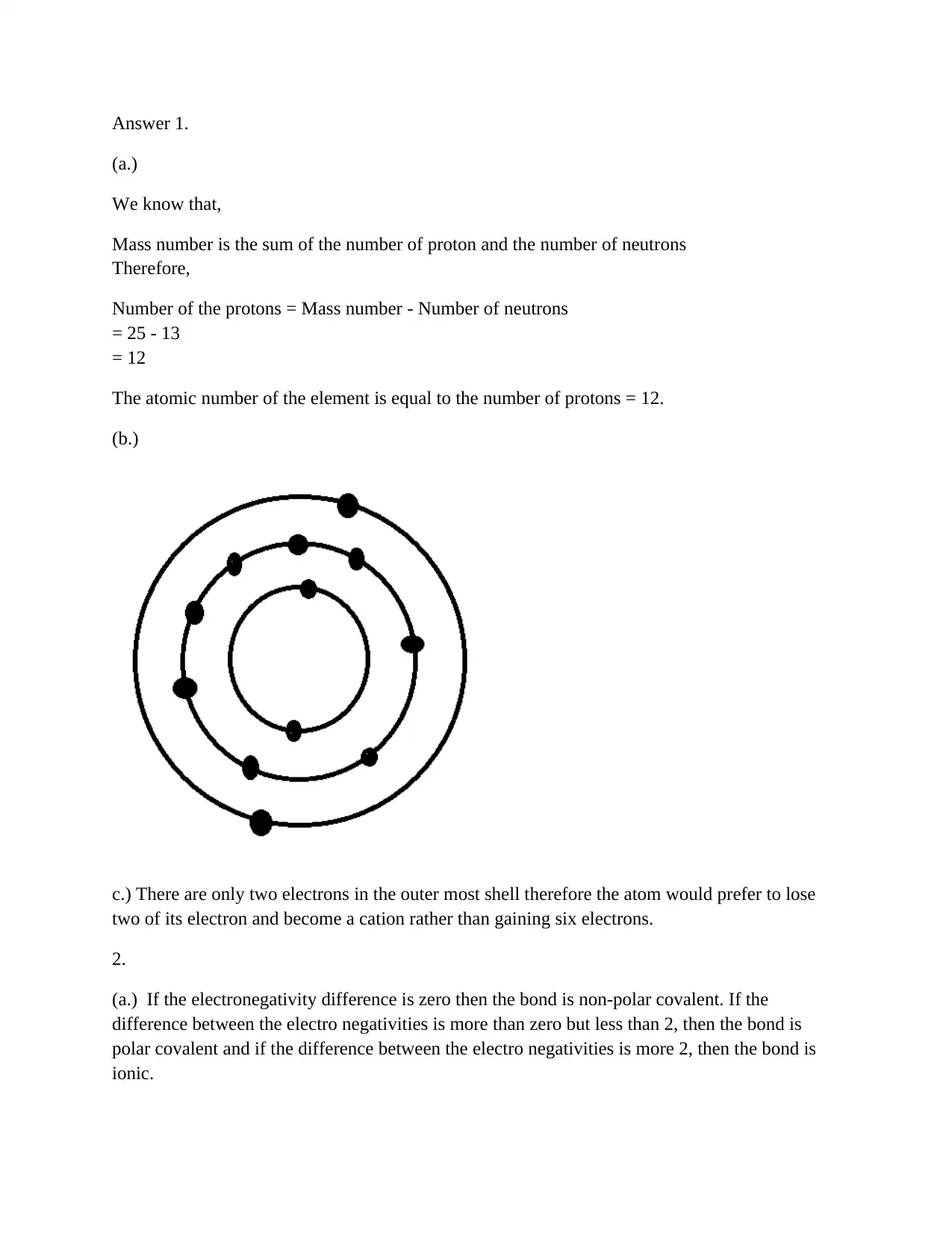
Chemistry Bonding and Solubility
VerifiedAdded on 2019/10/09
|3
|456
|194
Report
AI Summary
The assignment content discusses the concept of mass number, atomic number, and electronegativity difference in determining bond polarity. It also explains how sodium chloride (NaCl) is soluble in both water and methanol due to their polar nature.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.
1 out of 3
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)