Disease Pathophysiology and Concept Map
VerifiedAdded on 2022/10/16
|9
|2013
|309
AI Summary
This article discusses the risk factors, pathophysiology, clinical manifestations, and treatment of chronic kidney disease due to type 2 diabetes mellitus and hypertension. It also provides insights into diagnostic investigations and treatment options. The article includes a concept map that illustrates the relationship between risk factors, pathophysiology, clinical manifestations, and treatment.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: DISEASE PATHOPHYSIOLOGY AND CONCEPT MAP
DISEASE PATHOPHYSIOLOGY AND CONCEPT MAP
Name of the Student:
Name of the University:
Author note:
DISEASE PATHOPHYSIOLOGY AND CONCEPT MAP
Name of the Student:
Name of the University:
Author note:
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
1DISEASE PATHOPHYSIOLOGY AND CONCEPT MAP
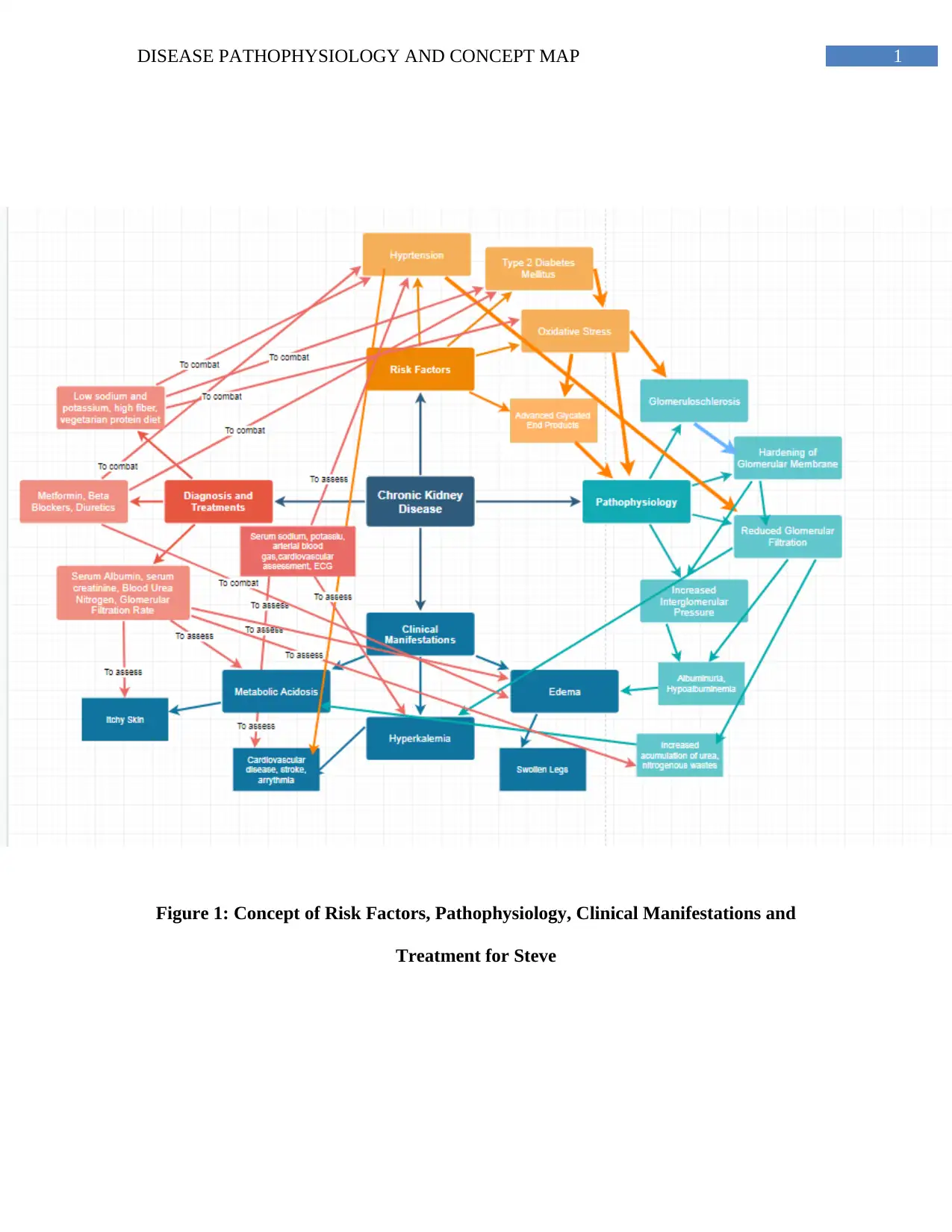
Figure 1: Concept of Risk Factors, Pathophysiology, Clinical Manifestations and
Treatment for Steve
Figure 1: Concept of Risk Factors, Pathophysiology, Clinical Manifestations and
Treatment for Steve

2DISEASE PATHOPHYSIOLOGY AND CONCEPT MAP
Risk factors and Pathophysiology
It has been evidenced that chronic conditions like type 2 diabetes mellitus (T2DM) and
hypertension are some of the strongest risk factors of chronic kidney disease (Webster, Nagler,
Morton & Masson, 2017).
Insulin resistance due to T2DM results in increased circulating levels of glucose in the
blood coupled by the activation of gluconeogenetic mechanisms resulting circulating levels of
fatty acids in the blood further results increased adipose tissue accumulation and oxidative
stress. Diabetes-associated oxidative stress occurs primarily due to oxidation of the accumulated
adipose tissues resulting in production of advanced glycation end products (AGEs) (Thomas,
Cooper & Zimmet, 2016). High blood glucose levels result in over expression of the glucose
transporter I (GLUT I) within the mesanglial cells of the kidney resulting in glycation within the
nephrons and associated symptoms of glomeruloschlerosis. Nodular glomeruloschlerosis is
accompanied by a gradual thickening of the glomerular basement membrane and increase in the
width of the podocytes’ slit membranes (Gharbi et al., 2016). The thickening is associated with
afferent arteriole dilation and efferent arteriole constriction followed by gradual hypofiltration of
the capillaries within the nephron resulting in decreased rate of glomerular filtration, gradual loss
of renal function, anuria and accumulation of nitrogenous wastes characteristic in chronic kidney
disease (Sulaiman, 2019). Additionally, the thickening of the basement membrane has been
associated with increased matrix in the mesanglia, its invasion within the capillaries of the
glomerulus resulting in formation of Kimmelstiel-Wilson nodules which completely decreases
filtration and causes chronic kidney disease (CKD) via consuming the glomerulus (Miranda-
Díaz, Pazarín-Villaseñor, Yanowsky-Escatell & Andrade-Sierra, 2016) (See Figure 1).
Risk factors and Pathophysiology
It has been evidenced that chronic conditions like type 2 diabetes mellitus (T2DM) and
hypertension are some of the strongest risk factors of chronic kidney disease (Webster, Nagler,
Morton & Masson, 2017).
Insulin resistance due to T2DM results in increased circulating levels of glucose in the
blood coupled by the activation of gluconeogenetic mechanisms resulting circulating levels of
fatty acids in the blood further results increased adipose tissue accumulation and oxidative
stress. Diabetes-associated oxidative stress occurs primarily due to oxidation of the accumulated
adipose tissues resulting in production of advanced glycation end products (AGEs) (Thomas,
Cooper & Zimmet, 2016). High blood glucose levels result in over expression of the glucose
transporter I (GLUT I) within the mesanglial cells of the kidney resulting in glycation within the
nephrons and associated symptoms of glomeruloschlerosis. Nodular glomeruloschlerosis is
accompanied by a gradual thickening of the glomerular basement membrane and increase in the
width of the podocytes’ slit membranes (Gharbi et al., 2016). The thickening is associated with
afferent arteriole dilation and efferent arteriole constriction followed by gradual hypofiltration of
the capillaries within the nephron resulting in decreased rate of glomerular filtration, gradual loss
of renal function, anuria and accumulation of nitrogenous wastes characteristic in chronic kidney
disease (Sulaiman, 2019). Additionally, the thickening of the basement membrane has been
associated with increased matrix in the mesanglia, its invasion within the capillaries of the
glomerulus resulting in formation of Kimmelstiel-Wilson nodules which completely decreases
filtration and causes chronic kidney disease (CKD) via consuming the glomerulus (Miranda-
Díaz, Pazarín-Villaseñor, Yanowsky-Escatell & Andrade-Sierra, 2016) (See Figure 1).

3DISEASE PATHOPHYSIOLOGY AND CONCEPT MAP
The pathophysiology of CKD due to T2DM is further aggravated due to hypertension.
Hypertension results in increased glomerular pressure via destruction of the blood vessels of the
kidney which further leads to an impairment of the renal system’s functioning associated with
the filtration of toxins, systemic waste accumulation and hence, CKD (Rossignol et al., 2015)
(See Figure 1).
Pathophysiology and Clinical Manifestations
Steve’s symptoms of edema can be attributed to hypoalbuminemia and proteinuria or
excretion of proteins like albumin in the urine. Albumin is a protein which is generally filtered
and does not undergo excretion in the urine. However, CKD associated with AGEs as mentioned
before, results in damage due to glomerular basement membrane, hindrances to normal filtration
mechanisms and excessive albumin urinary excretion (Park, Baek, Kim & Jung, 2017). Albumin
plays a key role in maintaining the oncotic pressure or colloidal osmotic pressure within the
fluids of the plasma. Thus its abnormal loss in the urine results in fluid accumulation within the
body and associated edema as observed in Steve (Alves et al., 2018) (See Figure 1).
Steve has presented symptoms of hyperkalemia. The kidneys play a key role in regulating
the metabolism of potassium in the body by initiating its excretion. Hindrances to filtration as a
result of CKD impairs the kidney’s ability to excrete potassium resulting in hyperkalemia.
Further, the loss of filtration due to CKD results in accumulation of phosphorous, nitrogenous
and uremic wastes in the body, which are linked to feelings of extreme fatigue and itchiness as
observed in Steve (Nakhoul et al., 2015). The kidneys are also responsible for maintenance of
acid-base balance of the body via the excretion of acidic ammonia and tubular reabsorption of
bicarbonate. However, loss of filtration due to CKD, results in accumulation of acidic
The pathophysiology of CKD due to T2DM is further aggravated due to hypertension.
Hypertension results in increased glomerular pressure via destruction of the blood vessels of the
kidney which further leads to an impairment of the renal system’s functioning associated with
the filtration of toxins, systemic waste accumulation and hence, CKD (Rossignol et al., 2015)
(See Figure 1).
Pathophysiology and Clinical Manifestations
Steve’s symptoms of edema can be attributed to hypoalbuminemia and proteinuria or
excretion of proteins like albumin in the urine. Albumin is a protein which is generally filtered
and does not undergo excretion in the urine. However, CKD associated with AGEs as mentioned
before, results in damage due to glomerular basement membrane, hindrances to normal filtration
mechanisms and excessive albumin urinary excretion (Park, Baek, Kim & Jung, 2017). Albumin
plays a key role in maintaining the oncotic pressure or colloidal osmotic pressure within the
fluids of the plasma. Thus its abnormal loss in the urine results in fluid accumulation within the
body and associated edema as observed in Steve (Alves et al., 2018) (See Figure 1).
Steve has presented symptoms of hyperkalemia. The kidneys play a key role in regulating
the metabolism of potassium in the body by initiating its excretion. Hindrances to filtration as a
result of CKD impairs the kidney’s ability to excrete potassium resulting in hyperkalemia.
Further, the loss of filtration due to CKD results in accumulation of phosphorous, nitrogenous
and uremic wastes in the body, which are linked to feelings of extreme fatigue and itchiness as
observed in Steve (Nakhoul et al., 2015). The kidneys are also responsible for maintenance of
acid-base balance of the body via the excretion of acidic ammonia and tubular reabsorption of
bicarbonate. However, loss of filtration due to CKD, results in accumulation of acidic
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

4DISEASE PATHOPHYSIOLOGY AND CONCEPT MAP
nitrogenous wastes and decreased update of alkaline bicarbonate resulting in metabolic acidosis
as observed in Steve (Collins et al., 2019) (See Figure 1).
Diagnostic Investigations and Treatment
Considering Steve’s symptoms of edema and its relevance to serum albumin, a blood test
of serum albumin and urine test measuring albumin is needed. Also, there is need to evaluate
Steve’s blood glucose and blood pressure levels in order to understand his present scale of
diabetic and hypertensive severity (Oshiro et al., 2018). Hypertension and Hyperkalemia is
associated with hypernatremia, arrhythmia and possible risk of stroke and cardiovascular
symptoms in Steve, thus calling for a need to conduct cardiovascular assessments, an
electrocardiogram (ECG) and serum sodium. CKD is associated with reduced filtration and
accumulation of excretory products like urea, creatinine and nitrogen in the blood which is why
Steve must also undergo diagnostic tests evaluating serum creatinine and blood urea nitrogen
(Bagalad et al., 2017). Steve must also be evaluated for his glomerular filtration rate, considering
reduced filtration in CKD, to evaluate his possible progression to end stage renal disease. An
assessment of Steve arterial blood gas results will shed light on his condition of metabolic
acidosis and bicarbonate status which has been clearly impaired due to CKD. Lastly, Steve must
be evaluated for his serum cholesterol and triglyceride levels and risk of cardiovascular disease
(Mills et al., 2016) (See Figure 1).
Steve’s diet must be free from potassium rich foods like dairy, seafood, fruits like
banana, oranges and peaches and vegetables like spinach and tomatoes. His diet must be free
from added sugars and processed foods rich in salt. Considering his symptoms of acidosis and
decreased urea filtration, Steve must reduce his dairy and meat consumption and opt for
nitrogenous wastes and decreased update of alkaline bicarbonate resulting in metabolic acidosis
as observed in Steve (Collins et al., 2019) (See Figure 1).
Diagnostic Investigations and Treatment
Considering Steve’s symptoms of edema and its relevance to serum albumin, a blood test
of serum albumin and urine test measuring albumin is needed. Also, there is need to evaluate
Steve’s blood glucose and blood pressure levels in order to understand his present scale of
diabetic and hypertensive severity (Oshiro et al., 2018). Hypertension and Hyperkalemia is
associated with hypernatremia, arrhythmia and possible risk of stroke and cardiovascular
symptoms in Steve, thus calling for a need to conduct cardiovascular assessments, an
electrocardiogram (ECG) and serum sodium. CKD is associated with reduced filtration and
accumulation of excretory products like urea, creatinine and nitrogen in the blood which is why
Steve must also undergo diagnostic tests evaluating serum creatinine and blood urea nitrogen
(Bagalad et al., 2017). Steve must also be evaluated for his glomerular filtration rate, considering
reduced filtration in CKD, to evaluate his possible progression to end stage renal disease. An
assessment of Steve arterial blood gas results will shed light on his condition of metabolic
acidosis and bicarbonate status which has been clearly impaired due to CKD. Lastly, Steve must
be evaluated for his serum cholesterol and triglyceride levels and risk of cardiovascular disease
(Mills et al., 2016) (See Figure 1).
Steve’s diet must be free from potassium rich foods like dairy, seafood, fruits like
banana, oranges and peaches and vegetables like spinach and tomatoes. His diet must be free
from added sugars and processed foods rich in salt. Considering his symptoms of acidosis and
decreased urea filtration, Steve must reduce his dairy and meat consumption and opt for

5DISEASE PATHOPHYSIOLOGY AND CONCEPT MAP
vegetarian proteins like soy, beans and legumes (Kalantar-Zadeh & Fouque, 2017). Enhancing
increase of fiber rich foods like green leafy vegetables and whole grains like oats can control
diabetic symptoms as well as reverse CKD associated inflammatory symptoms. Steve maybe
prescribe diuretics like furosemide, beta blockers and metformin to control his symptoms of
edema, hypertension and blood glucose (Crowley et al., 2017) (See Figure 1).
vegetarian proteins like soy, beans and legumes (Kalantar-Zadeh & Fouque, 2017). Enhancing
increase of fiber rich foods like green leafy vegetables and whole grains like oats can control
diabetic symptoms as well as reverse CKD associated inflammatory symptoms. Steve maybe
prescribe diuretics like furosemide, beta blockers and metformin to control his symptoms of
edema, hypertension and blood glucose (Crowley et al., 2017) (See Figure 1).

6DISEASE PATHOPHYSIOLOGY AND CONCEPT MAP
References
Alves, F. C., Sun, J., Qureshi, A. R., Dai, L., Snaedal, S., Bárány, P., ... & Stenvinkel, P. (2018).
The higher mortality associated with low serum albumin is dependent on systemic
inflammation in end-stage kidney disease. PLoS One, 13(1), e0190410. doi:
https://doi.org/10.1371/journal.pone.0190410.
Bagalad, B. S., Mohankumar, K. P., Madhushankari, G. S., Donoghue, M., & Kuberappa, P. H.
(2017). Diagnostic accuracy of salivary creatinine, urea, and potassium levels to assess
dialysis need in renal failure patients. Dental research journal, 14(1), 13. doi:
PMC5356383.
Collins, A. J., Pitt, B., Reaven, N., Funk, S., McGaughey, K., Wilson, D., & Bushinsky, D. A.
(2017). Association of serum potassium with all-cause mortality in patients with and
without heart failure, chronic kidney disease, and/or diabetes. American journal of
nephrology, 46(3), 213-221. doi: https://doi.org/10.1159/000479802.
Crowley, M. J., Diamantidis, C. J., McDuffie, J. R., Cameron, C. B., Stanifer, J. W., Mock, C.
K., ... & Williams, J. W. (2017). Clinical outcomes of metformin use in populations with
chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic
review. Annals of internal medicine, 166(3), 191-200. doi: 10.7326/M16-1901.
Gharbi, M. B., Elseviers, M., Zamd, M., Alaoui, A. B., Benahadi, N., Trabelssi, E. H., ... & De
Broe, M. E. (2016). Chronic kidney disease, hypertension, diabetes, and obesity in the
adult population of Morocco: how to avoid “over”-and “under”-diagnosis of
References
Alves, F. C., Sun, J., Qureshi, A. R., Dai, L., Snaedal, S., Bárány, P., ... & Stenvinkel, P. (2018).
The higher mortality associated with low serum albumin is dependent on systemic
inflammation in end-stage kidney disease. PLoS One, 13(1), e0190410. doi:
https://doi.org/10.1371/journal.pone.0190410.
Bagalad, B. S., Mohankumar, K. P., Madhushankari, G. S., Donoghue, M., & Kuberappa, P. H.
(2017). Diagnostic accuracy of salivary creatinine, urea, and potassium levels to assess
dialysis need in renal failure patients. Dental research journal, 14(1), 13. doi:
PMC5356383.
Collins, A. J., Pitt, B., Reaven, N., Funk, S., McGaughey, K., Wilson, D., & Bushinsky, D. A.
(2017). Association of serum potassium with all-cause mortality in patients with and
without heart failure, chronic kidney disease, and/or diabetes. American journal of
nephrology, 46(3), 213-221. doi: https://doi.org/10.1159/000479802.
Crowley, M. J., Diamantidis, C. J., McDuffie, J. R., Cameron, C. B., Stanifer, J. W., Mock, C.
K., ... & Williams, J. W. (2017). Clinical outcomes of metformin use in populations with
chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic
review. Annals of internal medicine, 166(3), 191-200. doi: 10.7326/M16-1901.
Gharbi, M. B., Elseviers, M., Zamd, M., Alaoui, A. B., Benahadi, N., Trabelssi, E. H., ... & De
Broe, M. E. (2016). Chronic kidney disease, hypertension, diabetes, and obesity in the
adult population of Morocco: how to avoid “over”-and “under”-diagnosis of
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7DISEASE PATHOPHYSIOLOGY AND CONCEPT MAP
CKD. Kidney international, 89(6), 1363-1371. doi:
https://doi.org/10.1016/j.kint.2016.02.019.
Kalantar-Zadeh, K., & Fouque, D. (2017). Nutritional management of chronic kidney
disease. New England Journal of Medicine, 377(18), 1765-1776. doi:
10.1056/NEJMra1700312.
Mills, K. T., Chen, J., Yang, W., Appel, L. J., Kusek, J. W., Alper, A., ... & Rahman, M. (2016).
Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney
disease. Jama, 315(20), 2200-2210. doi: 10.1001/jama.2016.4447.
Miranda-Díaz, A. G., Pazarín-Villaseñor, L., Yanowsky-Escatell, F. G., & Andrade-Sierra, J.
(2016). Oxidative stress in diabetic nephropathy with early chronic kidney
disease. Journal of diabetes research, 2016. Doi:
http://dx.doi.org/10.1155/2016/7047238.
Nakhoul, G. N., Huang, H., Arrigain, S., Jolly, S. E., Schold, J. D., Nally Jr, J. V., &
Navaneethan, S. D. (2015). Serum potassium, end-stage renal disease and mortality in
chronic kidney disease. American journal of nephrology, 41(6), 456-463. doi:
https://doi.org/10.1159/000437151.
Oshiro, S., Ishima, Y., Maeda, H., Honda, N., Bi, J., Kinoshita, R., ... & Miyamura, S. (2018).
Dual therapeutic effects of an albumin-based nitric oxide donor on 2 experimental models
of chronic kidney disease. Journal of pharmaceutical sciences, 107(3), 848-855. doi:
https://doi.org/10.1016/j.xphs.2017.10.023.
CKD. Kidney international, 89(6), 1363-1371. doi:
https://doi.org/10.1016/j.kint.2016.02.019.
Kalantar-Zadeh, K., & Fouque, D. (2017). Nutritional management of chronic kidney
disease. New England Journal of Medicine, 377(18), 1765-1776. doi:
10.1056/NEJMra1700312.
Mills, K. T., Chen, J., Yang, W., Appel, L. J., Kusek, J. W., Alper, A., ... & Rahman, M. (2016).
Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney
disease. Jama, 315(20), 2200-2210. doi: 10.1001/jama.2016.4447.
Miranda-Díaz, A. G., Pazarín-Villaseñor, L., Yanowsky-Escatell, F. G., & Andrade-Sierra, J.
(2016). Oxidative stress in diabetic nephropathy with early chronic kidney
disease. Journal of diabetes research, 2016. Doi:
http://dx.doi.org/10.1155/2016/7047238.
Nakhoul, G. N., Huang, H., Arrigain, S., Jolly, S. E., Schold, J. D., Nally Jr, J. V., &
Navaneethan, S. D. (2015). Serum potassium, end-stage renal disease and mortality in
chronic kidney disease. American journal of nephrology, 41(6), 456-463. doi:
https://doi.org/10.1159/000437151.
Oshiro, S., Ishima, Y., Maeda, H., Honda, N., Bi, J., Kinoshita, R., ... & Miyamura, S. (2018).
Dual therapeutic effects of an albumin-based nitric oxide donor on 2 experimental models
of chronic kidney disease. Journal of pharmaceutical sciences, 107(3), 848-855. doi:
https://doi.org/10.1016/j.xphs.2017.10.023.

8DISEASE PATHOPHYSIOLOGY AND CONCEPT MAP
Park, J. I., Baek, H., Kim, B. R., & Jung, H. H. (2017). Comparison of urine dipstick and
albumin: creatinine ratio for chronic kidney disease screening: A population-based
study. PloS one, 12(2), e0171106. doi: https://doi.org/10.1371/journal.pone.0171106.
Rossignol, P., Massy, Z. A., Azizi, M., Bakris, G., Ritz, E., Covic, A., ... & Mallamaci, F.
(2015). The double challenge of resistant hypertension and chronic kidney disease. The
Lancet, 386(10003), 1588-1598. doi: https://doi.org/10.1016/S0140-6736(15)00418-3.
Sulaiman, M. K. (2019). Diabetic nephropathy: recent advances in pathophysiology and
challenges in dietary management. Diabetology & metabolic syndrome, 11(1), 7. doi:
https://doi.org/10.1186/s13098-019-0403-4.
Thomas, M. C., Cooper, M. E., & Zimmet, P. (2016). Changing epidemiology of type 2 diabetes
mellitus and associated chronic kidney disease. Nature Reviews Nephrology, 12(2), 73.
doi: https://doi.org/10.1038/nrneph.2015.173.
Webster, A. C., Nagler, E. V., Morton, R. L., & Masson, P. (2017). Chronic kidney disease. The
lancet, 389(10075), 1238-1252. doi: https://doi.org/10.1016/S0140-6736(16)32064-5.
Park, J. I., Baek, H., Kim, B. R., & Jung, H. H. (2017). Comparison of urine dipstick and
albumin: creatinine ratio for chronic kidney disease screening: A population-based
study. PloS one, 12(2), e0171106. doi: https://doi.org/10.1371/journal.pone.0171106.
Rossignol, P., Massy, Z. A., Azizi, M., Bakris, G., Ritz, E., Covic, A., ... & Mallamaci, F.
(2015). The double challenge of resistant hypertension and chronic kidney disease. The
Lancet, 386(10003), 1588-1598. doi: https://doi.org/10.1016/S0140-6736(15)00418-3.
Sulaiman, M. K. (2019). Diabetic nephropathy: recent advances in pathophysiology and
challenges in dietary management. Diabetology & metabolic syndrome, 11(1), 7. doi:
https://doi.org/10.1186/s13098-019-0403-4.
Thomas, M. C., Cooper, M. E., & Zimmet, P. (2016). Changing epidemiology of type 2 diabetes
mellitus and associated chronic kidney disease. Nature Reviews Nephrology, 12(2), 73.
doi: https://doi.org/10.1038/nrneph.2015.173.
Webster, A. C., Nagler, E. V., Morton, R. L., & Masson, P. (2017). Chronic kidney disease. The
lancet, 389(10075), 1238-1252. doi: https://doi.org/10.1016/S0140-6736(16)32064-5.
1 out of 9
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.





