Effects of Solution Concentrations on Potato Osmosis Lab Report
VerifiedAdded on 2023/04/11
|11
|1651
|245
AI Summary
This lab report investigates the effects of varying sucrose solution concentrations on potato strips to demonstrate osmosis. The experiment involved preparing sucrose solutions of different molarities and observing the changes in mass and rigidity of potato strips immersed in these solutions for 20 minutes. The results indicated that potato strips in higher sucrose concentrations lost mass and became flaccid, while those in distilled water gained mass and became turgid. The report includes a detailed methodology, results table, a graph plotting concentration against change in mass, and a discussion of the findings in relation to osmosis principles. The conclusion supports the hypothesis that osmosis is controlled by solution concentration, with water moving from areas of low solute concentration to areas of high solute concentration. Desklib provides a platform for students to access similar lab reports and study resources.

Effects of Varying Concentrations of Solutions On the Potatoes Lab Report
By(name)
Course
Tutor
Institutional Affiliation
Date
By(name)
Course
Tutor
Institutional Affiliation
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Title: Effects of varying concentrations of solutions on the potatoes
Introduction
Osmosis is defined as the movement of molecules of water from high concentration to
low concentration through a semipermeable membrane (Owusu-Agyeman, Reinwald,
Jeihanipour, & Schäfer, 2019, p. 48). This is caused by the concentrations differences on the two
sides of membrane. Osmosis occurs in both plants and animals. For example, in human beings,
osmosis occurs majorly in the glomerulus of human kidneys. On the other hand, osmosis occurs
everywhere within the plant cells. The process of osmosis can be shown experimentally using a
potato and salt/glucose solutions. Osmotic pressure such as isotonic, hypertonic, and hypotonic
manipulate the direction of the movement of water molecules (Rastogi, 2018, p. 86).
Importance of Osmosis in Plants
1. Plants need osmosis to absorb water from the soil for survival.
2. Plant cells are also able to absorb water though osmosis, become turgid thus remain
upright.
Importance of Osmosis in Animals
1. Keeping the ion concentration balanced between the internal and external environment of
the cells
Introduction
Osmosis is defined as the movement of molecules of water from high concentration to
low concentration through a semipermeable membrane (Owusu-Agyeman, Reinwald,
Jeihanipour, & Schäfer, 2019, p. 48). This is caused by the concentrations differences on the two
sides of membrane. Osmosis occurs in both plants and animals. For example, in human beings,
osmosis occurs majorly in the glomerulus of human kidneys. On the other hand, osmosis occurs
everywhere within the plant cells. The process of osmosis can be shown experimentally using a
potato and salt/glucose solutions. Osmotic pressure such as isotonic, hypertonic, and hypotonic
manipulate the direction of the movement of water molecules (Rastogi, 2018, p. 86).
Importance of Osmosis in Plants
1. Plants need osmosis to absorb water from the soil for survival.
2. Plant cells are also able to absorb water though osmosis, become turgid thus remain
upright.
Importance of Osmosis in Animals
1. Keeping the ion concentration balanced between the internal and external environment of
the cells

2. Facilitating the transport of water molecules through capillaries to each cell in a body to
keep the cells firm, healthy, and functional
3. Osmoregulation process in human kidneys is facilitated by osmosis.
Osmosis in Plants
The environment surrounding the plant cells is always hypotonic and the plant cells tend
to have more concentrated fluids than the surrounding environment ("Reverse Osmosis," 2019,
p.98). This facilitates the movement of water molecules from the environment into the cells thus
raising the turgor pressure. This makes the plant cells and tissues rigid thus able to stand upright.
On the other hand, assuming the plant cells are in hypotonic environment, all the water would
leave the plant cells causing plasmolysis (Wang & Wang, 2019, p. 38). However, if the plants
are in isotonic environment, the cells would turn flaccid and wilt.
Osmosis in Animal cells
If an animal cell is placed in hypotonic solution, it would start absorbing water and later
burst, unlike the plant cells, animal cells lack the cell wall to prevent the cells from bursting
(Sivertsen, Holt, & Thelin, 2018, p. 39). While if the animal cells are placed in hypertonic
solutions, the cells would start diffusing water out and later shrivels. This explains why animal
cells should be kept constantly in an isotonic environment, otherwise, the animal cell would
either burst or shrivel (Zhang, Cheng, & Yang, 2014, p. 44).
Hypothesis, Aims and Objectives
Hypothesis: Osmosis is controlled by concentration of the solutions. The size and the mass of
the potato strips would reduce when submerged in a highly concentrated sucrose solution since
water inside the potato cells would leave out to create an equilibrium condition. On the other
keep the cells firm, healthy, and functional
3. Osmoregulation process in human kidneys is facilitated by osmosis.
Osmosis in Plants
The environment surrounding the plant cells is always hypotonic and the plant cells tend
to have more concentrated fluids than the surrounding environment ("Reverse Osmosis," 2019,
p.98). This facilitates the movement of water molecules from the environment into the cells thus
raising the turgor pressure. This makes the plant cells and tissues rigid thus able to stand upright.
On the other hand, assuming the plant cells are in hypotonic environment, all the water would
leave the plant cells causing plasmolysis (Wang & Wang, 2019, p. 38). However, if the plants
are in isotonic environment, the cells would turn flaccid and wilt.
Osmosis in Animal cells
If an animal cell is placed in hypotonic solution, it would start absorbing water and later
burst, unlike the plant cells, animal cells lack the cell wall to prevent the cells from bursting
(Sivertsen, Holt, & Thelin, 2018, p. 39). While if the animal cells are placed in hypertonic
solutions, the cells would start diffusing water out and later shrivels. This explains why animal
cells should be kept constantly in an isotonic environment, otherwise, the animal cell would
either burst or shrivel (Zhang, Cheng, & Yang, 2014, p. 44).
Hypothesis, Aims and Objectives
Hypothesis: Osmosis is controlled by concentration of the solutions. The size and the mass of
the potato strips would reduce when submerged in a highly concentrated sucrose solution since
water inside the potato cells would leave out to create an equilibrium condition. On the other
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

hand, if the cells are placed in low concentrated sucrose solution, its size and mass would
increase and become turgid and rigid (Altaee, Zaragoza, & Van Tonningen, 2014, p.52). These
natural osmotic pressures that determine the movement of water molecules are hypo-osmotic,
hyperosmotic and isosmotic. It has been proven experimentally that the movement of water
molecules is from the hyperosmotic solution to hypo-osmotic solution order to achieve isosmotic
solution where both sides of semipermeable membrane have equal amount of sucrose solutions
(Ismail, Khulbe, & Matsuura, 2019, p.98).
Aim: To investigate the effect of osmosis in potatoes placed in solutions with varying
concentrations
Objectives
1. Be able to prepare series of dilutions accurately and calculate molarities
2. To observe correctly and analyze the effects on potato placed in solutions with different
concentrations and to be able to conclude effectively.
3. To be able to produce calibration curve to estimate the water potential of potato tissue.
4. To gain knowledge and understanding of water movement by osmosis and be able to
describe the term hypotonic, hypertonic, and isotonic.
Method 2nd Experiment
Equipment
Potatoes (strips)
number 6 Cork borer
balances/weigh scale
water bath
increase and become turgid and rigid (Altaee, Zaragoza, & Van Tonningen, 2014, p.52). These
natural osmotic pressures that determine the movement of water molecules are hypo-osmotic,
hyperosmotic and isosmotic. It has been proven experimentally that the movement of water
molecules is from the hyperosmotic solution to hypo-osmotic solution order to achieve isosmotic
solution where both sides of semipermeable membrane have equal amount of sucrose solutions
(Ismail, Khulbe, & Matsuura, 2019, p.98).
Aim: To investigate the effect of osmosis in potatoes placed in solutions with varying
concentrations
Objectives
1. Be able to prepare series of dilutions accurately and calculate molarities
2. To observe correctly and analyze the effects on potato placed in solutions with different
concentrations and to be able to conclude effectively.
3. To be able to produce calibration curve to estimate the water potential of potato tissue.
4. To gain knowledge and understanding of water movement by osmosis and be able to
describe the term hypotonic, hypertonic, and isotonic.
Method 2nd Experiment
Equipment
Potatoes (strips)
number 6 Cork borer
balances/weigh scale
water bath
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

pipettes
sucrose solution
distilled water
scalpel
test tubes
test tube racks
napkin/tissues
petri dishes
stopwatches
ruler
black markers.
Procedure
1. The sucrose solutions were prepared in 6 test tubes and mixed thoroughly
2. Each test tube was labeled and placed in a water bath to equilibrate at 25 degrees celcius
3. While equilibrating, potato trips were prepared using number 6 cork borer to make
cylinders using the same potato
4. All the potato strips were measured using a ruler and cut to the same length
5. Excess water was dried off using tissue paper and initial mass of each potato strip
measured and recorded.
6. Each potato strip was placed in petri dishes labelled with concentrations to avoid
confusion
7. Each potato strip was placed in the correctly labelled solution test tubes in water bath and
left in the water bath for 20minutes to allow reaction to occur.
sucrose solution
distilled water
scalpel
test tubes
test tube racks
napkin/tissues
petri dishes
stopwatches
ruler
black markers.
Procedure
1. The sucrose solutions were prepared in 6 test tubes and mixed thoroughly
2. Each test tube was labeled and placed in a water bath to equilibrate at 25 degrees celcius
3. While equilibrating, potato trips were prepared using number 6 cork borer to make
cylinders using the same potato
4. All the potato strips were measured using a ruler and cut to the same length
5. Excess water was dried off using tissue paper and initial mass of each potato strip
measured and recorded.
6. Each potato strip was placed in petri dishes labelled with concentrations to avoid
confusion
7. Each potato strip was placed in the correctly labelled solution test tubes in water bath and
left in the water bath for 20minutes to allow reaction to occur.

8. After 20minutes, the samples were taken out and each strip removed and blotted dry for
excess water. Then, the samples were placed in the correctly labelled petri dish.
9. The final mass and length were measured and the observations recorded.
Method 1st Experiment
Equipment
3 potatoes (2 raw, 1 boiled)
Distilled water (ml)
Beaker/container
Sodium chloride/salt (g)
Scalpel
Stopwatch
Procedure
1. 3 potatoes, 2 raw and 1 boiled were prepared
2. The skins of the potatoes were peeled off and cut in halves
3. A hole was made in the middle of each potato and cut thinly in the base to make them flat
4. The beaker containing boiled potato filled with salt was labelled A, the beaker containing
raw potato filled with salt was labelled B and the beaker containing raw potato only
labelled C. The hollow cavities of potato A and B were filled.
5. Each potato was placed in a beaker and the beaker filled with equal amount of water
6. The initial observations were recorded
7. The samples were left for 20 minutes
8. After 20 minutes, the observations were carefully observed and recorded.
excess water. Then, the samples were placed in the correctly labelled petri dish.
9. The final mass and length were measured and the observations recorded.
Method 1st Experiment
Equipment
3 potatoes (2 raw, 1 boiled)
Distilled water (ml)
Beaker/container
Sodium chloride/salt (g)
Scalpel
Stopwatch
Procedure
1. 3 potatoes, 2 raw and 1 boiled were prepared
2. The skins of the potatoes were peeled off and cut in halves
3. A hole was made in the middle of each potato and cut thinly in the base to make them flat
4. The beaker containing boiled potato filled with salt was labelled A, the beaker containing
raw potato filled with salt was labelled B and the beaker containing raw potato only
labelled C. The hollow cavities of potato A and B were filled.
5. Each potato was placed in a beaker and the beaker filled with equal amount of water
6. The initial observations were recorded
7. The samples were left for 20 minutes
8. After 20 minutes, the observations were carefully observed and recorded.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
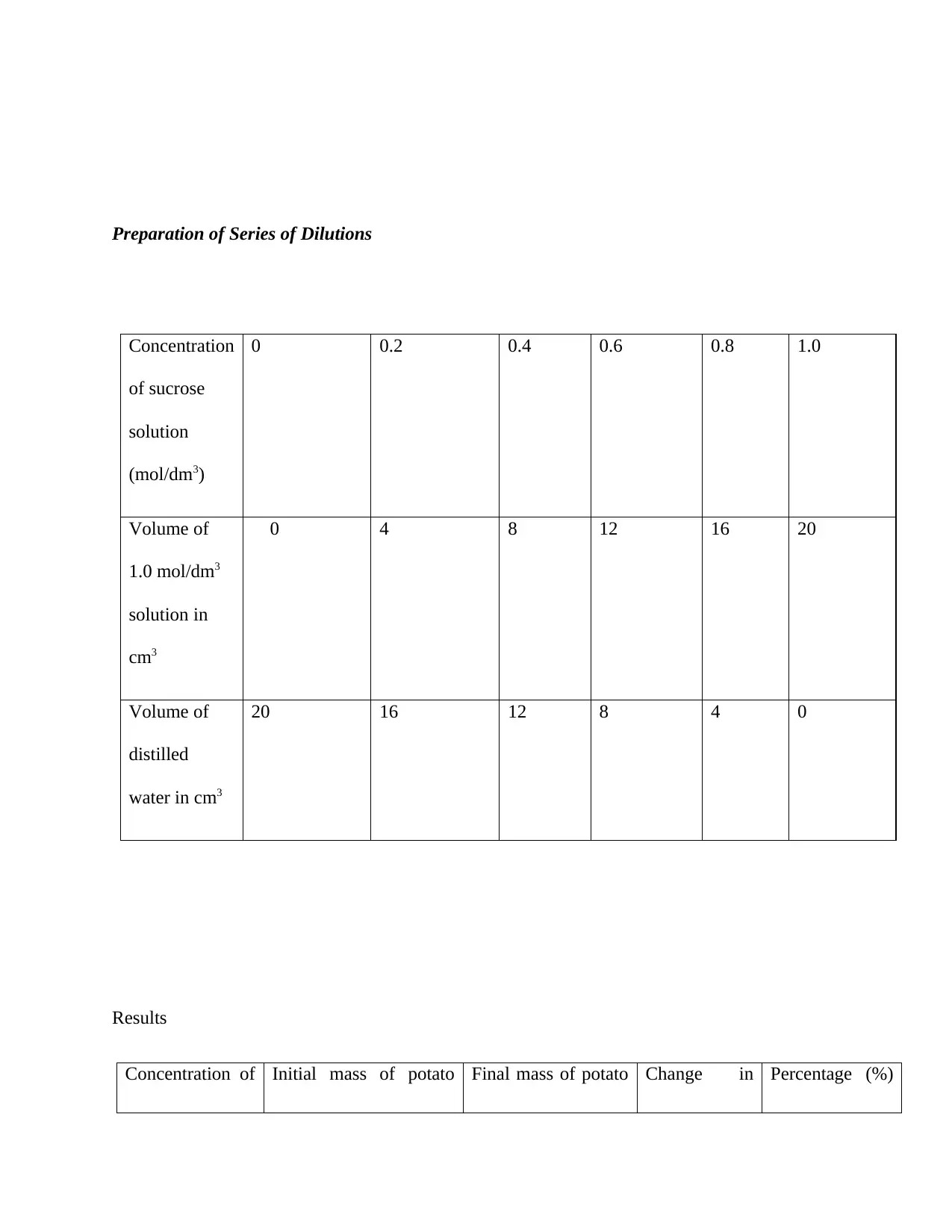
Preparation of Series of Dilutions
Concentration
of sucrose
solution
(mol/dm3)
0 0.2 0.4 0.6 0.8 1.0
Volume of
1.0 mol/dm3
solution in
cm3
0 4 8 12 16 20
Volume of
distilled
water in cm3
20 16 12 8 4 0
Results
Concentration of Initial mass of potato Final mass of potato Change in Percentage (%)
Concentration
of sucrose
solution
(mol/dm3)
0 0.2 0.4 0.6 0.8 1.0
Volume of
1.0 mol/dm3
solution in
cm3
0 4 8 12 16 20
Volume of
distilled
water in cm3
20 16 12 8 4 0
Results
Concentration of Initial mass of potato Final mass of potato Change in Percentage (%)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
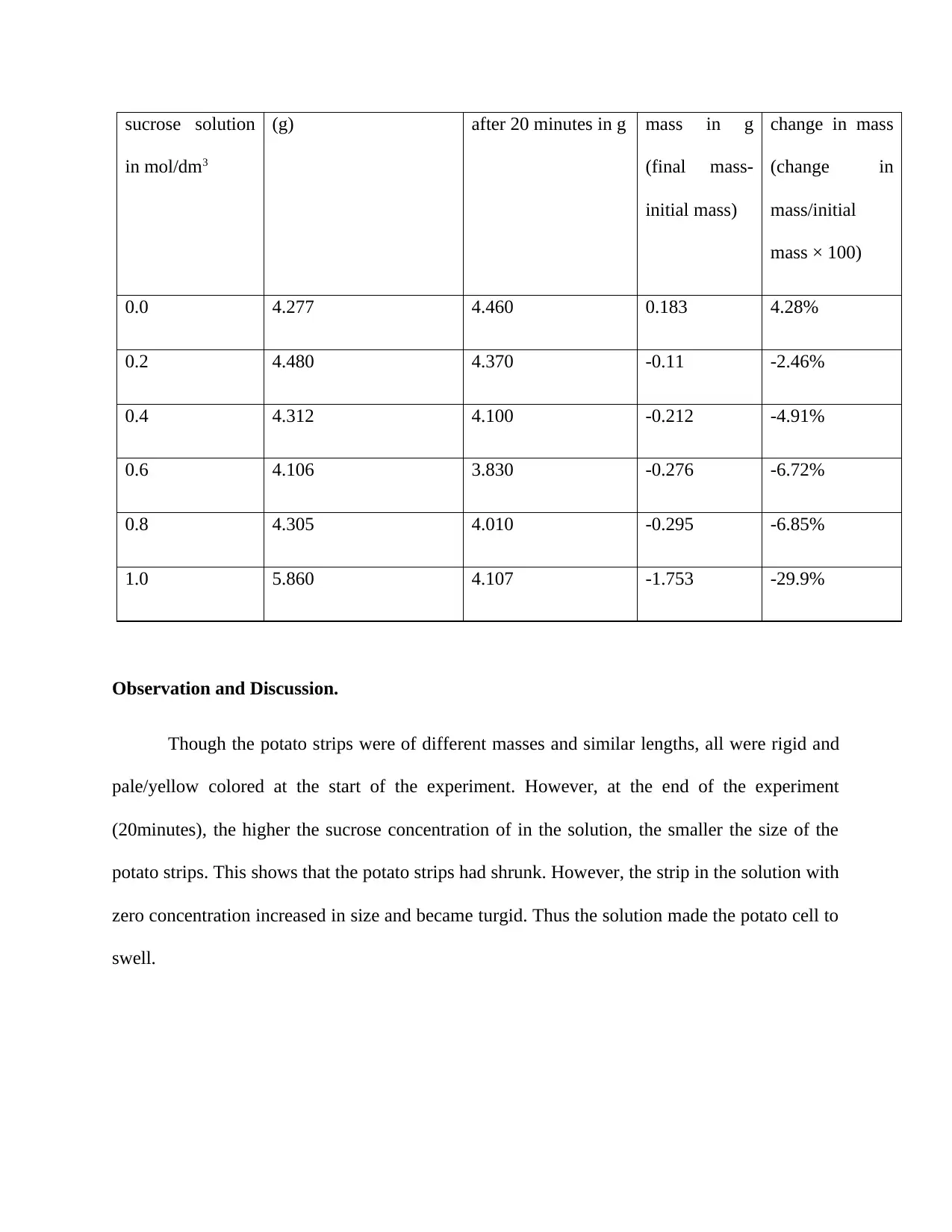
sucrose solution
in mol/dm3
(g) after 20 minutes in g mass in g
(final mass-
initial mass)
change in mass
(change in
mass/initial
mass × 100)
0.0 4.277 4.460 0.183 4.28%
0.2 4.480 4.370 -0.11 -2.46%
0.4 4.312 4.100 -0.212 -4.91%
0.6 4.106 3.830 -0.276 -6.72%
0.8 4.305 4.010 -0.295 -6.85%
1.0 5.860 4.107 -1.753 -29.9%
Observation and Discussion.
Though the potato strips were of different masses and similar lengths, all were rigid and
pale/yellow colored at the start of the experiment. However, at the end of the experiment
(20minutes), the higher the sucrose concentration of in the solution, the smaller the size of the
potato strips. This shows that the potato strips had shrunk. However, the strip in the solution with
zero concentration increased in size and became turgid. Thus the solution made the potato cell to
swell.
in mol/dm3
(g) after 20 minutes in g mass in g
(final mass-
initial mass)
change in mass
(change in
mass/initial
mass × 100)
0.0 4.277 4.460 0.183 4.28%
0.2 4.480 4.370 -0.11 -2.46%
0.4 4.312 4.100 -0.212 -4.91%
0.6 4.106 3.830 -0.276 -6.72%
0.8 4.305 4.010 -0.295 -6.85%
1.0 5.860 4.107 -1.753 -29.9%
Observation and Discussion.
Though the potato strips were of different masses and similar lengths, all were rigid and
pale/yellow colored at the start of the experiment. However, at the end of the experiment
(20minutes), the higher the sucrose concentration of in the solution, the smaller the size of the
potato strips. This shows that the potato strips had shrunk. However, the strip in the solution with
zero concentration increased in size and became turgid. Thus the solution made the potato cell to
swell.
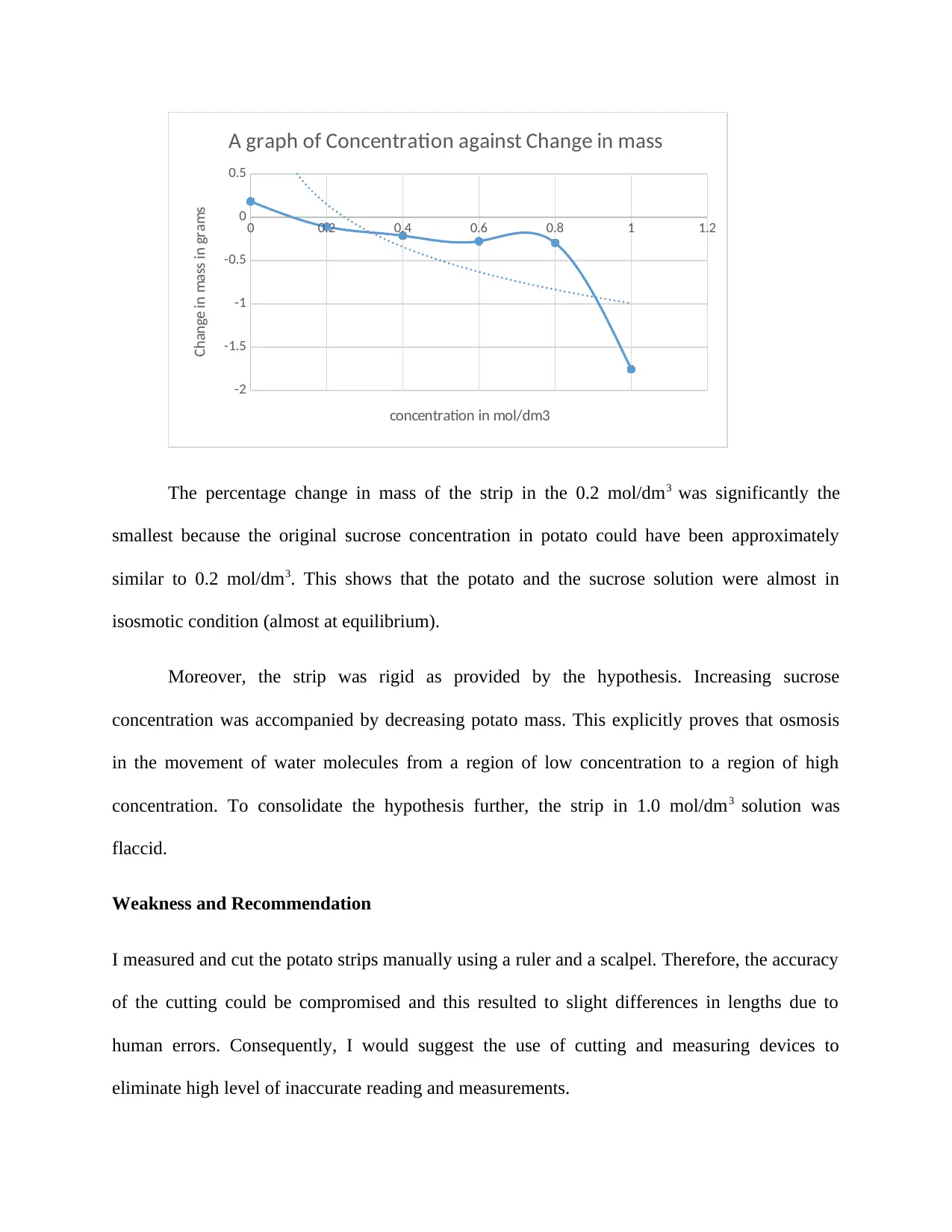
0 0.2 0.4 0.6 0.8 1 1.2
-2
-1.5
-1
-0.5
0
0.5
A graph of Concentration against Change in mass
concentration in mol/dm3
Change in mass in grams
The percentage change in mass of the strip in the 0.2 mol/dm3 was significantly the
smallest because the original sucrose concentration in potato could have been approximately
similar to 0.2 mol/dm3. This shows that the potato and the sucrose solution were almost in
isosmotic condition (almost at equilibrium).
Moreover, the strip was rigid as provided by the hypothesis. Increasing sucrose
concentration was accompanied by decreasing potato mass. This explicitly proves that osmosis
in the movement of water molecules from a region of low concentration to a region of high
concentration. To consolidate the hypothesis further, the strip in 1.0 mol/dm3 solution was
flaccid.
Weakness and Recommendation
I measured and cut the potato strips manually using a ruler and a scalpel. Therefore, the accuracy
of the cutting could be compromised and this resulted to slight differences in lengths due to
human errors. Consequently, I would suggest the use of cutting and measuring devices to
eliminate high level of inaccurate reading and measurements.
-2
-1.5
-1
-0.5
0
0.5
A graph of Concentration against Change in mass
concentration in mol/dm3
Change in mass in grams
The percentage change in mass of the strip in the 0.2 mol/dm3 was significantly the
smallest because the original sucrose concentration in potato could have been approximately
similar to 0.2 mol/dm3. This shows that the potato and the sucrose solution were almost in
isosmotic condition (almost at equilibrium).
Moreover, the strip was rigid as provided by the hypothesis. Increasing sucrose
concentration was accompanied by decreasing potato mass. This explicitly proves that osmosis
in the movement of water molecules from a region of low concentration to a region of high
concentration. To consolidate the hypothesis further, the strip in 1.0 mol/dm3 solution was
flaccid.
Weakness and Recommendation
I measured and cut the potato strips manually using a ruler and a scalpel. Therefore, the accuracy
of the cutting could be compromised and this resulted to slight differences in lengths due to
human errors. Consequently, I would suggest the use of cutting and measuring devices to
eliminate high level of inaccurate reading and measurements.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Conclusion
From the observations, we can conclude that the hypothesis was correctly stated. This is because
the mass of potato strips in the distilled water increased due to low sucrose concentration. While
the strips shrank (mass reduced) in high sucrose concentrated solutions. Based on this finding,
we can also conclude that if the length of the strips were to be measured then, their lengths
would reduce in the highly concentrated sucrose solutions and increase in the case of distilled
water. The experiment was thus a success.
References
From the observations, we can conclude that the hypothesis was correctly stated. This is because
the mass of potato strips in the distilled water increased due to low sucrose concentration. While
the strips shrank (mass reduced) in high sucrose concentrated solutions. Based on this finding,
we can also conclude that if the length of the strips were to be measured then, their lengths
would reduce in the highly concentrated sucrose solutions and increase in the case of distilled
water. The experiment was thus a success.
References
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Altaee, A., Zaragoza, G., & Van Tonningen, H. R. (2014). Comparison between Forward
Osmosis-Reverse Osmosis and Reverse Osmosis processes for seawater desalination.
Desalination, 336, 50-57.
Ismail, A. F., Khulbe, K. C., & Matsuura, T. (2019). RO Membrane Transport. Reverse Osmosis,
91-116.
Owusu-Agyeman, I., Reinwald, M., Jeihanipour, A., & Schäfer, A. I. (2019). Removal of
fluoride and natural organic matter from natural tropical brackish waters by
nanofiltration/reverse osmosis with varying water chemistry. Chemosphere, 217, 47-58.
Rastogi, N. K. (2018). Reverse Osmosis and Forward Osmosis for the Concentration of Fruit
Juices. Fruit Juices.
Reverse Osmosis. (2019).
Sivertsen, E., Holt, T., & Thelin, W. (2018). Concentration and Temperature Effects on Water
and Salt Permeabilities in Osmosis and Implications in Pressure-Retarded Osmosis.
Membranes, 8(3), 39.
Wang, Y., & Wang, R. (2019). Reverse Osmosis Membrane Separation Technology. Membrane
Separation Principles and Applications, 1-45.
Zhang, H., Cheng, S., & Yang, F. (2014). Use of a spacer to mitigate concentration polarization
during forward osmosis process. Desalination, 347, 112-119.
Osmosis-Reverse Osmosis and Reverse Osmosis processes for seawater desalination.
Desalination, 336, 50-57.
Ismail, A. F., Khulbe, K. C., & Matsuura, T. (2019). RO Membrane Transport. Reverse Osmosis,
91-116.
Owusu-Agyeman, I., Reinwald, M., Jeihanipour, A., & Schäfer, A. I. (2019). Removal of
fluoride and natural organic matter from natural tropical brackish waters by
nanofiltration/reverse osmosis with varying water chemistry. Chemosphere, 217, 47-58.
Rastogi, N. K. (2018). Reverse Osmosis and Forward Osmosis for the Concentration of Fruit
Juices. Fruit Juices.
Reverse Osmosis. (2019).
Sivertsen, E., Holt, T., & Thelin, W. (2018). Concentration and Temperature Effects on Water
and Salt Permeabilities in Osmosis and Implications in Pressure-Retarded Osmosis.
Membranes, 8(3), 39.
Wang, Y., & Wang, R. (2019). Reverse Osmosis Membrane Separation Technology. Membrane
Separation Principles and Applications, 1-45.
Zhang, H., Cheng, S., & Yang, F. (2014). Use of a spacer to mitigate concentration polarization
during forward osmosis process. Desalination, 347, 112-119.
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




