Chemistry Experiment: pH Testing and Chemical Reactions
Added on 2023-06-15
8 Pages1331 Words66 Views
End of preview
Want to access all the pages? Upload your documents or become a member.
Chemistry Assignment (Doc)
|11
|1811
|440
MULTIPLE CHOICE QUESTIONS.
|4
|233
|355
Chemistry Study Material
|10
|2814
|3
Comparison of SN1 and SN2 Mechanisms
|5
|1232
|75
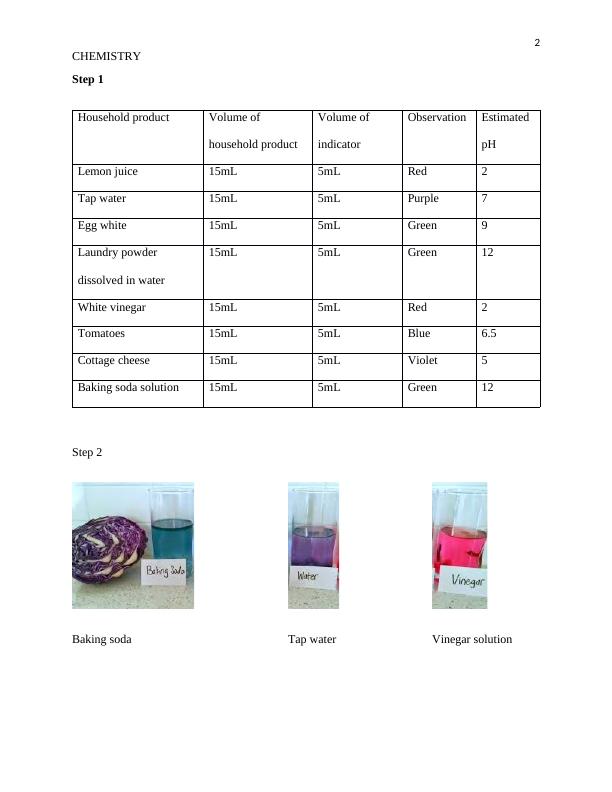
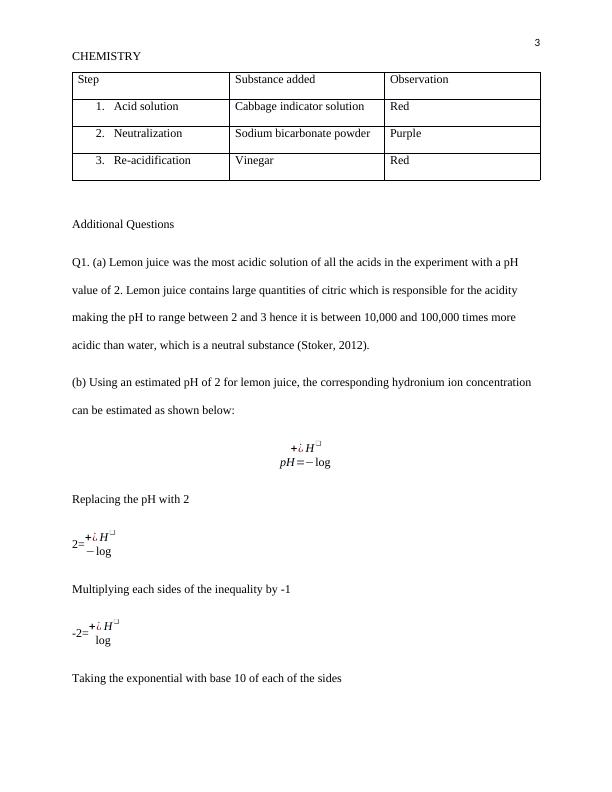
Measurement of Ph Using Indicators
|7
|1021
|283
No Possible Isomerism l Question Answers
|5
|834
|17