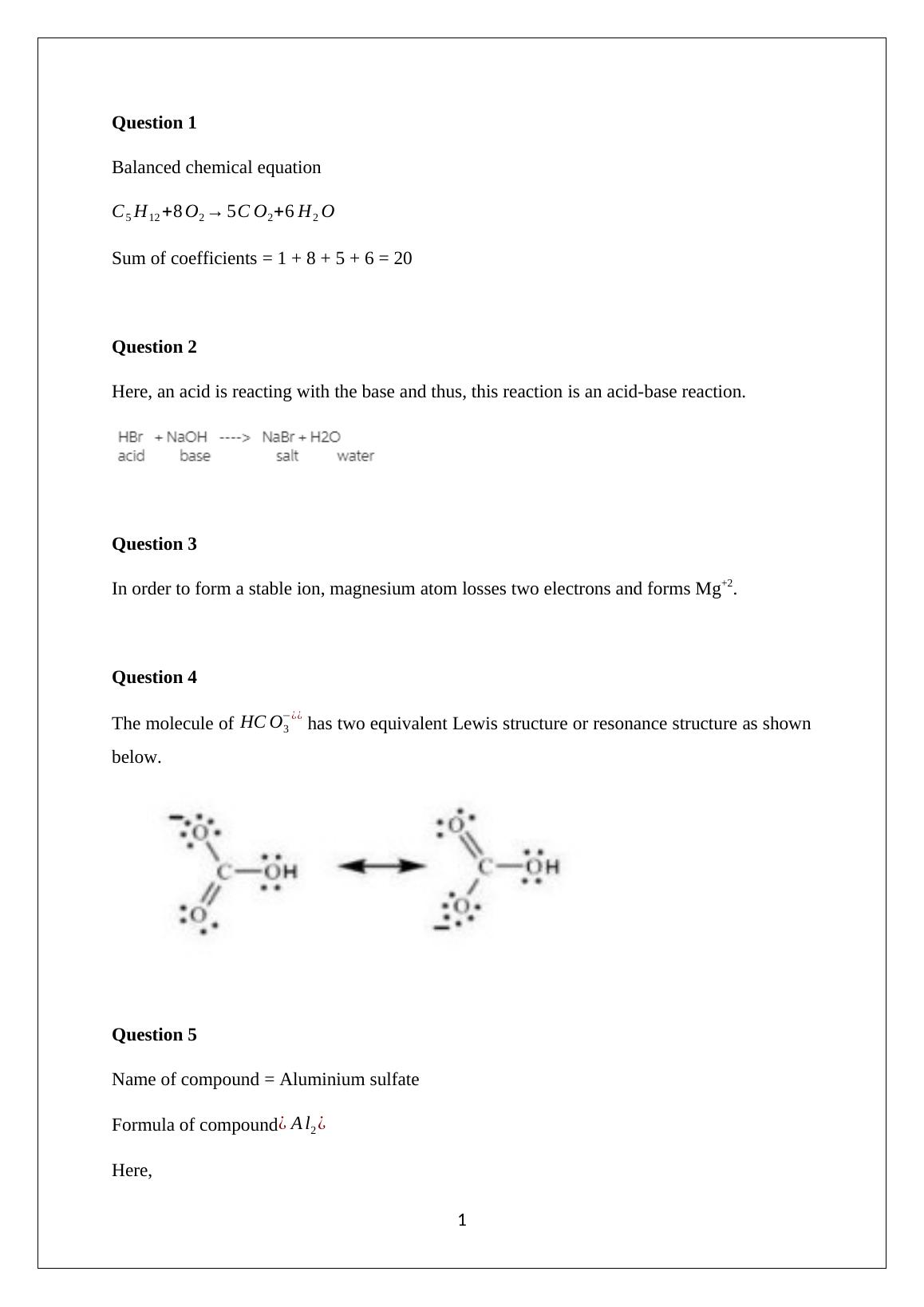
Balanced Chemical Equation
8 Pages734 Words20 Views
Added on 2022-09-16
Balanced Chemical Equation
Added on 2022-09-16
ShareRelated Documents
End of preview
Want to access all the pages? Upload your documents or become a member.
Molar Mass of Compound
|9
|860
|20
Solutions for Order number 1022789
|5
|646
|81
Assignment on Chemistry - Number of atoms
|5
|802
|15