industry will
have grown to revenues of $7.8 billion in the next three years (Page, 2015). The market analysis
will focus on three key groups of customers:
Sickle Cell Anaemia Patients
Thalassaemia Patients
Pregnant women

FEASIBILITY - MEDICAL WEARABLE 9
5.1.2 Market Segmentation
The market will be segmented according to three areas:
Sickle Cell Anaemia Patients – Sickle cell anaemia is characterized by various symptoms, which
usually lead to painful episodes, jaundice, fatigue, stroke and wounds that do not heal. The
disease is characterized by sickle shaped red blood cells, which are unable to carry oxygen
sufficiently like normal cells. Sickle cell patient also suffer from low hemoglobin hence frequent
transfusions due to anaemia. The pain episodes and other manifestations can be triggered by
strenuous activity, dehydration, exposure to cold, infections and anaemia. The wearable device
will be able to detect changes in a patient’s body in case they are dehydrated, are exposed to
cold, have an infection or blood count has gone down (Jee & Sohn, 2015). Early detection will
enable a patient to visit the hospital in time hence avoid painful episodes or other complications
that arise from delay in treatment.
Thalassaemia Patients-In Thalassaemia is characterized by red blood cells being destroyed very
quickly hence leading to anaemia. This leads to jaundice, fatigue, deformities of bones, slow
growth, swollen abdomen and dark urine. The patient has to be blood transfused due to anaemia.
The wearable technology will be able to detect the breakdown rate of red blood cells hence
patients will be alerted and go to hospital on time (Jee & Sohn, 2015). It will also detect if the
hemoglobin level is slow or the patient has another complication.
Pregnant Women – Pregnant women have to be tested if they want to know if their unborn
children are suffering from any hemoglobin disorders (Jee & Sohn, 2015). The wearable device
will enable detection of any hemoglobin issues that the unborn child may have hence assisting
the mothers to know earlier on and make health management arrangements for their unborn
children.
5.1.2 Market Segmentation
The market will be segmented according to three areas:
Sickle Cell Anaemia Patients – Sickle cell anaemia is characterized by various symptoms, which
usually lead to painful episodes, jaundice, fatigue, stroke and wounds that do not heal. The
disease is characterized by sickle shaped red blood cells, which are unable to carry oxygen
sufficiently like normal cells. Sickle cell patient also suffer from low hemoglobin hence frequent
transfusions due to anaemia. The pain episodes and other manifestations can be triggered by
strenuous activity, dehydration, exposure to cold, infections and anaemia. The wearable device
will be able to detect changes in a patient’s body in case they are dehydrated, are exposed to
cold, have an infection or blood count has gone down (Jee & Sohn, 2015). Early detection will
enable a patient to visit the hospital in time hence avoid painful episodes or other complications
that arise from delay in treatment.
Thalassaemia Patients-In Thalassaemia is characterized by red blood cells being destroyed very
quickly hence leading to anaemia. This leads to jaundice, fatigue, deformities of bones, slow
growth, swollen abdomen and dark urine. The patient has to be blood transfused due to anaemia.
The wearable technology will be able to detect the breakdown rate of red blood cells hence
patients will be alerted and go to hospital on time (Jee & Sohn, 2015). It will also detect if the
hemoglobin level is slow or the patient has another complication.
Pregnant Women – Pregnant women have to be tested if they want to know if their unborn
children are suffering from any hemoglobin disorders (Jee & Sohn, 2015). The wearable device
will enable detection of any hemoglobin issues that the unborn child may have hence assisting
the mothers to know earlier on and make health management arrangements for their unborn
children.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
FEASIBILITY - MEDICAL WEARABLE 10
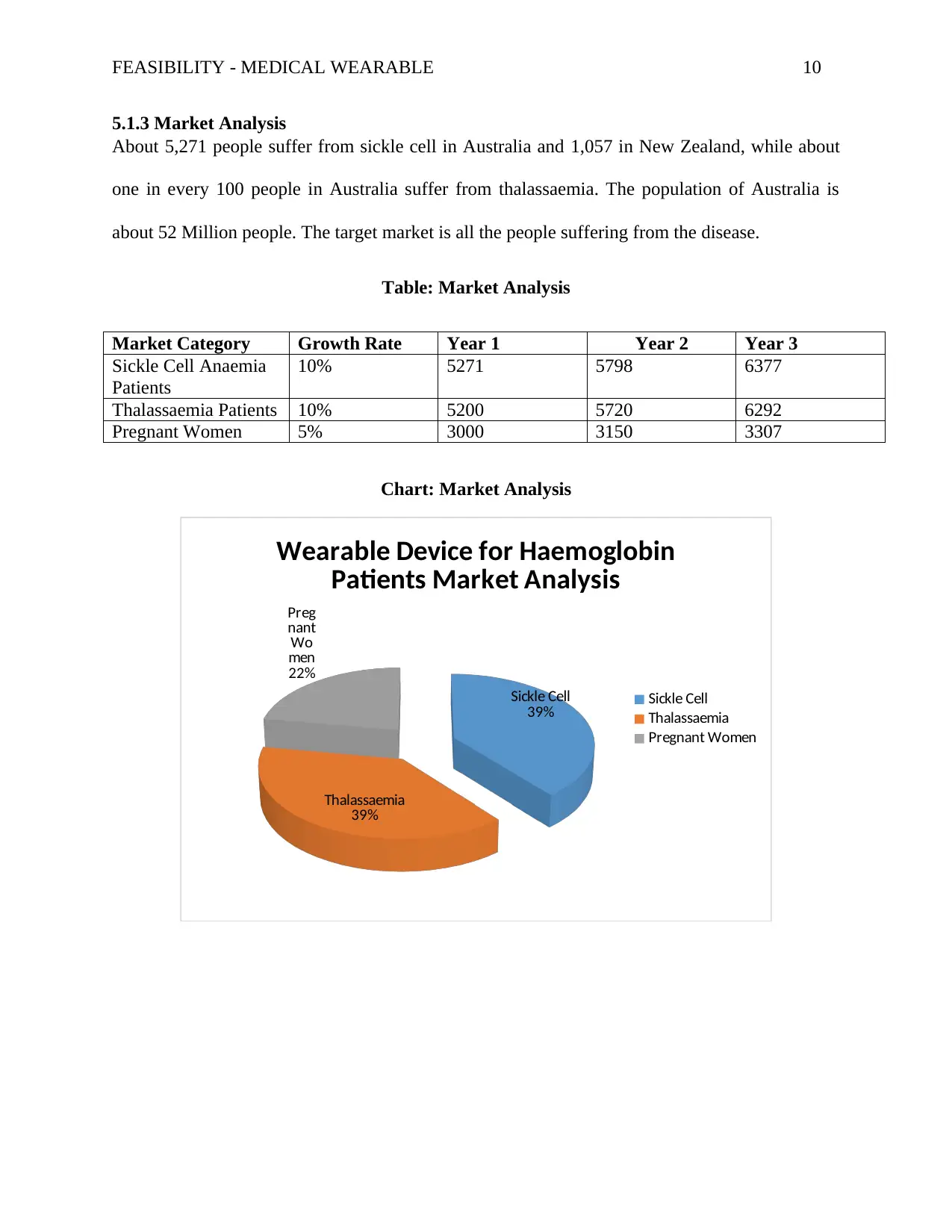
5.1.3 Market Analysis
About 5,271 people suffer from sickle cell in Australia and 1,057 in New Zealand, while about
one in every 100 people in Australia suffer from thalassaemia. The population of Australia is
about 52 Million people. The target market is all the people suffering from the disease.
Table: Market Analysis
Market Category Growth Rate Year 1 Year 2 Year 3
Sickle Cell Anaemia
Patients
10% 5271 5798 6377
Thalassaemia Patients 10% 5200 5720 6292
Pregnant Women 5% 3000 3150 3307
Chart: Market Analysis
Sickle Cell
39%
Thalassaemia
39%
Preg
nant
Wo
men
22%
Wearable Device for Haemoglobin
Patients Market Analysis
Sickle Cell
Thalassaemia
Pregnant Women
5.1.3 Market Analysis
About 5,271 people suffer from sickle cell in Australia and 1,057 in New Zealand, while about
one in every 100 people in Australia suffer from thalassaemia. The population of Australia is
about 52 Million people. The target market is all the people suffering from the disease.
Table: Market Analysis
Market Category Growth Rate Year 1 Year 2 Year 3
Sickle Cell Anaemia
Patients
10% 5271 5798 6377
Thalassaemia Patients 10% 5200 5720 6292
Pregnant Women 5% 3000 3150 3307
Chart: Market Analysis
Sickle Cell
39%
Thalassaemia
39%
Preg
nant
Wo
men
22%
Wearable Device for Haemoglobin
Patients Market Analysis
Sickle Cell
Thalassaemia
Pregnant Women
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
FEASIBILITY - MEDICAL WEARABLE 11
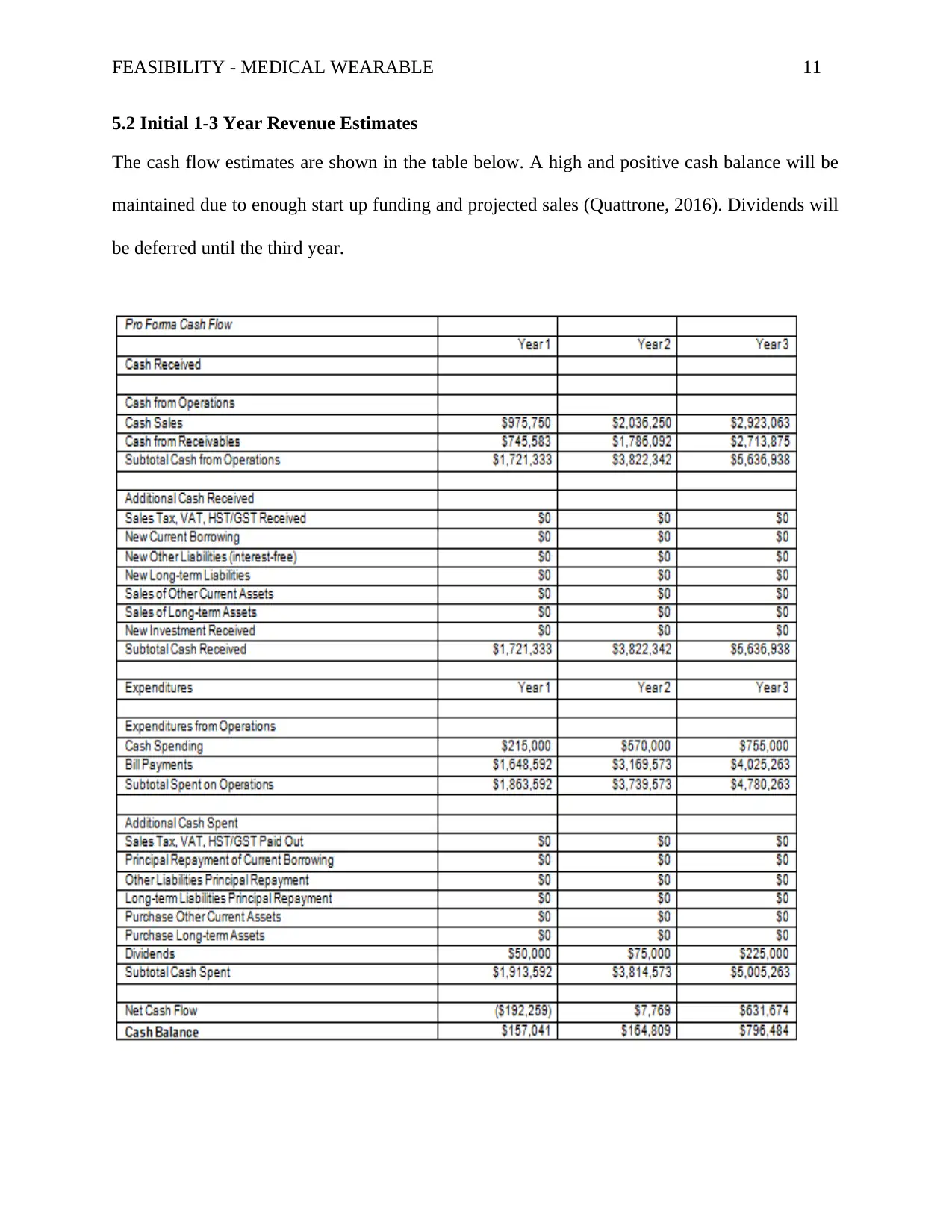
5.2 Initial 1-3 Year Revenue Estimates
The cash flow estimates are shown in the table below. A high and positive cash balance will be
maintained due to enough start up funding and projected sales (Quattrone, 2016). Dividends will
be deferred until the third year.
5.2 Initial 1-3 Year Revenue Estimates
The cash flow estimates are shown in the table below. A high and positive cash balance will be
maintained due to enough start up funding and projected sales (Quattrone, 2016). Dividends will
be deferred until the third year.
FEASIBILITY - MEDICAL WEARABLE 12
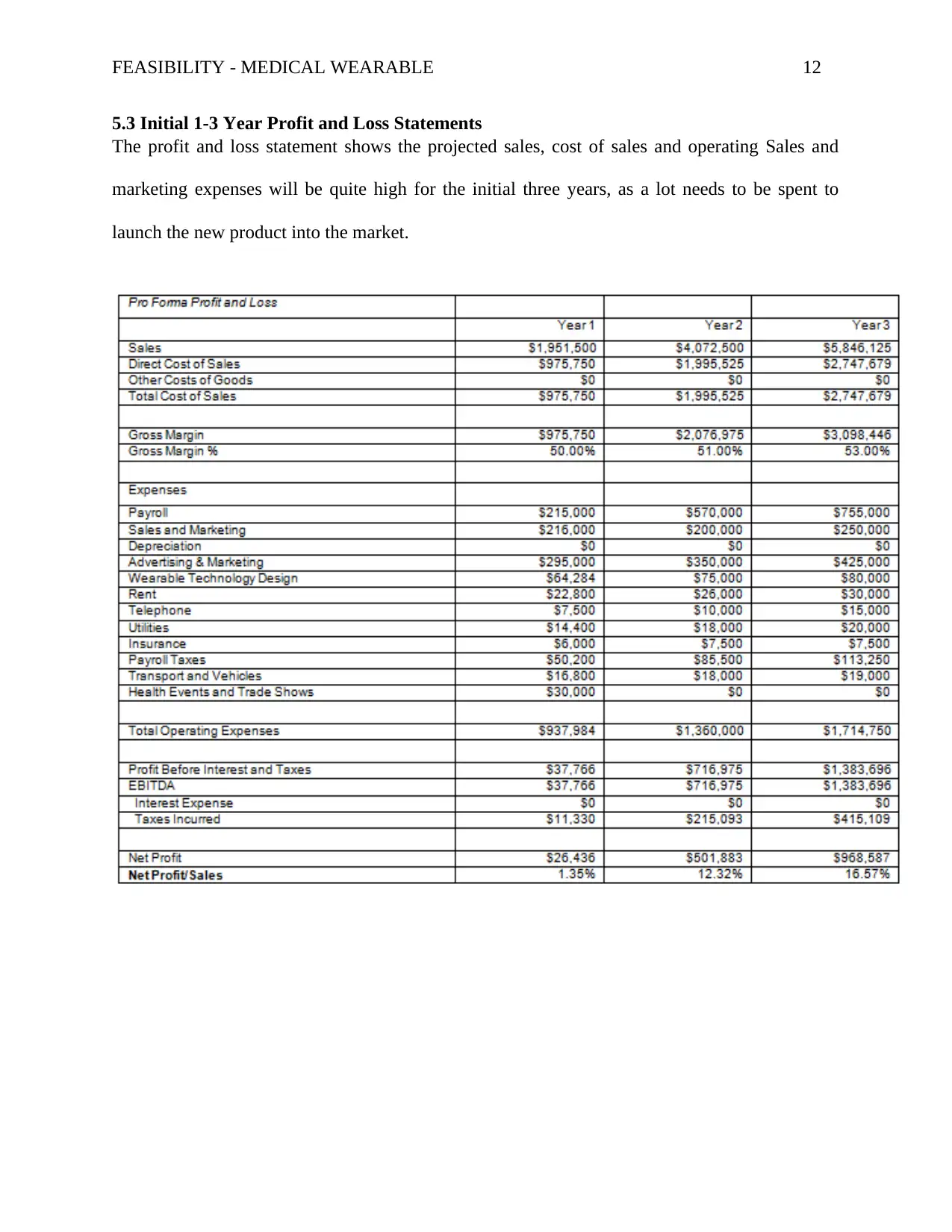
5.3 Initial 1-3 Year Profit and Loss Statements
The profit and loss statement shows the projected sales, cost of sales and operating Sales and
marketing expenses will be quite high for the initial three years, as a lot needs to be spent to
launch the new product into the market.
5.3 Initial 1-3 Year Profit and Loss Statements
The profit and loss statement shows the projected sales, cost of sales and operating Sales and
marketing expenses will be quite high for the initial three years, as a lot needs to be spent to
launch the new product into the market.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 19
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
