Steady State Kinetic Study: Investigating Yeast Alcohol Dehydrogenase
VerifiedAdded on 2024/04/03
|13
|3976
|191
Practical Assignment
AI Summary
This practical assignment focuses on the steady-state kinetics of yeast alcohol dehydrogenase (YAD). The experiments involve determining the initial rate of YAD with ethanol as a substrate, identifying which alcohols act as substrates or non-substrates, and testing for inhibitors of YAD oxidation of ethanol. The protocol includes spectrophotometric measurements to observe NADH production and calculate enzyme activity. Students design a kinetic study to determine parameters like Km, Vmax, Kmapp, and Ki, using provided raw data to analyze enzyme inhibition modes via Lineweaver-Burk plots. The lab report requires tables and figures presenting the experimental data, along with a discussion on the relationship between alcohol structure and YAD active site activity. The report should adhere to a specific format, including an abstract, introduction, methods, results, discussion, conclusion, and references, with a word limit for the main sections.

Department of Biomedical Sciences
Manual of Enzyme Kinetic Practical
Steady State Kinetic Study of Enzyme Activity
1. Background
In these sessions, you will investigate the steady state kinetics of yeast alcohol dehydrogenase
(YAD). You will have lectures explaining the theory behind this work and should refer to your
notes on these to help you understand your study.
As its name suggests, YAD catalyses the dehydrogenation of alcohols. The reaction is shown
below. YAD can use several different alcohols as substrates. The R group in the reaction below
could be H-, CH3, etc (see Table 1).
R-CH2OH + NAD+ <----> R-CHO + NADH + H+
The NADH generated absorbs light at 340nm and we use this property as a signal for the
progress of the reaction. Using a spectrophotometer, we observe the increase in absorbance at
340nm (A340) associated with production of NADH.
2. Experimental Objectives
The aim of these experiments is to determine some of the properties of an enzyme (YAD) by
measuring its rate of catalysis under steady state conditions. In particular:
A. Determine the initial rate of YAD under the standard assay conditions i.e. with ethanol as
the substrate. For this value you can calculate the specific activity of YAD with ethanol
as a substrate (μmoles/min/mg protein)
B. Determine which of the alcohols provided are substrates for YAD and which are not (Non-
substrates) and explain the structural features required for a substrate of YAD and what
structural features may alter YAD activity with alcohols that are substrates.
C. Determine which alcohols are inhibitors of YAD oxidation of ethanol and suggest what
structural features make an alcohol an inhibitor of YAD.
D. Determine the kinetic properties of ethanol in the absence or presence of an inhibitor
(Km, Vmax, Kmapp, Ki).
3. Experimental Protocol
3.1. Determination of enzyme rate (refer to Appendix 1 for
preparation and calculation sheet)
3.1.1. Reagents and Apparatus
Spectrophotometer
plastic cuvettes (1cm light path)
1
Manual of Enzyme Kinetic Practical
Steady State Kinetic Study of Enzyme Activity
1. Background
In these sessions, you will investigate the steady state kinetics of yeast alcohol dehydrogenase
(YAD). You will have lectures explaining the theory behind this work and should refer to your
notes on these to help you understand your study.
As its name suggests, YAD catalyses the dehydrogenation of alcohols. The reaction is shown
below. YAD can use several different alcohols as substrates. The R group in the reaction below
could be H-, CH3, etc (see Table 1).
R-CH2OH + NAD+ <----> R-CHO + NADH + H+
The NADH generated absorbs light at 340nm and we use this property as a signal for the
progress of the reaction. Using a spectrophotometer, we observe the increase in absorbance at
340nm (A340) associated with production of NADH.
2. Experimental Objectives
The aim of these experiments is to determine some of the properties of an enzyme (YAD) by
measuring its rate of catalysis under steady state conditions. In particular:
A. Determine the initial rate of YAD under the standard assay conditions i.e. with ethanol as
the substrate. For this value you can calculate the specific activity of YAD with ethanol
as a substrate (μmoles/min/mg protein)
B. Determine which of the alcohols provided are substrates for YAD and which are not (Non-
substrates) and explain the structural features required for a substrate of YAD and what
structural features may alter YAD activity with alcohols that are substrates.
C. Determine which alcohols are inhibitors of YAD oxidation of ethanol and suggest what
structural features make an alcohol an inhibitor of YAD.
D. Determine the kinetic properties of ethanol in the absence or presence of an inhibitor
(Km, Vmax, Kmapp, Ki).
3. Experimental Protocol
3.1. Determination of enzyme rate (refer to Appendix 1 for
preparation and calculation sheet)
3.1.1. Reagents and Apparatus
Spectrophotometer
plastic cuvettes (1cm light path)
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Department of Biomedical Sciences
Manual of Enzyme Kinetic Practical
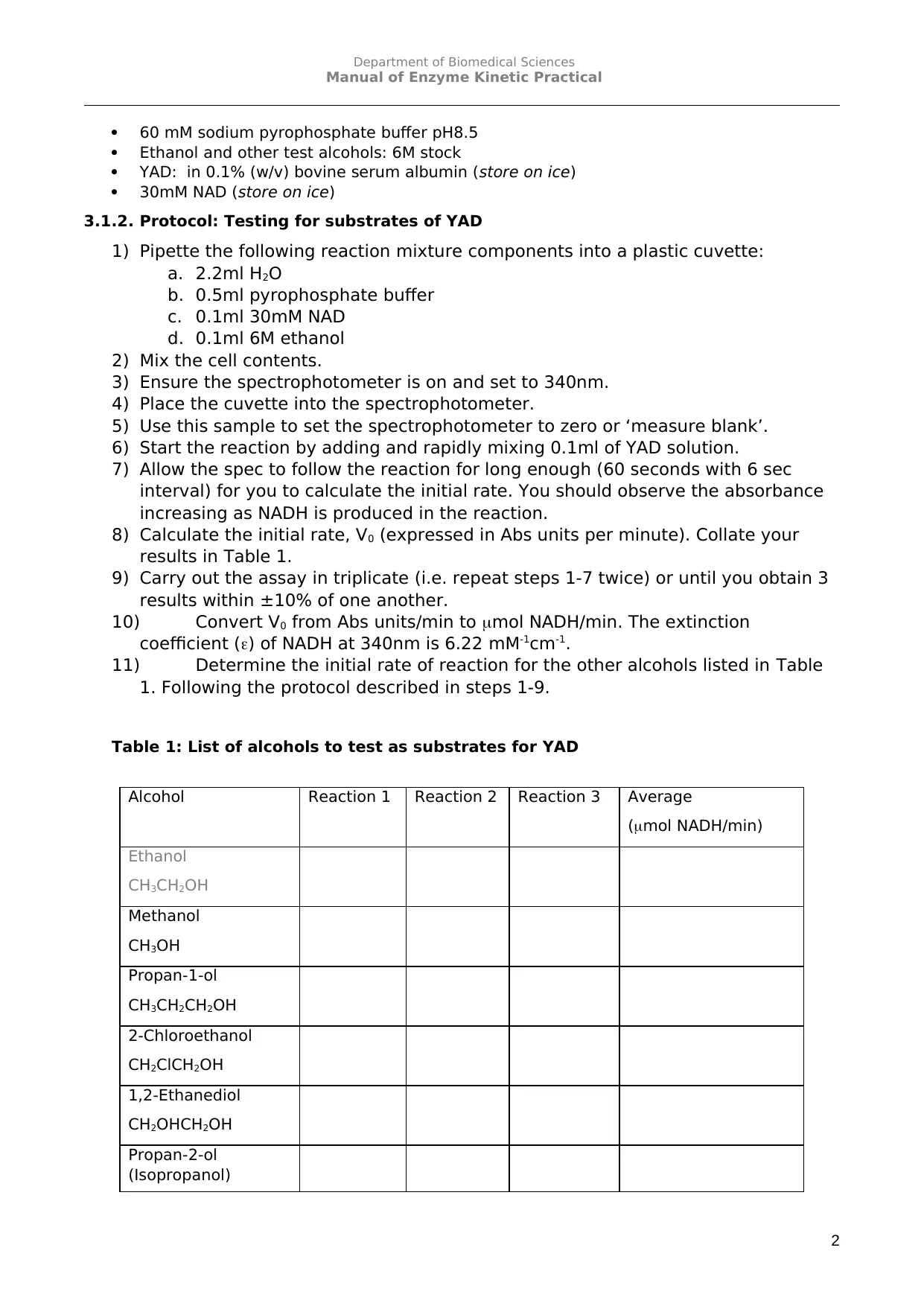
60 mM sodium pyrophosphate buffer pH8.5
Ethanol and other test alcohols: 6M stock
YAD: in 0.1% (w/v) bovine serum albumin (store on ice)
30mM NAD (store on ice)
3.1.2. Protocol: Testing for substrates of YAD
1) Pipette the following reaction mixture components into a plastic cuvette:
a. 2.2ml H2O
b. 0.5ml pyrophosphate buffer
c. 0.1ml 30mM NAD
d. 0.1ml 6M ethanol
2) Mix the cell contents.
3) Ensure the spectrophotometer is on and set to 340nm.
4) Place the cuvette into the spectrophotometer.
5) Use this sample to set the spectrophotometer to zero or ‘measure blank’.
6) Start the reaction by adding and rapidly mixing 0.1ml of YAD solution.
7) Allow the spec to follow the reaction for long enough (60 seconds with 6 sec
interval) for you to calculate the initial rate. You should observe the absorbance
increasing as NADH is produced in the reaction.
8) Calculate the initial rate, V0 (expressed in Abs units per minute). Collate your
results in Table 1.
9) Carry out the assay in triplicate (i.e. repeat steps 1-7 twice) or until you obtain 3
results within ±10% of one another.
10) Convert V0 from Abs units/min to mol NADH/min. The extinction
coefficient () of NADH at 340nm is 6.22 mM-1cm-1.
11) Determine the initial rate of reaction for the other alcohols listed in Table
1. Following the protocol described in steps 1-9.
Table 1: List of alcohols to test as substrates for YAD
Alcohol Reaction 1 Reaction 2 Reaction 3 Average
(mol NADH/min)
Ethanol
CH3CH2OH
Methanol
CH3OH
Propan-1-ol
CH3CH2CH2OH
2-Chloroethanol
CH2ClCH2OH
1,2-Ethanediol
CH2OHCH2OH
Propan-2-ol
(Isopropanol)
2
Manual of Enzyme Kinetic Practical
60 mM sodium pyrophosphate buffer pH8.5
Ethanol and other test alcohols: 6M stock
YAD: in 0.1% (w/v) bovine serum albumin (store on ice)
30mM NAD (store on ice)
3.1.2. Protocol: Testing for substrates of YAD
1) Pipette the following reaction mixture components into a plastic cuvette:
a. 2.2ml H2O
b. 0.5ml pyrophosphate buffer
c. 0.1ml 30mM NAD
d. 0.1ml 6M ethanol
2) Mix the cell contents.
3) Ensure the spectrophotometer is on and set to 340nm.
4) Place the cuvette into the spectrophotometer.
5) Use this sample to set the spectrophotometer to zero or ‘measure blank’.
6) Start the reaction by adding and rapidly mixing 0.1ml of YAD solution.
7) Allow the spec to follow the reaction for long enough (60 seconds with 6 sec
interval) for you to calculate the initial rate. You should observe the absorbance
increasing as NADH is produced in the reaction.
8) Calculate the initial rate, V0 (expressed in Abs units per minute). Collate your
results in Table 1.
9) Carry out the assay in triplicate (i.e. repeat steps 1-7 twice) or until you obtain 3
results within ±10% of one another.
10) Convert V0 from Abs units/min to mol NADH/min. The extinction
coefficient () of NADH at 340nm is 6.22 mM-1cm-1.
11) Determine the initial rate of reaction for the other alcohols listed in Table
1. Following the protocol described in steps 1-9.
Table 1: List of alcohols to test as substrates for YAD
Alcohol Reaction 1 Reaction 2 Reaction 3 Average
(mol NADH/min)
Ethanol
CH3CH2OH
Methanol
CH3OH
Propan-1-ol
CH3CH2CH2OH
2-Chloroethanol
CH2ClCH2OH
1,2-Ethanediol
CH2OHCH2OH
Propan-2-ol
(Isopropanol)
2
Department of Biomedical Sciences
Manual of Enzyme Kinetic Practical
CH3CHOHCH3
2-Propen-1-ol
(Allyl alcohol)
CH2=CHCH2OH
1-Cyclopropyl-
methanol
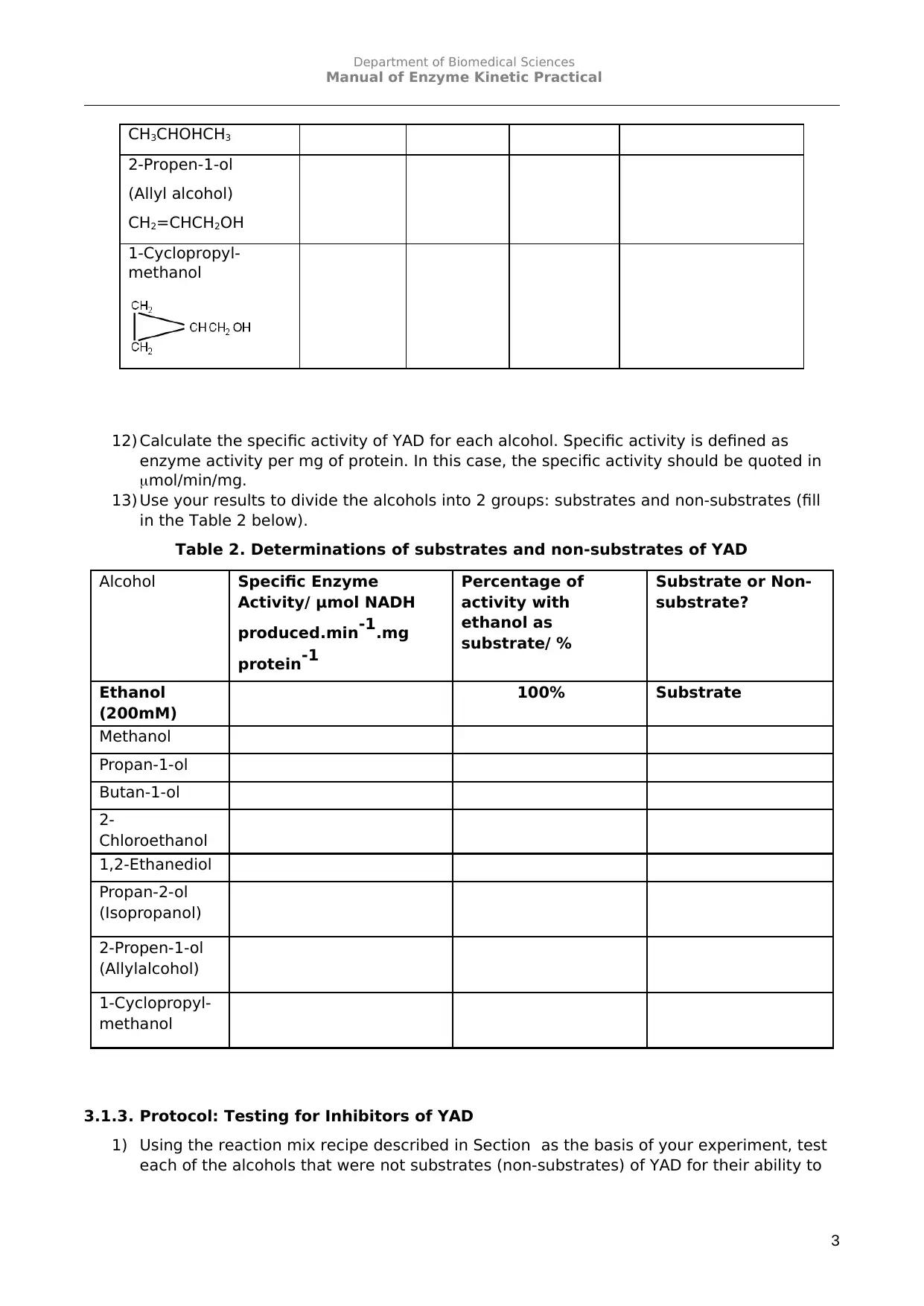
12) Calculate the specific activity of YAD for each alcohol. Specific activity is defined as
enzyme activity per mg of protein. In this case, the specific activity should be quoted in
mol/min/mg.
13) Use your results to divide the alcohols into 2 groups: substrates and non-substrates (fill
in the Table 2 below).
Table 2. Determinations of substrates and non-substrates of YAD
Alcohol Specific Enzyme
Activity/ μmol NADH
produced.min-1.mg
protein-1
Percentage of
activity with
ethanol as
substrate/ %
Substrate or Non-
substrate?
Ethanol
(200mM)
100% Substrate
Methanol
Propan-1-ol
Butan-1-ol
2-
Chloroethanol
1,2-Ethanediol
Propan-2-ol
(Isopropanol)
2-Propen-1-ol
(Allylalcohol)
1-Cyclopropyl-
methanol
3.1.3. Protocol: Testing for Inhibitors of YAD
1) Using the reaction mix recipe described in Section as the basis of your experiment, test
each of the alcohols that were not substrates (non-substrates) of YAD for their ability to
3
Manual of Enzyme Kinetic Practical
CH3CHOHCH3
2-Propen-1-ol
(Allyl alcohol)
CH2=CHCH2OH
1-Cyclopropyl-
methanol
12) Calculate the specific activity of YAD for each alcohol. Specific activity is defined as
enzyme activity per mg of protein. In this case, the specific activity should be quoted in
mol/min/mg.
13) Use your results to divide the alcohols into 2 groups: substrates and non-substrates (fill
in the Table 2 below).
Table 2. Determinations of substrates and non-substrates of YAD
Alcohol Specific Enzyme
Activity/ μmol NADH
produced.min-1.mg
protein-1
Percentage of
activity with
ethanol as
substrate/ %
Substrate or Non-
substrate?
Ethanol
(200mM)
100% Substrate
Methanol
Propan-1-ol
Butan-1-ol
2-
Chloroethanol
1,2-Ethanediol
Propan-2-ol
(Isopropanol)
2-Propen-1-ol
(Allylalcohol)
1-Cyclopropyl-
methanol
3.1.3. Protocol: Testing for Inhibitors of YAD
1) Using the reaction mix recipe described in Section as the basis of your experiment, test
each of the alcohols that were not substrates (non-substrates) of YAD for their ability to
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Department of Biomedical Sciences
Manual of Enzyme Kinetic Practical
inhibit the reduction of ethanol by YAD. You will need to make the changes described in
points 2 and 3 (below).
2) Use a non-saturating concentration of ethanol (10mM).
a. Calculate how much you need to dilute the ethanol stock to achieve a final
concentration of 10mM, when 0.1ml of the diluted stock is added.
3) Measure the initial rate at the new concentration of ethanol in the absence of other
alcohols.
4) Measure the initial rate in the presence of each of the non-substrate alcohols by adding
0.1ml of the 6M stock of each of the alcohols you want to test to the reaction mix.
a. You will need to reduce the volume of H2O by 0.1ml to accommodate the extra
volume.
5) Keep a table of your results and use these results to determine which alcohols are
inhibitors of YAD (similar to Table 1).
6) Use your results to identify the inhibitor (fill in the Table 3 below).
Table 3. Identification of inhibitor of YAD using ethanol as the substrate
Alcohols Specific Enzyme
Activity/ μmol
NADH
produced.min-1.mg
protein-1
Percentage of
activity with
ethanol as
substrate/ %
Non-substrate or
inhibitor
Ethanol only
(10mM)
100% N/A
Ethanol with
Ethanol with
Ethanol with
3.2. Design a study to determine the kinetic properties of YAD
using ethanol as the substrate in the absence or presence of an
inhibitor (Km, Vmax, Kmapp, Ki).
1. Select ethanol and the inhibitor.
2. Using the methods of the type used in section , design a kinetic study to learn more
about the inhibitor.
3. For example, to determine the enzyme inhibition mode and kinetic parameters (Km,
Vmax, Kmapp, Ki) by using Lineweaver-burk plot.
4. Simulated raw data will be provided (See Appendix 2).
3.3. Lab Report
Your report should include the following data:
A table showing the measured rate of NADH production for each alcohol at saturating
final concentration (200mM). The data should be presented with units of mol/min/mg
(i.e. mol NADH produced per minute per mg of YAD) and as a percentage of the activity
obtained with ethanol.
4
Manual of Enzyme Kinetic Practical
inhibit the reduction of ethanol by YAD. You will need to make the changes described in
points 2 and 3 (below).
2) Use a non-saturating concentration of ethanol (10mM).
a. Calculate how much you need to dilute the ethanol stock to achieve a final
concentration of 10mM, when 0.1ml of the diluted stock is added.
3) Measure the initial rate at the new concentration of ethanol in the absence of other
alcohols.
4) Measure the initial rate in the presence of each of the non-substrate alcohols by adding
0.1ml of the 6M stock of each of the alcohols you want to test to the reaction mix.
a. You will need to reduce the volume of H2O by 0.1ml to accommodate the extra
volume.
5) Keep a table of your results and use these results to determine which alcohols are
inhibitors of YAD (similar to Table 1).
6) Use your results to identify the inhibitor (fill in the Table 3 below).
Table 3. Identification of inhibitor of YAD using ethanol as the substrate
Alcohols Specific Enzyme
Activity/ μmol
NADH
produced.min-1.mg
protein-1
Percentage of
activity with
ethanol as
substrate/ %
Non-substrate or
inhibitor
Ethanol only
(10mM)
100% N/A
Ethanol with
Ethanol with
Ethanol with
3.2. Design a study to determine the kinetic properties of YAD
using ethanol as the substrate in the absence or presence of an
inhibitor (Km, Vmax, Kmapp, Ki).
1. Select ethanol and the inhibitor.
2. Using the methods of the type used in section , design a kinetic study to learn more
about the inhibitor.
3. For example, to determine the enzyme inhibition mode and kinetic parameters (Km,
Vmax, Kmapp, Ki) by using Lineweaver-burk plot.
4. Simulated raw data will be provided (See Appendix 2).
3.3. Lab Report
Your report should include the following data:
A table showing the measured rate of NADH production for each alcohol at saturating
final concentration (200mM). The data should be presented with units of mol/min/mg
(i.e. mol NADH produced per minute per mg of YAD) and as a percentage of the activity
obtained with ethanol.
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Department of Biomedical Sciences
Manual of Enzyme Kinetic Practical
A table showing the effect of non-substrate alcohols on the activity of YAD with ethanol
as a substrate at sub saturating concentration of ethanol (10mM). The data should be
presented as above.
Figures showing the data obtained from your designed kinetic study. These should be in
the form of the appropriate graphs.
A table showing the kinetic parameters determined from your designed study.
Your report should address the following question: ‘What do the data obtained from your
kinetic studies tell you about the structure of the YAD active site?’
Think about how the structures of alcohols that are substrates for YAD compared with those that
are non-substrates or that inhibit the reaction.
Reports must adhere to the format described below. The newly developed marking rubric will be
used (See Appendix 3)
1. Report title, your name and affiliation
2. Abstract (200 word max)
3. Introduction: concise background information.
4. Methods: concise description of the methods used.
5. Results: Concise description of the data obtained. This section should include figures and
tables as required to display the data you obtained. Figures might include images or
graphs. Each table and/or figure must be numbered and given a short explanatory title
and legend.
6. Discussion: A concise evaluation and interpretation of the data shown in the results
section. Make sure to comment on any particular questions you have been asked to
consider.
7. Conclusion
8. References: use Harvard style
9. Format, grammar ect.
Maximum of 1500 words for sections 3-7, excluding figure legends.
Appendix 1
Steady state kinetic study of an enzyme: yeast alcohol dehydrogenase (YAD) –
Calculation and preparation sheet
A. Determine the initial rate of YAD with ethanol (200mM final concentration) as
substrate under the standard assay conditions.
5
Manual of Enzyme Kinetic Practical
A table showing the effect of non-substrate alcohols on the activity of YAD with ethanol
as a substrate at sub saturating concentration of ethanol (10mM). The data should be
presented as above.
Figures showing the data obtained from your designed kinetic study. These should be in
the form of the appropriate graphs.
A table showing the kinetic parameters determined from your designed study.
Your report should address the following question: ‘What do the data obtained from your
kinetic studies tell you about the structure of the YAD active site?’
Think about how the structures of alcohols that are substrates for YAD compared with those that
are non-substrates or that inhibit the reaction.
Reports must adhere to the format described below. The newly developed marking rubric will be
used (See Appendix 3)
1. Report title, your name and affiliation
2. Abstract (200 word max)
3. Introduction: concise background information.
4. Methods: concise description of the methods used.
5. Results: Concise description of the data obtained. This section should include figures and
tables as required to display the data you obtained. Figures might include images or
graphs. Each table and/or figure must be numbered and given a short explanatory title
and legend.
6. Discussion: A concise evaluation and interpretation of the data shown in the results
section. Make sure to comment on any particular questions you have been asked to
consider.
7. Conclusion
8. References: use Harvard style
9. Format, grammar ect.
Maximum of 1500 words for sections 3-7, excluding figure legends.
Appendix 1
Steady state kinetic study of an enzyme: yeast alcohol dehydrogenase (YAD) –
Calculation and preparation sheet
A. Determine the initial rate of YAD with ethanol (200mM final concentration) as
substrate under the standard assay conditions.
5
Department of Biomedical Sciences
Manual of Enzyme Kinetic Practical
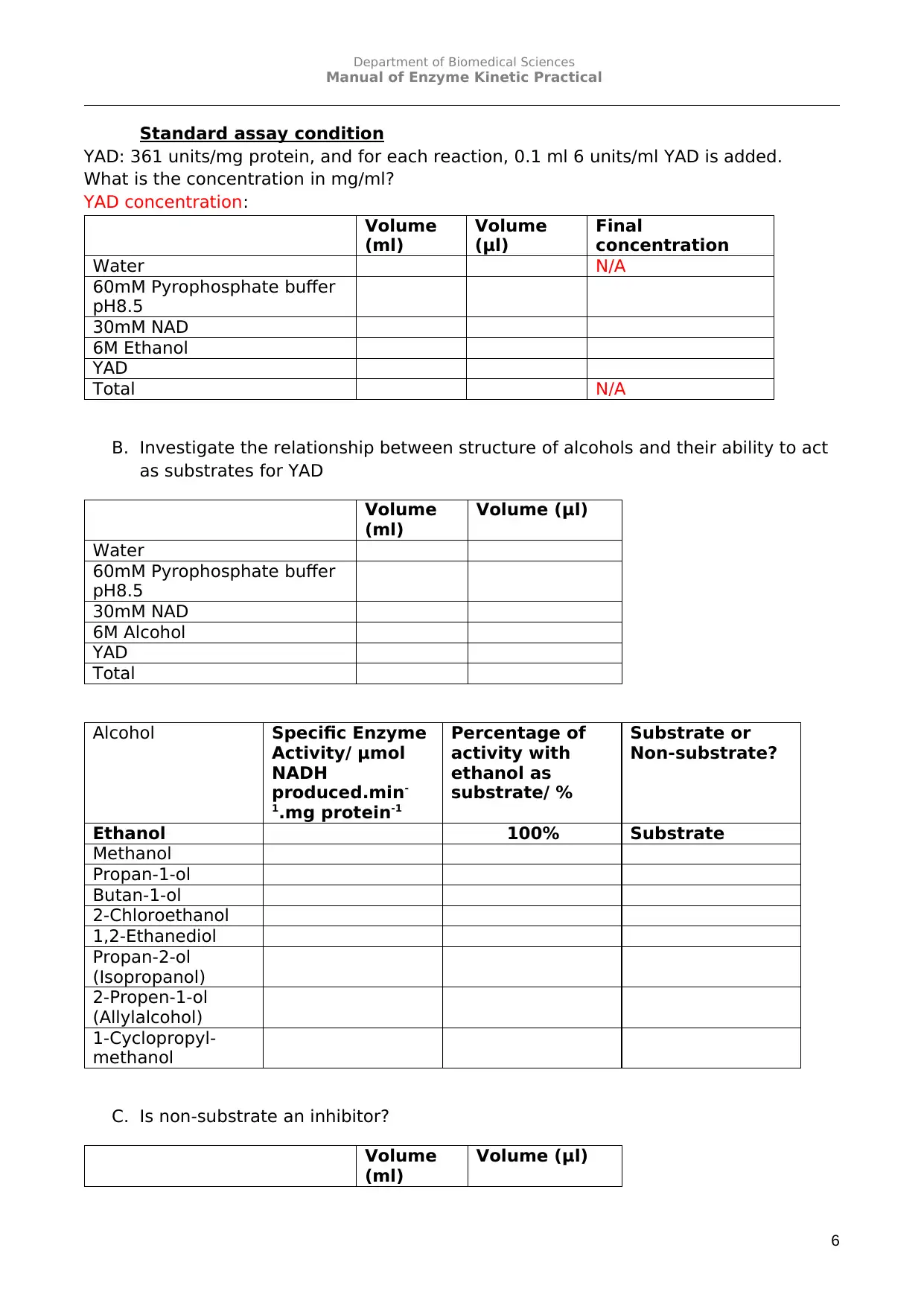
Standard assay condition
YAD: 361 units/mg protein, and for each reaction, 0.1 ml 6 units/ml YAD is added.
What is the concentration in mg/ml?
YAD concentration:
Volume
(ml)
Volume
(μl)
Final
concentration
Water N/A
60mM Pyrophosphate buffer
pH8.5
30mM NAD
6M Ethanol
YAD
Total N/A
B. Investigate the relationship between structure of alcohols and their ability to act
as substrates for YAD
Volume
(ml)
Volume (μl)
Water
60mM Pyrophosphate buffer
pH8.5
30mM NAD
6M Alcohol
YAD
Total
Alcohol Specific Enzyme
Activity/ μmol
NADH
produced.min-
1.mg protein-1
Percentage of
activity with
ethanol as
substrate/ %
Substrate or
Non-substrate?
Ethanol 100% Substrate
Methanol
Propan-1-ol
Butan-1-ol
2-Chloroethanol
1,2-Ethanediol
Propan-2-ol
(Isopropanol)
2-Propen-1-ol
(Allylalcohol)
1-Cyclopropyl-
methanol
C. Is non-substrate an inhibitor?
Volume
(ml)
Volume (μl)
6
Manual of Enzyme Kinetic Practical
Standard assay condition
YAD: 361 units/mg protein, and for each reaction, 0.1 ml 6 units/ml YAD is added.
What is the concentration in mg/ml?
YAD concentration:
Volume
(ml)
Volume
(μl)
Final
concentration
Water N/A
60mM Pyrophosphate buffer
pH8.5
30mM NAD
6M Ethanol
YAD
Total N/A
B. Investigate the relationship between structure of alcohols and their ability to act
as substrates for YAD
Volume
(ml)
Volume (μl)
Water
60mM Pyrophosphate buffer
pH8.5
30mM NAD
6M Alcohol
YAD
Total
Alcohol Specific Enzyme
Activity/ μmol
NADH
produced.min-
1.mg protein-1
Percentage of
activity with
ethanol as
substrate/ %
Substrate or
Non-substrate?
Ethanol 100% Substrate
Methanol
Propan-1-ol
Butan-1-ol
2-Chloroethanol
1,2-Ethanediol
Propan-2-ol
(Isopropanol)
2-Propen-1-ol
(Allylalcohol)
1-Cyclopropyl-
methanol
C. Is non-substrate an inhibitor?
Volume
(ml)
Volume (μl)
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Department of Biomedical Sciences
Manual of Enzyme Kinetic Practical
Water
60mM Pyrophosphate buffer
pH8.5
30mM NAD
6M Non-substrate/water
YAD
6M Ethanol
Total
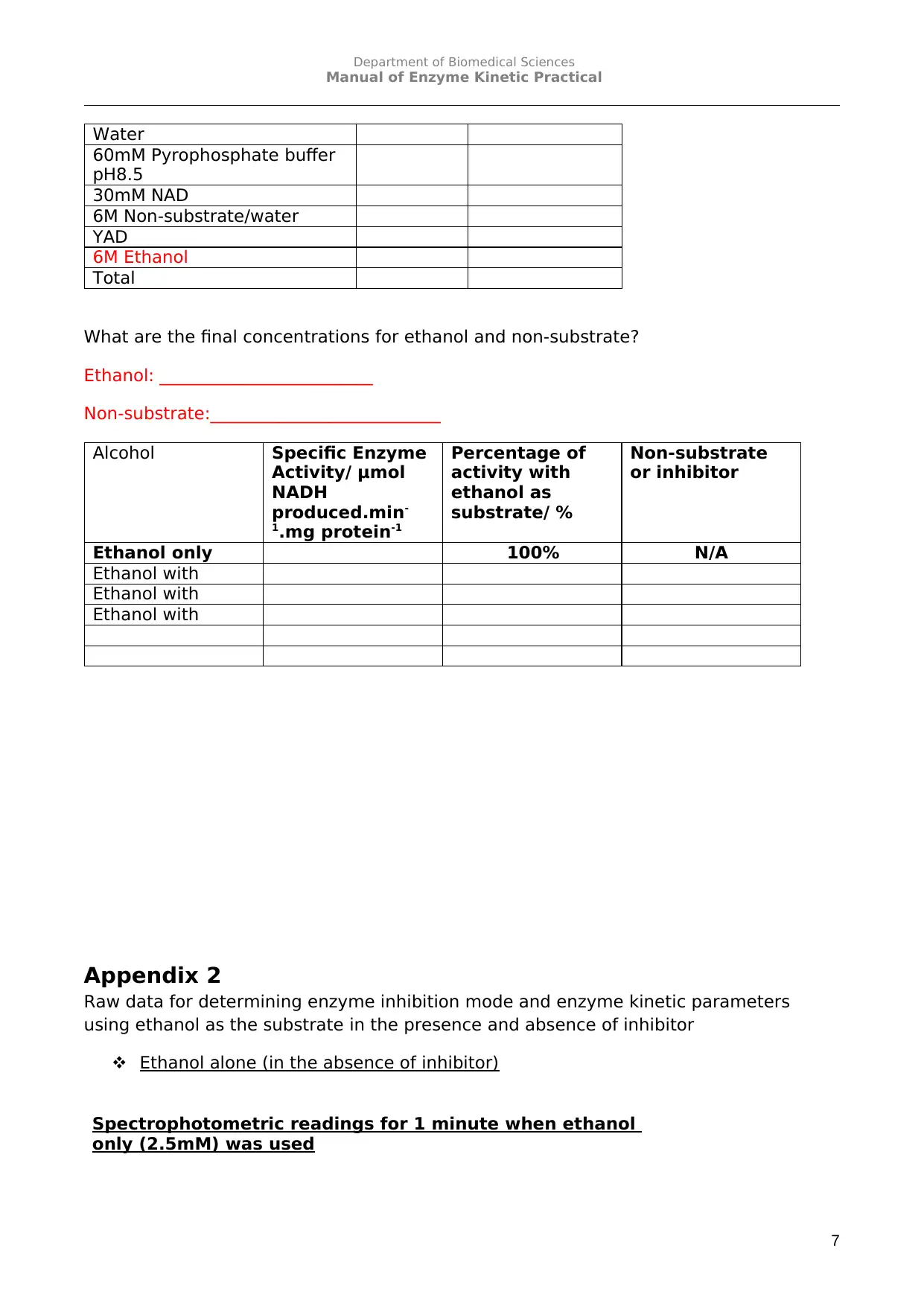
What are the final concentrations for ethanol and non-substrate?
Ethanol: _________________________
Non-substrate:___________________________
Alcohol Specific Enzyme
Activity/ μmol
NADH
produced.min-
1.mg protein-1
Percentage of
activity with
ethanol as
substrate/ %
Non-substrate
or inhibitor
Ethanol only 100% N/A
Ethanol with
Ethanol with
Ethanol with
Appendix 2
Raw data for determining enzyme inhibition mode and enzyme kinetic parameters
using ethanol as the substrate in the presence and absence of inhibitor
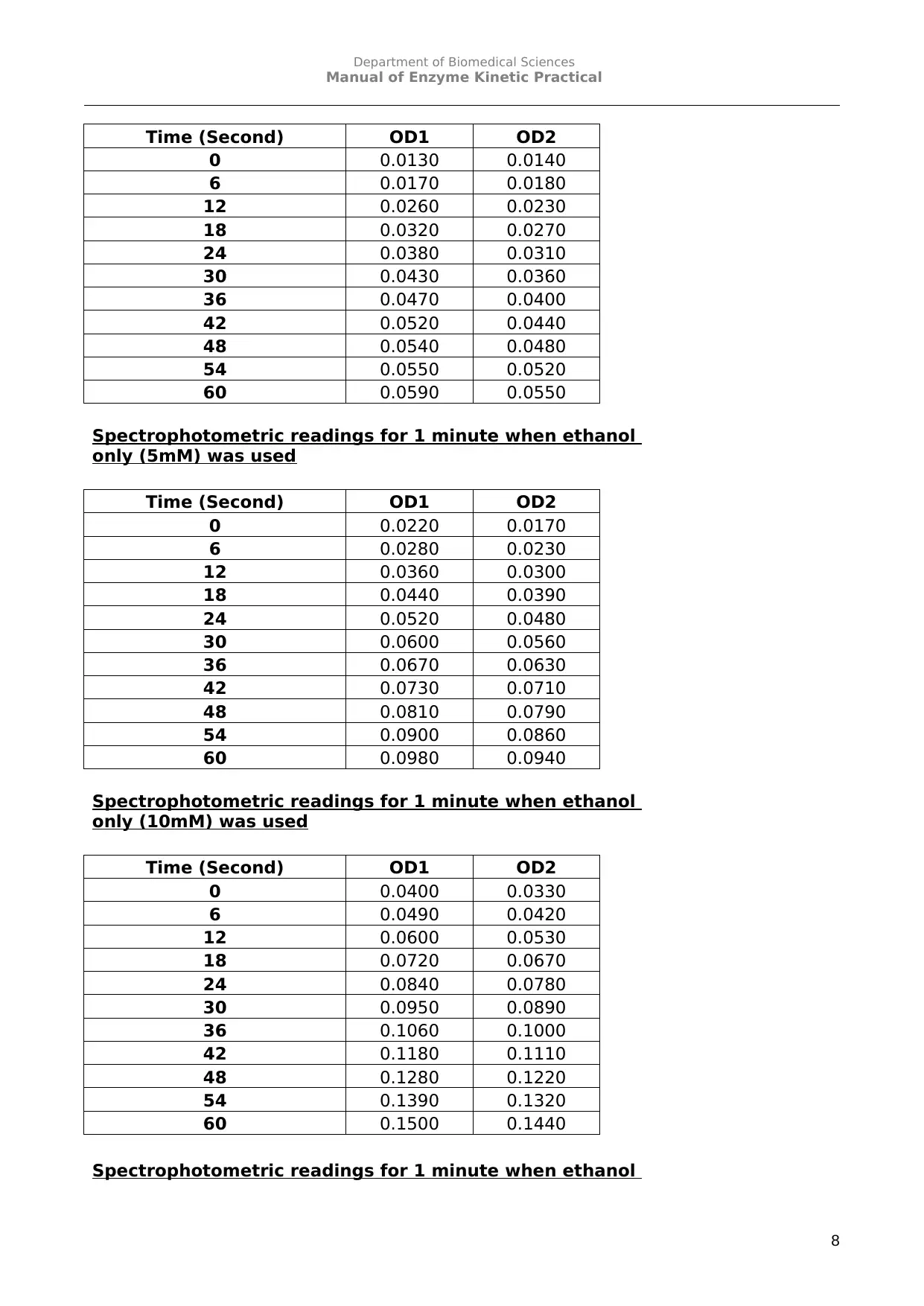
Ethanol alone (in the absence of inhibitor)
Spectrophotometric readings for 1 minute when ethanol
only (2.5mM) was used
7
Manual of Enzyme Kinetic Practical
Water
60mM Pyrophosphate buffer
pH8.5
30mM NAD
6M Non-substrate/water
YAD
6M Ethanol
Total
What are the final concentrations for ethanol and non-substrate?
Ethanol: _________________________
Non-substrate:___________________________
Alcohol Specific Enzyme
Activity/ μmol
NADH
produced.min-
1.mg protein-1
Percentage of
activity with
ethanol as
substrate/ %
Non-substrate
or inhibitor
Ethanol only 100% N/A
Ethanol with
Ethanol with
Ethanol with
Appendix 2
Raw data for determining enzyme inhibition mode and enzyme kinetic parameters
using ethanol as the substrate in the presence and absence of inhibitor
Ethanol alone (in the absence of inhibitor)
Spectrophotometric readings for 1 minute when ethanol
only (2.5mM) was used
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Department of Biomedical Sciences
Manual of Enzyme Kinetic Practical
Time (Second) OD1 OD2
0 0.0130 0.0140
6 0.0170 0.0180
12 0.0260 0.0230
18 0.0320 0.0270
24 0.0380 0.0310
30 0.0430 0.0360
36 0.0470 0.0400
42 0.0520 0.0440
48 0.0540 0.0480
54 0.0550 0.0520
60 0.0590 0.0550
Spectrophotometric readings for 1 minute when ethanol
only (5mM) was used
Time (Second) OD1 OD2
0 0.0220 0.0170
6 0.0280 0.0230
12 0.0360 0.0300
18 0.0440 0.0390
24 0.0520 0.0480
30 0.0600 0.0560
36 0.0670 0.0630
42 0.0730 0.0710
48 0.0810 0.0790
54 0.0900 0.0860
60 0.0980 0.0940
Spectrophotometric readings for 1 minute when ethanol
only (10mM) was used
Time (Second) OD1 OD2
0 0.0400 0.0330
6 0.0490 0.0420
12 0.0600 0.0530
18 0.0720 0.0670
24 0.0840 0.0780
30 0.0950 0.0890
36 0.1060 0.1000
42 0.1180 0.1110
48 0.1280 0.1220
54 0.1390 0.1320
60 0.1500 0.1440
Spectrophotometric readings for 1 minute when ethanol
8
Manual of Enzyme Kinetic Practical
Time (Second) OD1 OD2
0 0.0130 0.0140
6 0.0170 0.0180
12 0.0260 0.0230
18 0.0320 0.0270
24 0.0380 0.0310
30 0.0430 0.0360
36 0.0470 0.0400
42 0.0520 0.0440
48 0.0540 0.0480
54 0.0550 0.0520
60 0.0590 0.0550
Spectrophotometric readings for 1 minute when ethanol
only (5mM) was used
Time (Second) OD1 OD2
0 0.0220 0.0170
6 0.0280 0.0230
12 0.0360 0.0300
18 0.0440 0.0390
24 0.0520 0.0480
30 0.0600 0.0560
36 0.0670 0.0630
42 0.0730 0.0710
48 0.0810 0.0790
54 0.0900 0.0860
60 0.0980 0.0940
Spectrophotometric readings for 1 minute when ethanol
only (10mM) was used
Time (Second) OD1 OD2
0 0.0400 0.0330
6 0.0490 0.0420
12 0.0600 0.0530
18 0.0720 0.0670
24 0.0840 0.0780
30 0.0950 0.0890
36 0.1060 0.1000
42 0.1180 0.1110
48 0.1280 0.1220
54 0.1390 0.1320
60 0.1500 0.1440
Spectrophotometric readings for 1 minute when ethanol
8
Department of Biomedical Sciences
Manual of Enzyme Kinetic Practical
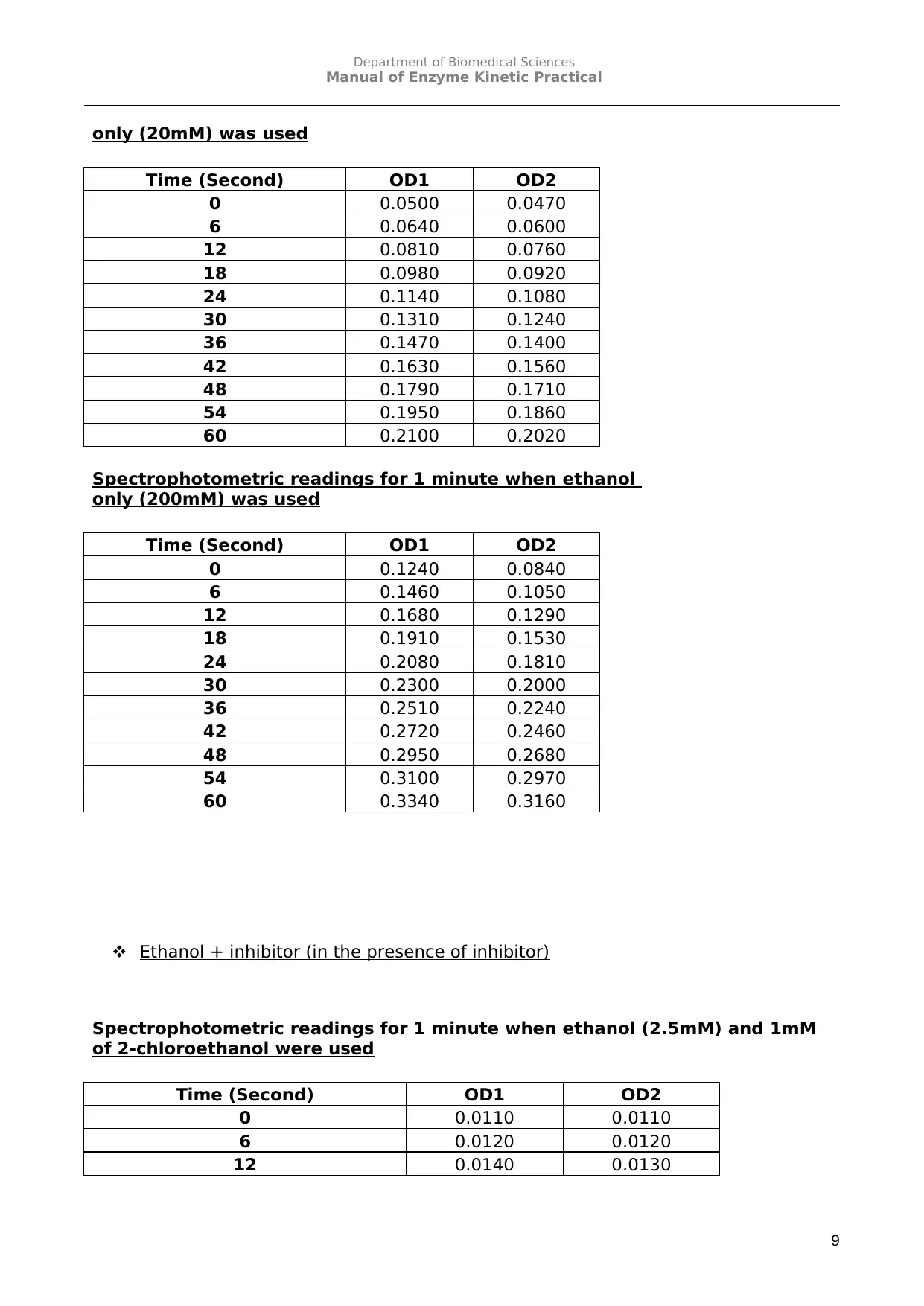
only (20mM) was used
Time (Second) OD1 OD2
0 0.0500 0.0470
6 0.0640 0.0600
12 0.0810 0.0760
18 0.0980 0.0920
24 0.1140 0.1080
30 0.1310 0.1240
36 0.1470 0.1400
42 0.1630 0.1560
48 0.1790 0.1710
54 0.1950 0.1860
60 0.2100 0.2020
Spectrophotometric readings for 1 minute when ethanol
only (200mM) was used
Time (Second) OD1 OD2
0 0.1240 0.0840
6 0.1460 0.1050
12 0.1680 0.1290
18 0.1910 0.1530
24 0.2080 0.1810
30 0.2300 0.2000
36 0.2510 0.2240
42 0.2720 0.2460
48 0.2950 0.2680
54 0.3100 0.2970
60 0.3340 0.3160
Ethanol + inhibitor (in the presence of inhibitor)
Spectrophotometric readings for 1 minute when ethanol (2.5mM) and 1mM
of 2-chloroethanol were used
Time (Second) OD1 OD2
0 0.0110 0.0110
6 0.0120 0.0120
12 0.0140 0.0130
9
Manual of Enzyme Kinetic Practical
only (20mM) was used
Time (Second) OD1 OD2
0 0.0500 0.0470
6 0.0640 0.0600
12 0.0810 0.0760
18 0.0980 0.0920
24 0.1140 0.1080
30 0.1310 0.1240
36 0.1470 0.1400
42 0.1630 0.1560
48 0.1790 0.1710
54 0.1950 0.1860
60 0.2100 0.2020
Spectrophotometric readings for 1 minute when ethanol
only (200mM) was used
Time (Second) OD1 OD2
0 0.1240 0.0840
6 0.1460 0.1050
12 0.1680 0.1290
18 0.1910 0.1530
24 0.2080 0.1810
30 0.2300 0.2000
36 0.2510 0.2240
42 0.2720 0.2460
48 0.2950 0.2680
54 0.3100 0.2970
60 0.3340 0.3160
Ethanol + inhibitor (in the presence of inhibitor)
Spectrophotometric readings for 1 minute when ethanol (2.5mM) and 1mM
of 2-chloroethanol were used
Time (Second) OD1 OD2
0 0.0110 0.0110
6 0.0120 0.0120
12 0.0140 0.0130
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Department of Biomedical Sciences
Manual of Enzyme Kinetic Practical
18 0.0160 0.0140
24 0.0180 0.0150
30 0.0200 0.0160
36 0.0220 0.0170
42 0.0240 0.0190
48 0.0260 0.0200
54 0.0280 0.0210
60 0.0300 0.0230
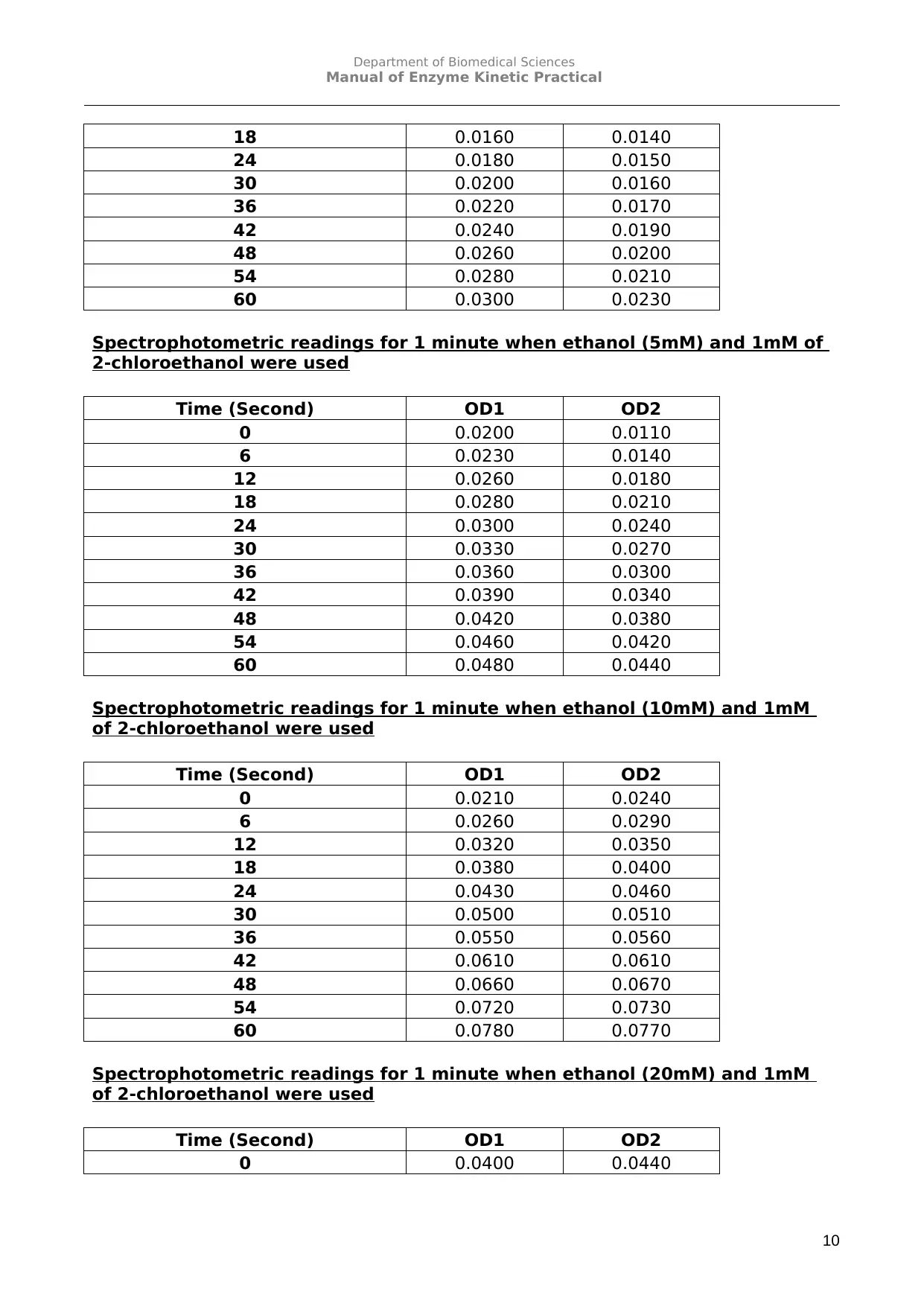
Spectrophotometric readings for 1 minute when ethanol (5mM) and 1mM of
2-chloroethanol were used
Time (Second) OD1 OD2
0 0.0200 0.0110
6 0.0230 0.0140
12 0.0260 0.0180
18 0.0280 0.0210
24 0.0300 0.0240
30 0.0330 0.0270
36 0.0360 0.0300
42 0.0390 0.0340
48 0.0420 0.0380
54 0.0460 0.0420
60 0.0480 0.0440
Spectrophotometric readings for 1 minute when ethanol (10mM) and 1mM
of 2-chloroethanol were used
Time (Second) OD1 OD2
0 0.0210 0.0240
6 0.0260 0.0290
12 0.0320 0.0350
18 0.0380 0.0400
24 0.0430 0.0460
30 0.0500 0.0510
36 0.0550 0.0560
42 0.0610 0.0610
48 0.0660 0.0670
54 0.0720 0.0730
60 0.0780 0.0770
Spectrophotometric readings for 1 minute when ethanol (20mM) and 1mM
of 2-chloroethanol were used
Time (Second) OD1 OD2
0 0.0400 0.0440
10
Manual of Enzyme Kinetic Practical
18 0.0160 0.0140
24 0.0180 0.0150
30 0.0200 0.0160
36 0.0220 0.0170
42 0.0240 0.0190
48 0.0260 0.0200
54 0.0280 0.0210
60 0.0300 0.0230
Spectrophotometric readings for 1 minute when ethanol (5mM) and 1mM of
2-chloroethanol were used
Time (Second) OD1 OD2
0 0.0200 0.0110
6 0.0230 0.0140
12 0.0260 0.0180
18 0.0280 0.0210
24 0.0300 0.0240
30 0.0330 0.0270
36 0.0360 0.0300
42 0.0390 0.0340
48 0.0420 0.0380
54 0.0460 0.0420
60 0.0480 0.0440
Spectrophotometric readings for 1 minute when ethanol (10mM) and 1mM
of 2-chloroethanol were used
Time (Second) OD1 OD2
0 0.0210 0.0240
6 0.0260 0.0290
12 0.0320 0.0350
18 0.0380 0.0400
24 0.0430 0.0460
30 0.0500 0.0510
36 0.0550 0.0560
42 0.0610 0.0610
48 0.0660 0.0670
54 0.0720 0.0730
60 0.0780 0.0770
Spectrophotometric readings for 1 minute when ethanol (20mM) and 1mM
of 2-chloroethanol were used
Time (Second) OD1 OD2
0 0.0400 0.0440
10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Department of Biomedical Sciences
Manual of Enzyme Kinetic Practical
6 0.0480 0.0520
12 0.0560 0.0610
18 0.0640 0.0700
24 0.0730 0.0770
30 0.0800 0.0850
36 0.0880 0.0930
42 0.0950 0.1020
48 0.1020 0.1080
54 0.1090 0.1160
60 0.1180 0.1260
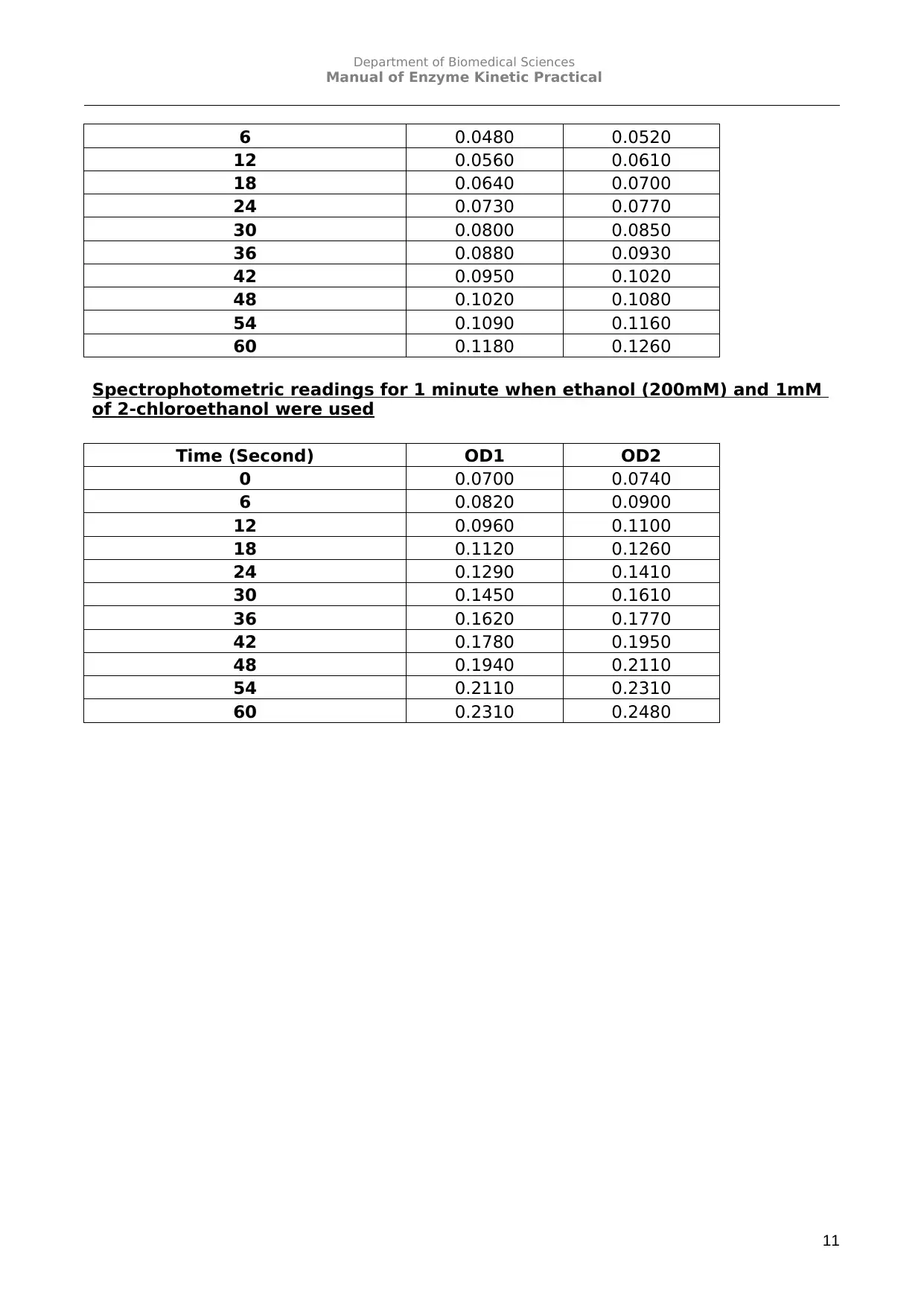
Spectrophotometric readings for 1 minute when ethanol (200mM) and 1mM
of 2-chloroethanol were used
Time (Second) OD1 OD2
0 0.0700 0.0740
6 0.0820 0.0900
12 0.0960 0.1100
18 0.1120 0.1260
24 0.1290 0.1410
30 0.1450 0.1610
36 0.1620 0.1770
42 0.1780 0.1950
48 0.1940 0.2110
54 0.2110 0.2310
60 0.2310 0.2480
11
Manual of Enzyme Kinetic Practical
6 0.0480 0.0520
12 0.0560 0.0610
18 0.0640 0.0700
24 0.0730 0.0770
30 0.0800 0.0850
36 0.0880 0.0930
42 0.0950 0.1020
48 0.1020 0.1080
54 0.1090 0.1160
60 0.1180 0.1260
Spectrophotometric readings for 1 minute when ethanol (200mM) and 1mM
of 2-chloroethanol were used
Time (Second) OD1 OD2
0 0.0700 0.0740
6 0.0820 0.0900
12 0.0960 0.1100
18 0.1120 0.1260
24 0.1290 0.1410
30 0.1450 0.1610
36 0.1620 0.1770
42 0.1780 0.1950
48 0.1940 0.2110
54 0.2110 0.2310
60 0.2310 0.2480
11
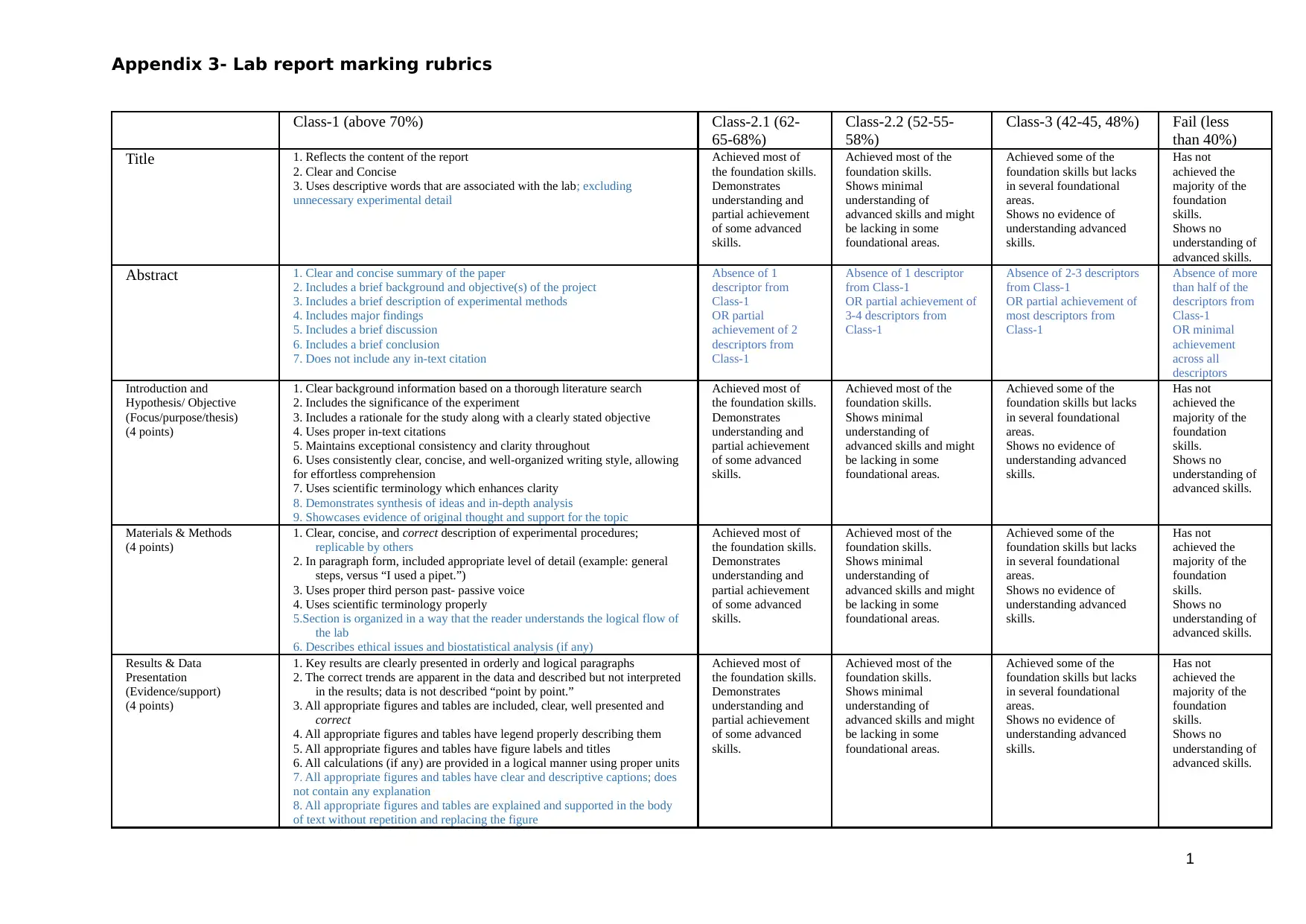
Appendix 3- Lab report marking rubrics
Class-1 (above 70%) Class-2.1 (62-
65-68%)
Class-2.2 (52-55-
58%)
Class-3 (42-45, 48%) Fail (less
than 40%)
Title 1. Reflects the content of the report
2. Clear and Concise
3. Uses descriptive words that are associated with the lab; excluding
unnecessary experimental detail
Achieved most of
the foundation skills.
Demonstrates
understanding and
partial achievement
of some advanced
skills.
Achieved most of the
foundation skills.
Shows minimal
understanding of
advanced skills and might
be lacking in some
foundational areas.
Achieved some of the
foundation skills but lacks
in several foundational
areas.
Shows no evidence of
understanding advanced
skills.
Has not
achieved the
majority of the
foundation
skills.
Shows no
understanding of
advanced skills.
Abstract 1. Clear and concise summary of the paper
2. Includes a brief background and objective(s) of the project
3. Includes a brief description of experimental methods
4. Includes major findings
5. Includes a brief discussion
6. Includes a brief conclusion
7. Does not include any in-text citation
Absence of 1
descriptor from
Class-1
OR partial
achievement of 2
descriptors from
Class-1
Absence of 1 descriptor
from Class-1
OR partial achievement of
3-4 descriptors from
Class-1
Absence of 2-3 descriptors
from Class-1
OR partial achievement of
most descriptors from
Class-1
Absence of more
than half of the
descriptors from
Class-1
OR minimal
achievement
across all
descriptors
Introduction and
Hypothesis/ Objective
(Focus/purpose/thesis)
(4 points)
1. Clear background information based on a thorough literature search
2. Includes the significance of the experiment
3. Includes a rationale for the study along with a clearly stated objective
4. Uses proper in-text citations
5. Maintains exceptional consistency and clarity throughout
6. Uses consistently clear, concise, and well-organized writing style, allowing
for effortless comprehension
7. Uses scientific terminology which enhances clarity
8. Demonstrates synthesis of ideas and in-depth analysis
9. Showcases evidence of original thought and support for the topic
Achieved most of
the foundation skills.
Demonstrates
understanding and
partial achievement
of some advanced
skills.
Achieved most of the
foundation skills.
Shows minimal
understanding of
advanced skills and might
be lacking in some
foundational areas.
Achieved some of the
foundation skills but lacks
in several foundational
areas.
Shows no evidence of
understanding advanced
skills.
Has not
achieved the
majority of the
foundation
skills.
Shows no
understanding of
advanced skills.
Materials & Methods
(4 points)
1. Clear, concise, and correct description of experimental procedures;
replicable by others
2. In paragraph form, included appropriate level of detail (example: general
steps, versus “I used a pipet.”)
3. Uses proper third person past- passive voice
4. Uses scientific terminology properly
5.Section is organized in a way that the reader understands the logical flow of
the lab
6. Describes ethical issues and biostatistical analysis (if any)
Achieved most of
the foundation skills.
Demonstrates
understanding and
partial achievement
of some advanced
skills.
Achieved most of the
foundation skills.
Shows minimal
understanding of
advanced skills and might
be lacking in some
foundational areas.
Achieved some of the
foundation skills but lacks
in several foundational
areas.
Shows no evidence of
understanding advanced
skills.
Has not
achieved the
majority of the
foundation
skills.
Shows no
understanding of
advanced skills.
Results & Data
Presentation
(Evidence/support)
(4 points)
1. Key results are clearly presented in orderly and logical paragraphs
2. The correct trends are apparent in the data and described but not interpreted
in the results; data is not described “point by point.”
3. All appropriate figures and tables are included, clear, well presented and
correct
4. All appropriate figures and tables have legend properly describing them
5. All appropriate figures and tables have figure labels and titles
6. All calculations (if any) are provided in a logical manner using proper units
7. All appropriate figures and tables have clear and descriptive captions; does
not contain any explanation
8. All appropriate figures and tables are explained and supported in the body
of text without repetition and replacing the figure
Achieved most of
the foundation skills.
Demonstrates
understanding and
partial achievement
of some advanced
skills.
Achieved most of the
foundation skills.
Shows minimal
understanding of
advanced skills and might
be lacking in some
foundational areas.
Achieved some of the
foundation skills but lacks
in several foundational
areas.
Shows no evidence of
understanding advanced
skills.
Has not
achieved the
majority of the
foundation
skills.
Shows no
understanding of
advanced skills.
1
Class-1 (above 70%) Class-2.1 (62-
65-68%)
Class-2.2 (52-55-
58%)
Class-3 (42-45, 48%) Fail (less
than 40%)
Title 1. Reflects the content of the report
2. Clear and Concise
3. Uses descriptive words that are associated with the lab; excluding
unnecessary experimental detail
Achieved most of
the foundation skills.
Demonstrates
understanding and
partial achievement
of some advanced
skills.
Achieved most of the
foundation skills.
Shows minimal
understanding of
advanced skills and might
be lacking in some
foundational areas.
Achieved some of the
foundation skills but lacks
in several foundational
areas.
Shows no evidence of
understanding advanced
skills.
Has not
achieved the
majority of the
foundation
skills.
Shows no
understanding of
advanced skills.
Abstract 1. Clear and concise summary of the paper
2. Includes a brief background and objective(s) of the project
3. Includes a brief description of experimental methods
4. Includes major findings
5. Includes a brief discussion
6. Includes a brief conclusion
7. Does not include any in-text citation
Absence of 1
descriptor from
Class-1
OR partial
achievement of 2
descriptors from
Class-1
Absence of 1 descriptor
from Class-1
OR partial achievement of
3-4 descriptors from
Class-1
Absence of 2-3 descriptors
from Class-1
OR partial achievement of
most descriptors from
Class-1
Absence of more
than half of the
descriptors from
Class-1
OR minimal
achievement
across all
descriptors
Introduction and
Hypothesis/ Objective
(Focus/purpose/thesis)
(4 points)
1. Clear background information based on a thorough literature search
2. Includes the significance of the experiment
3. Includes a rationale for the study along with a clearly stated objective
4. Uses proper in-text citations
5. Maintains exceptional consistency and clarity throughout
6. Uses consistently clear, concise, and well-organized writing style, allowing
for effortless comprehension
7. Uses scientific terminology which enhances clarity
8. Demonstrates synthesis of ideas and in-depth analysis
9. Showcases evidence of original thought and support for the topic
Achieved most of
the foundation skills.
Demonstrates
understanding and
partial achievement
of some advanced
skills.
Achieved most of the
foundation skills.
Shows minimal
understanding of
advanced skills and might
be lacking in some
foundational areas.
Achieved some of the
foundation skills but lacks
in several foundational
areas.
Shows no evidence of
understanding advanced
skills.
Has not
achieved the
majority of the
foundation
skills.
Shows no
understanding of
advanced skills.
Materials & Methods
(4 points)
1. Clear, concise, and correct description of experimental procedures;
replicable by others
2. In paragraph form, included appropriate level of detail (example: general
steps, versus “I used a pipet.”)
3. Uses proper third person past- passive voice
4. Uses scientific terminology properly
5.Section is organized in a way that the reader understands the logical flow of
the lab
6. Describes ethical issues and biostatistical analysis (if any)
Achieved most of
the foundation skills.
Demonstrates
understanding and
partial achievement
of some advanced
skills.
Achieved most of the
foundation skills.
Shows minimal
understanding of
advanced skills and might
be lacking in some
foundational areas.
Achieved some of the
foundation skills but lacks
in several foundational
areas.
Shows no evidence of
understanding advanced
skills.
Has not
achieved the
majority of the
foundation
skills.
Shows no
understanding of
advanced skills.
Results & Data
Presentation
(Evidence/support)
(4 points)
1. Key results are clearly presented in orderly and logical paragraphs
2. The correct trends are apparent in the data and described but not interpreted
in the results; data is not described “point by point.”
3. All appropriate figures and tables are included, clear, well presented and
correct
4. All appropriate figures and tables have legend properly describing them
5. All appropriate figures and tables have figure labels and titles
6. All calculations (if any) are provided in a logical manner using proper units
7. All appropriate figures and tables have clear and descriptive captions; does
not contain any explanation
8. All appropriate figures and tables are explained and supported in the body
of text without repetition and replacing the figure
Achieved most of
the foundation skills.
Demonstrates
understanding and
partial achievement
of some advanced
skills.
Achieved most of the
foundation skills.
Shows minimal
understanding of
advanced skills and might
be lacking in some
foundational areas.
Achieved some of the
foundation skills but lacks
in several foundational
areas.
Shows no evidence of
understanding advanced
skills.
Has not
achieved the
majority of the
foundation
skills.
Shows no
understanding of
advanced skills.
1
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 13
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
