Lab Report: Determining Glucose Levels in Urine Samples
VerifiedAdded on 2023/01/23
|5
|1300
|87
Report
AI Summary
This lab report details an experiment to determine the concentration of glucose in urine samples using the GOD-PAP assay and a spectrophotometer. The introduction provides background information on glucose, its importance, and the health implications of abnormal levels, particularly in urine. The GOD-PAP assay, a colorimetric method, is described, along with the principle of spectrophotometry, including Beer-Lambert's law. The methods section (to be filled in by the author) would outline the experimental procedure. The results section presents a standard curve generated from known glucose concentrations and absorbance values, along with the calculation of glucose concentrations in unknown urine samples. The discussion analyzes the accuracy of the standard curve, adherence to Beer-Lambert's law, and potential sources of error. The report includes tables of absorbance values and the equation used for concentration calculations. The conclusion summarizes the findings and their significance.

DETERMINING THE LEVEL OF GLUCOSE IN URINE SAMPLE USING DOG-
PAP ASSAY THROUGH SPECTROPHOT0METER
INTRODUCTION:-
Glucose is one of the simplest forms of all carbohydrates belonging to
monosaccharides. Glucose in the form of foods rich in carbohydrates like grains,
starchy vegetables, dairy, whole fruit, etc. are body’s desired source of energy.
Since glucose is an important energy source for our body and unhealthy levels of
glucose due to disorders of carbohydrate metabolism can lead to serious
diseases conditions which can be treatable or even may lead to permanent
diseased condition. Level of glucose both in blood and urine is an important thing
to be monitored (Kathleen, 2017). Not having a normal level of glucose in urine is
a sigh of health problem. The most common of which is diabetes in which there
is an elevated level of glucose in urine. Diabetes is a condition in which body is
not being able to regulate glucose levels properly either due to insufficient
production of insulin or insulin resistance. To check the level of glucose in urine
is a simple and quick way to understand the abnormality of glucose in urine
(Dabra, 2018}. GOD-PAP assay is an enzymatic colorimetric method used to
detect the level of glucose in the urine samples. Glucose oxidase (GOD) oxidises
glucose present in the sample to form hydrogen peroxide. This hydrogen
peroxide reacts with phenol and 4-aminoantipyrine under catalysis of peroxidase
(PAP) to form a red coloured product Quinoneimine as indicator. The reading is
taken at (492 – 550 nm) in a spectrophotometer (Spectrum, n.d).
Glucose + 2 H2O + O2 Gluconic acid + H2O2
2 H2O2 +Phenol + 4-amino-antipyrine 4 H2O + Quinoneimine
A spectrophotometer is a device that measures the amount of light absorbed at
various wavelengths by the sample in terms of photons after it passes through
the sample. Considering the range of wavelength of source of light, it can be
classified into Ultraviolet spectrum, Visible spectrum and Infrared spectrum of
the electromagnetic spectrum.
Spectrophotometer is designed on the principle of photometry which states that
When a beam of light is of intensity I0 passes through a sample solution, a part of
it is reflected (Ir), a part is absorbed (Ia) and rest of the light is transmitted (It)
Thus, I0 = Ir + Ia + It
In case of any photometers Ir is kept constant by using identical cells and
moreover the readings of I0 and It are enough to determine the value of Ia. The
relationship between the amount of light absorbed and the concentration of the
substance is based on the two fundamental laws of photometry.
GOD
PAP
PAP ASSAY THROUGH SPECTROPHOT0METER
INTRODUCTION:-
Glucose is one of the simplest forms of all carbohydrates belonging to
monosaccharides. Glucose in the form of foods rich in carbohydrates like grains,
starchy vegetables, dairy, whole fruit, etc. are body’s desired source of energy.
Since glucose is an important energy source for our body and unhealthy levels of
glucose due to disorders of carbohydrate metabolism can lead to serious
diseases conditions which can be treatable or even may lead to permanent
diseased condition. Level of glucose both in blood and urine is an important thing
to be monitored (Kathleen, 2017). Not having a normal level of glucose in urine is
a sigh of health problem. The most common of which is diabetes in which there
is an elevated level of glucose in urine. Diabetes is a condition in which body is
not being able to regulate glucose levels properly either due to insufficient
production of insulin or insulin resistance. To check the level of glucose in urine
is a simple and quick way to understand the abnormality of glucose in urine
(Dabra, 2018}. GOD-PAP assay is an enzymatic colorimetric method used to
detect the level of glucose in the urine samples. Glucose oxidase (GOD) oxidises
glucose present in the sample to form hydrogen peroxide. This hydrogen
peroxide reacts with phenol and 4-aminoantipyrine under catalysis of peroxidase
(PAP) to form a red coloured product Quinoneimine as indicator. The reading is
taken at (492 – 550 nm) in a spectrophotometer (Spectrum, n.d).
Glucose + 2 H2O + O2 Gluconic acid + H2O2
2 H2O2 +Phenol + 4-amino-antipyrine 4 H2O + Quinoneimine
A spectrophotometer is a device that measures the amount of light absorbed at
various wavelengths by the sample in terms of photons after it passes through
the sample. Considering the range of wavelength of source of light, it can be
classified into Ultraviolet spectrum, Visible spectrum and Infrared spectrum of
the electromagnetic spectrum.
Spectrophotometer is designed on the principle of photometry which states that
When a beam of light is of intensity I0 passes through a sample solution, a part of
it is reflected (Ir), a part is absorbed (Ia) and rest of the light is transmitted (It)
Thus, I0 = Ir + Ia + It
In case of any photometers Ir is kept constant by using identical cells and
moreover the readings of I0 and It are enough to determine the value of Ia. The
relationship between the amount of light absorbed and the concentration of the
substance is based on the two fundamental laws of photometry.
GOD
PAP
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Beer’s Law
This law states that the amount of light absorbed is directly proportional to the
concentration of the solute in the solution.
Mathematical representation as,
Log10 I0/It = asc
as = Index of Absorbency
c = Concentration of Solution
Lambert’s Law
The Lambert’s law states that the amount of light absorbed is directly
proportional to the length or thickness of the solution under analysis.
Mathematical representation as,
A = Log10 I0/It = asb
A = Absorbance of test
as = Absorbance of standard
b = length / thickness of the solution
Log10 I0 / It = asbc
If b is kept constant then,
Log10 I0/It = asc
The absorbency index as is defined as
as = A/cl
c = concentration of the absorbing material in gm/liter.
l = distance traversed by the light in solution in cm.
Therefore,
The working principle of the Spectrophotometer is based on Beer-Lambert’s law
which states that the amount of light absorbed by a colored solution is directly
proportional to the concentration of the solution and the length of a light path
through the solution (Batra, 2018).
This law states that the amount of light absorbed is directly proportional to the
concentration of the solute in the solution.
Mathematical representation as,
Log10 I0/It = asc
as = Index of Absorbency
c = Concentration of Solution
Lambert’s Law
The Lambert’s law states that the amount of light absorbed is directly
proportional to the length or thickness of the solution under analysis.
Mathematical representation as,
A = Log10 I0/It = asb
A = Absorbance of test
as = Absorbance of standard
b = length / thickness of the solution
Log10 I0 / It = asbc
If b is kept constant then,
Log10 I0/It = asc
The absorbency index as is defined as
as = A/cl
c = concentration of the absorbing material in gm/liter.
l = distance traversed by the light in solution in cm.
Therefore,
The working principle of the Spectrophotometer is based on Beer-Lambert’s law
which states that the amount of light absorbed by a colored solution is directly
proportional to the concentration of the solution and the length of a light path
through the solution (Batra, 2018).
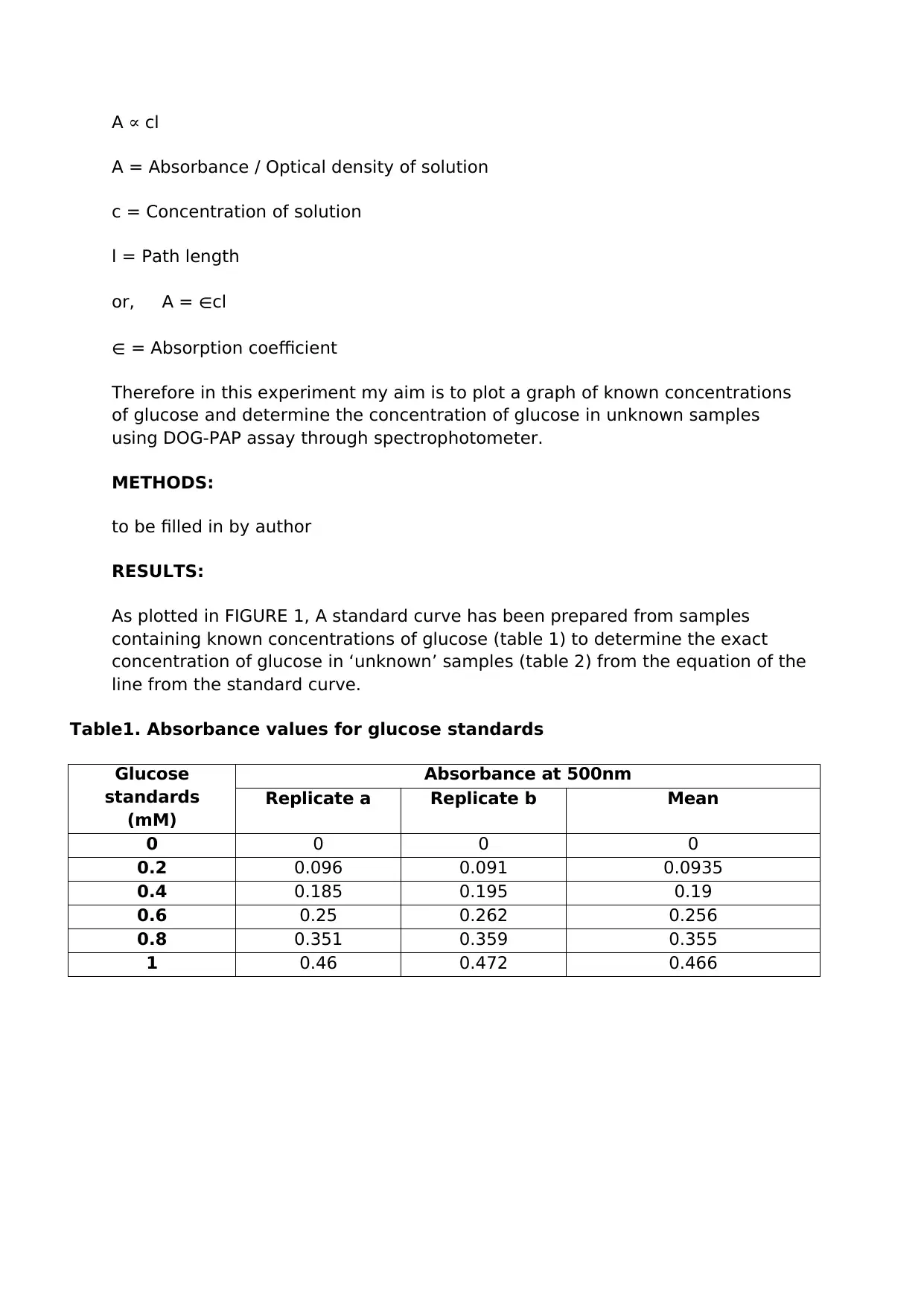
A ∝ cl
A = Absorbance / Optical density of solution
c = Concentration of solution
l = Path length
or, A = ∈cl
∈ = Absorption coefficient
Therefore in this experiment my aim is to plot a graph of known concentrations
of glucose and determine the concentration of glucose in unknown samples
using DOG-PAP assay through spectrophotometer.
METHODS:
to be filled in by author
RESULTS:
As plotted in FIGURE 1, A standard curve has been prepared from samples
containing known concentrations of glucose (table 1) to determine the exact
concentration of glucose in ‘unknown’ samples (table 2) from the equation of the
line from the standard curve.
Table1. Absorbance values for glucose standards
Glucose
standards
(mM)
Absorbance at 500nm
Replicate a Replicate b Mean
0 0 0 0
0.2 0.096 0.091 0.0935
0.4 0.185 0.195 0.19
0.6 0.25 0.262 0.256
0.8 0.351 0.359 0.355
1 0.46 0.472 0.466
A = Absorbance / Optical density of solution
c = Concentration of solution
l = Path length
or, A = ∈cl
∈ = Absorption coefficient
Therefore in this experiment my aim is to plot a graph of known concentrations
of glucose and determine the concentration of glucose in unknown samples
using DOG-PAP assay through spectrophotometer.
METHODS:
to be filled in by author
RESULTS:
As plotted in FIGURE 1, A standard curve has been prepared from samples
containing known concentrations of glucose (table 1) to determine the exact
concentration of glucose in ‘unknown’ samples (table 2) from the equation of the
line from the standard curve.
Table1. Absorbance values for glucose standards
Glucose
standards
(mM)
Absorbance at 500nm
Replicate a Replicate b Mean
0 0 0 0
0.2 0.096 0.091 0.0935
0.4 0.185 0.195 0.19
0.6 0.25 0.262 0.256
0.8 0.351 0.359 0.355
1 0.46 0.472 0.466
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
0 0.2 0.4 0.6 0.8 1 1.2
0
0.05
0.1
0.15
0.2
0.25
0.3
0.35
0.4
0.45
0.5
0
0.093500000000
0002
0.19
0.256
0.355
0.466
f(x) = 0.453772727272727 x
R² = 0.998769871050725
O.D at 500nm
O.D at 500nm
Linear (O.D at 500nm)
Linear (O.D at 500nm)
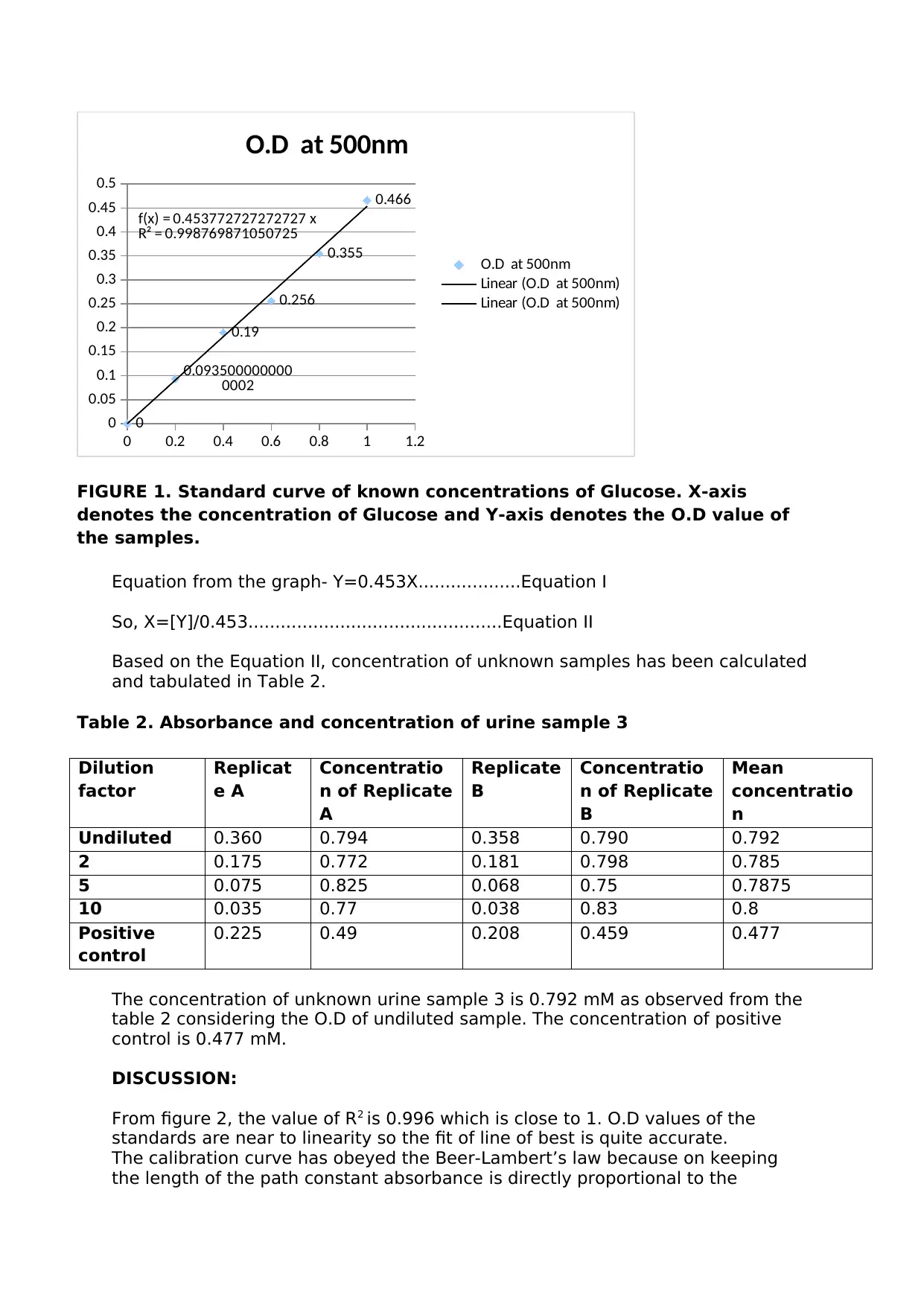
FIGURE 1. Standard curve of known concentrations of Glucose. X-axis
denotes the concentration of Glucose and Y-axis denotes the O.D value of
the samples.
Equation from the graph- Y=0.453X...................Equation I
So, X=[Y]/0.453...............................................Equation II
Based on the Equation II, concentration of unknown samples has been calculated
and tabulated in Table 2.
Table 2. Absorbance and concentration of urine sample 3
Dilution
factor
Replicat
e A
Concentratio
n of Replicate
A
Replicate
B
Concentratio
n of Replicate
B
Mean
concentratio
n
Undiluted 0.360 0.794 0.358 0.790 0.792
2 0.175 0.772 0.181 0.798 0.785
5 0.075 0.825 0.068 0.75 0.7875
10 0.035 0.77 0.038 0.83 0.8
Positive
control
0.225 0.49 0.208 0.459 0.477
The concentration of unknown urine sample 3 is 0.792 mM as observed from the
table 2 considering the O.D of undiluted sample. The concentration of positive
control is 0.477 mM.
DISCUSSION:
From figure 2, the value of R2 is 0.996 which is close to 1. O.D values of the
standards are near to linearity so the fit of line of best is quite accurate.
The calibration curve has obeyed the Beer-Lambert’s law because on keeping
the length of the path constant absorbance is directly proportional to the
0
0.05
0.1
0.15
0.2
0.25
0.3
0.35
0.4
0.45
0.5
0
0.093500000000
0002
0.19
0.256
0.355
0.466
f(x) = 0.453772727272727 x
R² = 0.998769871050725
O.D at 500nm
O.D at 500nm
Linear (O.D at 500nm)
Linear (O.D at 500nm)
FIGURE 1. Standard curve of known concentrations of Glucose. X-axis
denotes the concentration of Glucose and Y-axis denotes the O.D value of
the samples.
Equation from the graph- Y=0.453X...................Equation I
So, X=[Y]/0.453...............................................Equation II
Based on the Equation II, concentration of unknown samples has been calculated
and tabulated in Table 2.
Table 2. Absorbance and concentration of urine sample 3
Dilution
factor
Replicat
e A
Concentratio
n of Replicate
A
Replicate
B
Concentratio
n of Replicate
B
Mean
concentratio
n
Undiluted 0.360 0.794 0.358 0.790 0.792
2 0.175 0.772 0.181 0.798 0.785
5 0.075 0.825 0.068 0.75 0.7875
10 0.035 0.77 0.038 0.83 0.8
Positive
control
0.225 0.49 0.208 0.459 0.477
The concentration of unknown urine sample 3 is 0.792 mM as observed from the
table 2 considering the O.D of undiluted sample. The concentration of positive
control is 0.477 mM.
DISCUSSION:
From figure 2, the value of R2 is 0.996 which is close to 1. O.D values of the
standards are near to linearity so the fit of line of best is quite accurate.
The calibration curve has obeyed the Beer-Lambert’s law because on keeping
the length of the path constant absorbance is directly proportional to the
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

concentration of the solution. Moreover the O.D values of duplicates are similar
to each other except for positive control which is slightly variable which can be
due to pipetting error. The O.D of undiluted sample of the urine sample 3 is quite
near to the O.D of the standard (0.8mM). So the O.D of undiluted urine sample 3
was considered for caalulating the concentration.
REFERENCES:
Kathleen,P. (2017, March). Everything You Need to Know About Glucose.
Retrieved from https://www.healthline.com/health/glucose
Debra, S., Lauren, R. (2018, June). Urine Glucose Test. Retrieved from
https://www.healthline.com/health/glucose-test-urine
GLUCOSE - Liquizyme GOD - PAP (Single Reagent). Retrieved from
http://spectrum-diagnostics.com/data/Glucose(GOD-PAP).pdf
Batra, S. (2018, February). SPECTROPHOTOMETER – PRINCIPLE,
COMPONENTS, WORKING & APPLICATION. Retrieved from
https://paramedicsworld.com/biochemistry-practicals/demonstration-of-
spectrophotometer-principle-components-working-applications/medical-
paramedical-studynotes#.XMdwirczbIU
Word count excluding tables, figures and method section= 901
to each other except for positive control which is slightly variable which can be
due to pipetting error. The O.D of undiluted sample of the urine sample 3 is quite
near to the O.D of the standard (0.8mM). So the O.D of undiluted urine sample 3
was considered for caalulating the concentration.
REFERENCES:
Kathleen,P. (2017, March). Everything You Need to Know About Glucose.
Retrieved from https://www.healthline.com/health/glucose
Debra, S., Lauren, R. (2018, June). Urine Glucose Test. Retrieved from
https://www.healthline.com/health/glucose-test-urine
GLUCOSE - Liquizyme GOD - PAP (Single Reagent). Retrieved from
http://spectrum-diagnostics.com/data/Glucose(GOD-PAP).pdf
Batra, S. (2018, February). SPECTROPHOTOMETER – PRINCIPLE,
COMPONENTS, WORKING & APPLICATION. Retrieved from
https://paramedicsworld.com/biochemistry-practicals/demonstration-of-
spectrophotometer-principle-components-working-applications/medical-
paramedical-studynotes#.XMdwirczbIU
Word count excluding tables, figures and method section= 901
1 out of 5
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

