Core Principles of Chemistry.
11 Pages2310 Words82 Views
Added on 2023-01-18
Core Principles of Chemistry.
Added on 2023-01-18
ShareRelated Documents
Core Principles of Chemistry

TAQ 1: Trends across periodic table and in group 1 and 7
Trends down group 1 (Alkali Metals)
Group 1 elements (Alkali metals) are a group of chemical elements from the s-block of
the periodic table having similar physical and chemical properties. They are silvery in color,
shiny and soft. Group 1 element are highly reactive at STP (standard temperature and pressure)
and ready to lose their outermost electron to form cation. The group 1 elements includes; lithium
(Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr) (Kostiner,
2003). Table 1 below shows the atomic radius, ionization energy, melting and boiling point of
Alkali metals
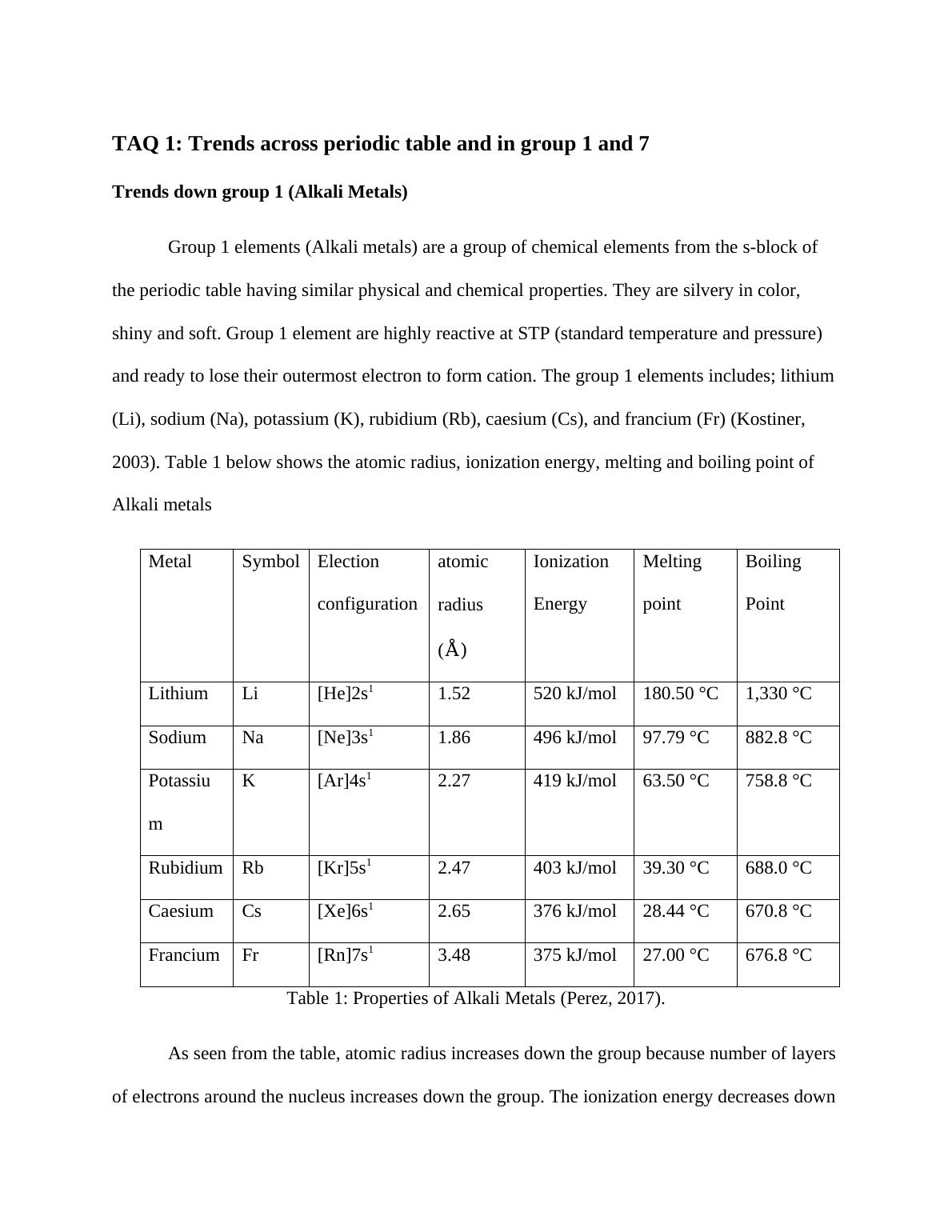
Metal Symbol Election
configuration
atomic
radius (Å)
Ionization
Energy
Melting
point
Boiling
Point
Lithium Li [He]2s1 1.52 520 kJ/mol 180.50 °C 1,330 °C
Sodium Na [Ne]3s1 1.86 496 kJ/mol 97.79 °C 882.8 °C
Potassiu
m
K [Ar]4s1 2.27 419 kJ/mol 63.50 °C 758.8 °C
Rubidium Rb [Kr]5s1 2.47 403 kJ/mol 39.30 °C 688.0 °C
Caesium Cs [Xe]6s1 2.65 376 kJ/mol 28.44 °C 670.8 °C
Francium Fr [Rn]7s1 3.48 375 kJ/mol 27.00 °C 676.8 °C
Table 1: Properties of Alkali Metals (Perez, 2017).
As seen from the table, atomic radius increases down the group because number of layers
of electrons around the nucleus increases down the group. The ionization energy decreases down
the group because any bonding electron pair becomes farther from the metal nucleus, and so is
Trends down group 1 (Alkali Metals)
Group 1 elements (Alkali metals) are a group of chemical elements from the s-block of
the periodic table having similar physical and chemical properties. They are silvery in color,
shiny and soft. Group 1 element are highly reactive at STP (standard temperature and pressure)
and ready to lose their outermost electron to form cation. The group 1 elements includes; lithium
(Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr) (Kostiner,
2003). Table 1 below shows the atomic radius, ionization energy, melting and boiling point of
Alkali metals
Metal Symbol Election
configuration
atomic
radius (Å)
Ionization
Energy
Melting
point
Boiling
Point
Lithium Li [He]2s1 1.52 520 kJ/mol 180.50 °C 1,330 °C
Sodium Na [Ne]3s1 1.86 496 kJ/mol 97.79 °C 882.8 °C
Potassiu
m
K [Ar]4s1 2.27 419 kJ/mol 63.50 °C 758.8 °C
Rubidium Rb [Kr]5s1 2.47 403 kJ/mol 39.30 °C 688.0 °C
Caesium Cs [Xe]6s1 2.65 376 kJ/mol 28.44 °C 670.8 °C
Francium Fr [Rn]7s1 3.48 375 kJ/mol 27.00 °C 676.8 °C
Table 1: Properties of Alkali Metals (Perez, 2017).
As seen from the table, atomic radius increases down the group because number of layers
of electrons around the nucleus increases down the group. The ionization energy decreases down
the group because any bonding electron pair becomes farther from the metal nucleus, and so is
less strongly attracted towards the nucleus. Both melting and boiling point decreases down the
group since the metals have metallic bond which depends on the nucleus attraction. As the size
of atoms increase down the group, the distance between the nuclei and delocalized electrons
increases hence decreasing the electronic bond, thus MP and BP decreases down the group
(Anon., n.d.).
Trends down group 7 (Halogens)
Group 7 elements (Halogens) are the family of chemical elements which include fluorine
(F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). This element exists as diatomic
molecules. Table 2 below shows some physical and chemical trends down the group.
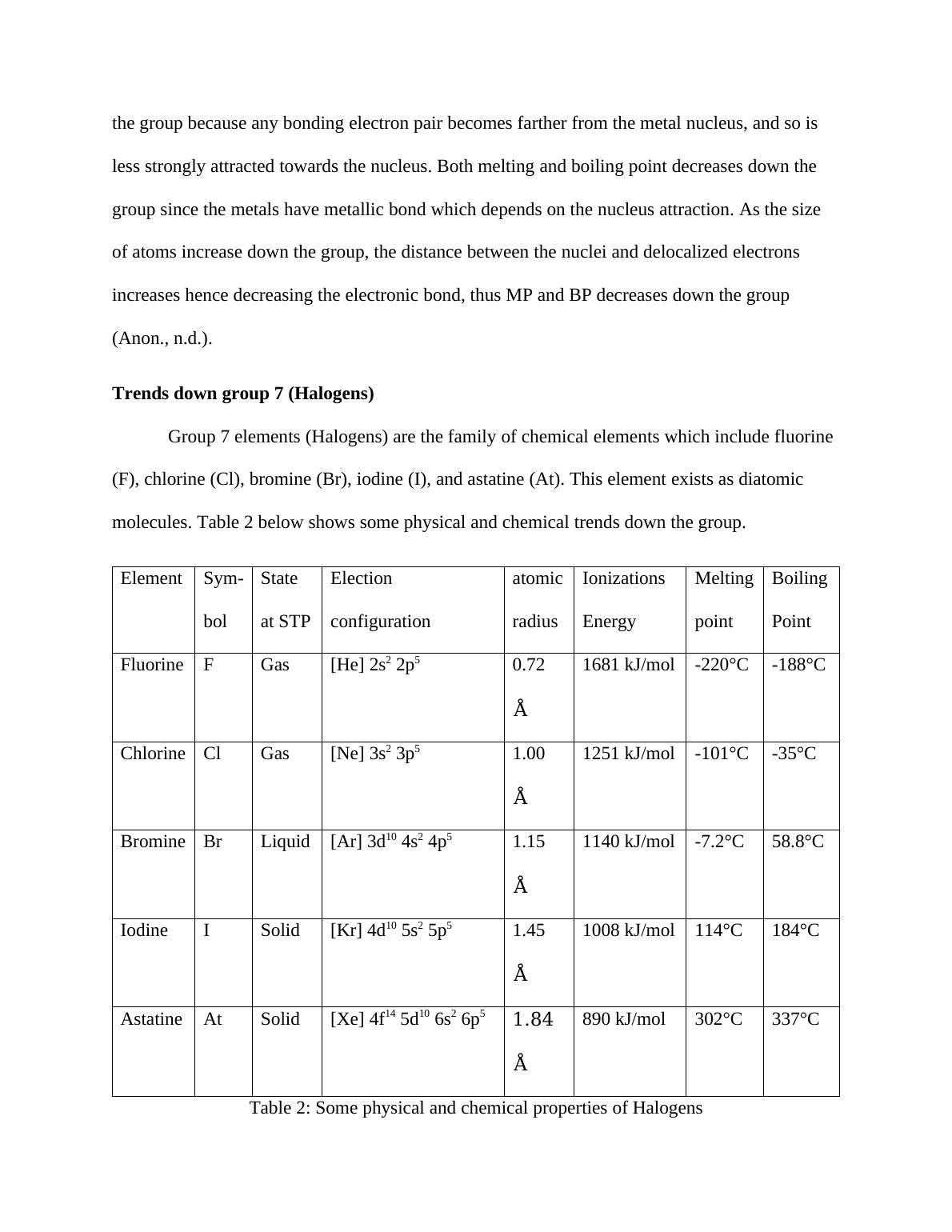
Element Sym-
bol
State
at STP
Election
configuration
atomic
radius
Ionizations
Energy
Melting
point
Boiling
Point
Fluorine F Gas [He] 2s2 2p5 0.72 Å 1681 kJ/mol -220°C -188°C
Chlorine Cl Gas [Ne] 3s2 3p5 1.00 Å 1251 kJ/mol -101°C -35°C
Bromine Br Liquid [Ar] 3d10 4s2 4p5 1.15 Å 1140 kJ/mol -7.2°C 58.8°C
Iodine I Solid [Kr] 4d10 5s2 5p5 1.45 Å 1008 kJ/mol 114°C 184°C
Astatine At Solid [Xe] 4f14 5d10 6s2 6p5 1.84Å 890 kJ/mol 302°C 337°C
Table 2: Some physical and chemical properties of Halogens
As from table 2, the atomic radius increases down the group due to increase in energy
level. The MP and BP increase down the group, since, the strength of the induced dipole-dipole
interactions (van der Waals forces) increases due to increase in the number of electrons down the
group. This trend indicates why the physical state of the halogens changes from gaseous (F) to
solid (At) down the group. As the BP increases down the group, the volatility. on the other hand
group since the metals have metallic bond which depends on the nucleus attraction. As the size
of atoms increase down the group, the distance between the nuclei and delocalized electrons
increases hence decreasing the electronic bond, thus MP and BP decreases down the group
(Anon., n.d.).
Trends down group 7 (Halogens)
Group 7 elements (Halogens) are the family of chemical elements which include fluorine
(F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). This element exists as diatomic
molecules. Table 2 below shows some physical and chemical trends down the group.
Element Sym-
bol
State
at STP
Election
configuration
atomic
radius
Ionizations
Energy
Melting
point
Boiling
Point
Fluorine F Gas [He] 2s2 2p5 0.72 Å 1681 kJ/mol -220°C -188°C
Chlorine Cl Gas [Ne] 3s2 3p5 1.00 Å 1251 kJ/mol -101°C -35°C
Bromine Br Liquid [Ar] 3d10 4s2 4p5 1.15 Å 1140 kJ/mol -7.2°C 58.8°C
Iodine I Solid [Kr] 4d10 5s2 5p5 1.45 Å 1008 kJ/mol 114°C 184°C
Astatine At Solid [Xe] 4f14 5d10 6s2 6p5 1.84Å 890 kJ/mol 302°C 337°C
Table 2: Some physical and chemical properties of Halogens
As from table 2, the atomic radius increases down the group due to increase in energy
level. The MP and BP increase down the group, since, the strength of the induced dipole-dipole
interactions (van der Waals forces) increases due to increase in the number of electrons down the
group. This trend indicates why the physical state of the halogens changes from gaseous (F) to
solid (At) down the group. As the BP increases down the group, the volatility. on the other hand
decreases down the group. As the atomic radius increases down the group, the force required to
remove an electron decreases, thus the ionization energy decreases down the group (Anon., n.d.).
Trends across period III
Table 3 shows some physical and chemical properties of period 3 elements
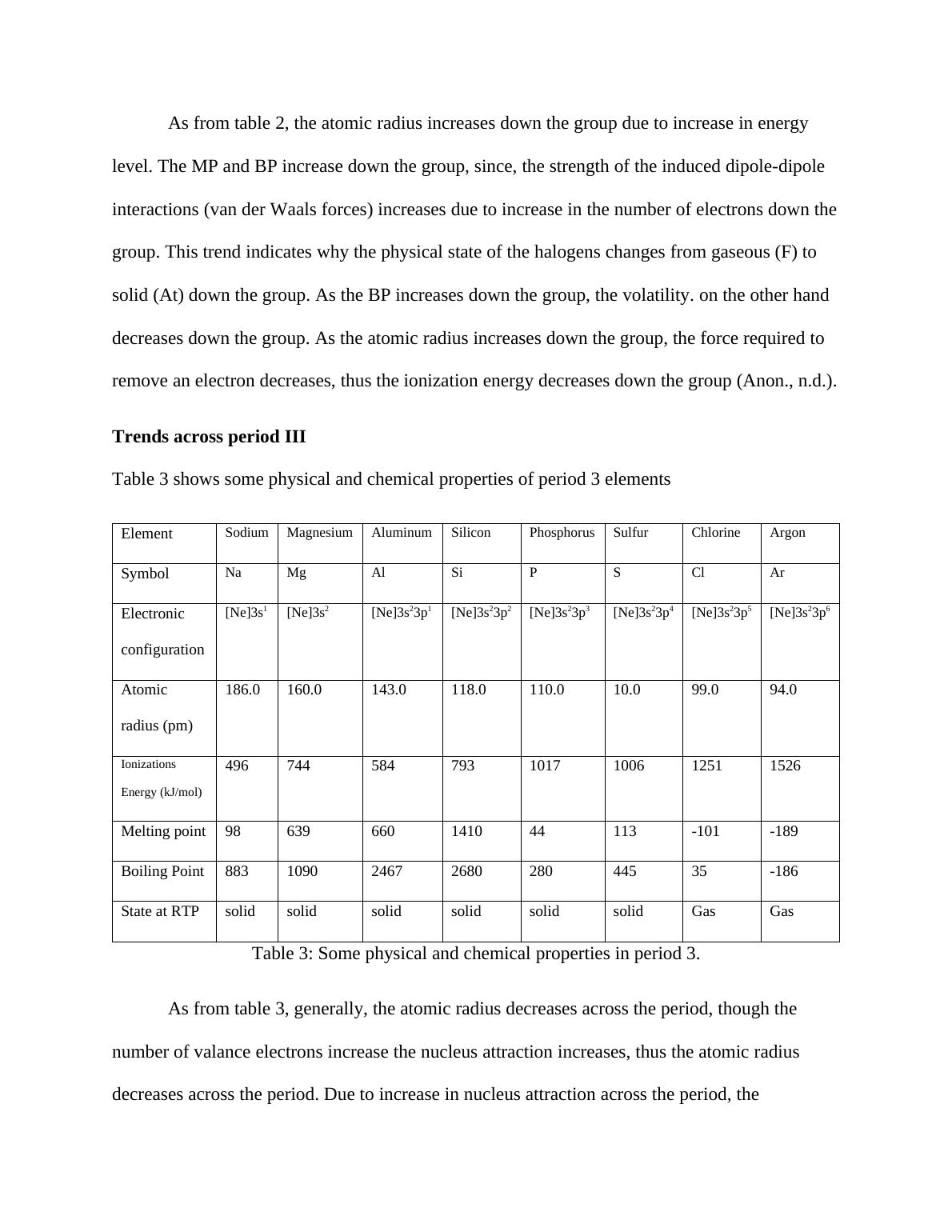
Element Sodium Magnesium Aluminum Silicon Phosphorus Sulfur Chlorine Argon
Symbol Na Mg Al Si P S Cl Ar
Electronic
configuration
[Ne]3s1 [Ne]3s2 [Ne]3s23p1 [Ne]3s23p2 [Ne]3s23p3 [Ne]3s23p4 [Ne]3s23p5 [Ne]3s23p6
Atomic
radius (pm)
186.0 160.0 143.0 118.0 110.0 10.0 99.0 94.0
Ionizations
Energy (kJ/mol)
496 744 584 793 1017 1006 1251 1526
Melting point 98 639 660 1410 44 113 -101 -189
Boiling Point 883 1090 2467 2680 280 445 35 -186
State at RTP solid solid solid solid solid solid Gas Gas
Table 3: Some physical and chemical properties in period 3.
As from table 3, generally, the atomic radius decreases across the period, though the
number of valance electrons increase the nucleus attraction increases, thus the atomic radius
decreases across the period. Due to increase in nucleus attraction across the period, the
Ionizations Energy increases across the periodic table. BP and MP depends on wheather the
elements are metal, metalloid or non-metal. Na, Mg and Al are metals, the strength of metallic
bond increases across the period, thus the MP and BP increases. Si is a metalloid and has very
high MP and BP since its atom is held by strong covalent bonds. P, S, Cl and Ar are non-metal
which are held together by van der Waals forces. P exist as P4 molecule, S as S8 molecule, Cl as
remove an electron decreases, thus the ionization energy decreases down the group (Anon., n.d.).
Trends across period III
Table 3 shows some physical and chemical properties of period 3 elements
Element Sodium Magnesium Aluminum Silicon Phosphorus Sulfur Chlorine Argon
Symbol Na Mg Al Si P S Cl Ar
Electronic
configuration
[Ne]3s1 [Ne]3s2 [Ne]3s23p1 [Ne]3s23p2 [Ne]3s23p3 [Ne]3s23p4 [Ne]3s23p5 [Ne]3s23p6
Atomic
radius (pm)
186.0 160.0 143.0 118.0 110.0 10.0 99.0 94.0
Ionizations
Energy (kJ/mol)
496 744 584 793 1017 1006 1251 1526
Melting point 98 639 660 1410 44 113 -101 -189
Boiling Point 883 1090 2467 2680 280 445 35 -186
State at RTP solid solid solid solid solid solid Gas Gas
Table 3: Some physical and chemical properties in period 3.
As from table 3, generally, the atomic radius decreases across the period, though the
number of valance electrons increase the nucleus attraction increases, thus the atomic radius
decreases across the period. Due to increase in nucleus attraction across the period, the
Ionizations Energy increases across the periodic table. BP and MP depends on wheather the
elements are metal, metalloid or non-metal. Na, Mg and Al are metals, the strength of metallic
bond increases across the period, thus the MP and BP increases. Si is a metalloid and has very
high MP and BP since its atom is held by strong covalent bonds. P, S, Cl and Ar are non-metal
which are held together by van der Waals forces. P exist as P4 molecule, S as S8 molecule, Cl as
End of preview
Want to access all the pages? Upload your documents or become a member.
Related Documents
Core Principles of Chemistrylg...
|11
|2290
|66
Properties and Trends of Alkali Metals: Lithium, Sodium and Potassiumlg...
|6
|700
|329
