Enthalpy and entropy Reaction change in Physical Chemistry
VerifiedAdded on 2023/06/09
|12
|2284
|433
AI Summary
This article discusses Enthalpy and Entropy Reaction Change in Physical Chemistry. It covers topics such as the second law of thermodynamics, latent heat of evaporation, standard electrode potential, solubility equilibrium, Le Chatelier’s principle, and more. It also includes a graph of aspartate of decarboxylase catalysis and the effect of inhibitors on the Michaelis constant.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Physical chemistry 1
Topic: Physical chemistry
Paper type: Course work
Word count: 1900
Pages: 7 pages
Referencing style: Oxford
Education level:
Enthalpy and entropy Reaction change
Name of the writer:
Name of the institution:
Date:
Topic: Physical chemistry
Paper type: Course work
Word count: 1900
Pages: 7 pages
Referencing style: Oxford
Education level:
Enthalpy and entropy Reaction change
Name of the writer:
Name of the institution:
Date:
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Physical chemistry 1
Enthalpy and entropy Reaction change
Question 1 (a)
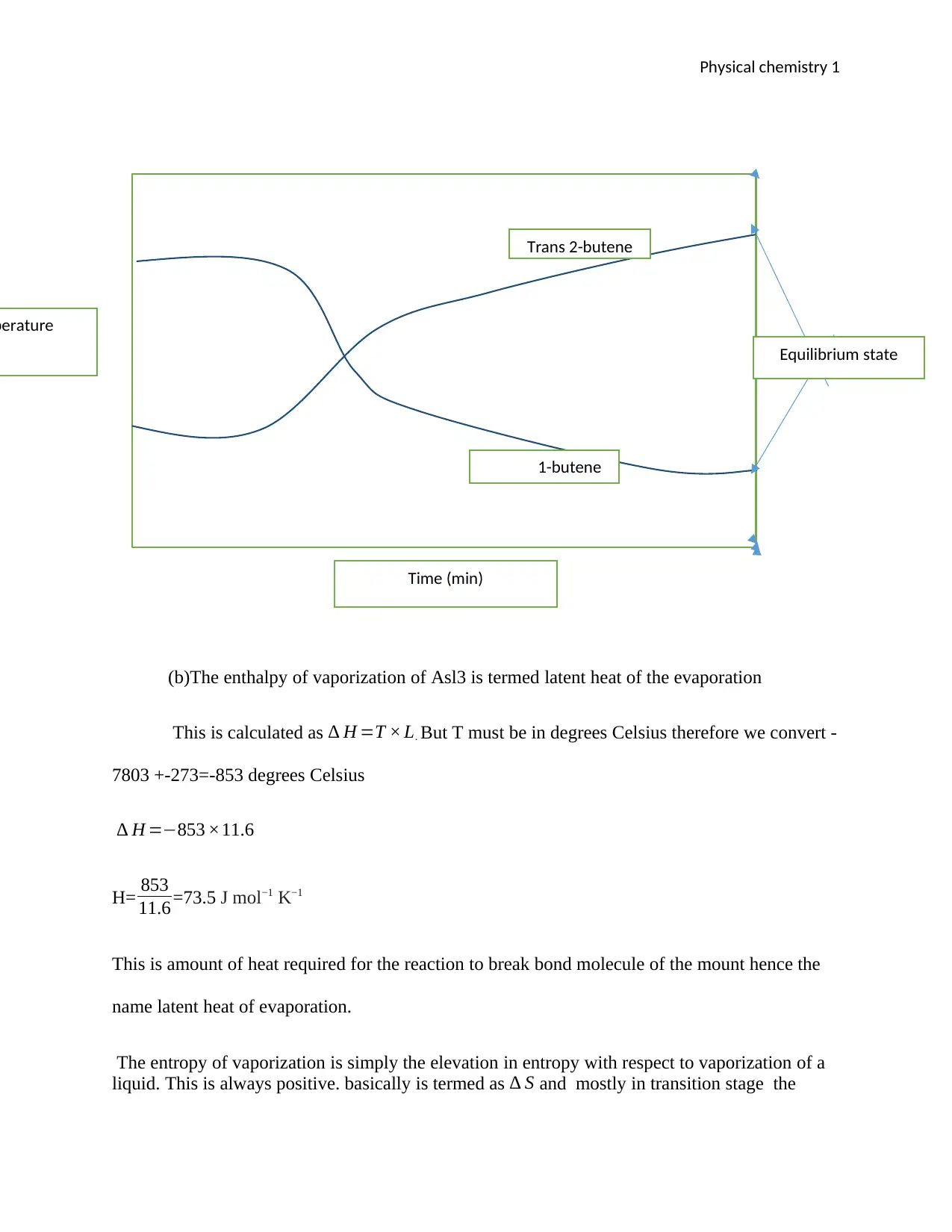
(i) The enthalpy and entropy of the reaction Change in respect to temperature. This is
determined using the second law of the thermodynamics which illustrates “total
entropy of an isolated system can never decrease over time”. Therefore, the
accumulative entropy cannot vary in perfect scenario where the reaction is in a steady
state of equality. This cannot be continuous process since the reaction is endothermic
therefore it ends up losing heat from the surrounding. When 1-bitene and trans-2-
butene reacts the temperature will increase in the beaker. Therefore, you can easily
compute the enthalpy and entropy of the reaction can be solved using Gibbs free
energy question: ∆G = ∆H - T∆S. Where ∆G, is the highest amount of the job that a
reaction can do for the environments. Ultimately mobile energy is same difference
between the thermal energy of the entropy change during the reaction and the entropy
change during the reaction. Since heat energy change to is termed as chemical
bonding and entropy is related to the problem of the system. Therefore, in calculation
the net ionic reaction of the entropy. The H is the amount of heat in the beaker so the
expected sign will be positive since heat is being absorbed from the surrounding to
the beaker during the reaction hence we refer this kind of reaction as endothermic
reaction. The magnitude of T∆S in the reaction at equilibrium given the maximum
time in minutes is illustrated in the figure below.
(ii) The reaction is at equilibrium, and it will not proceed to the right product side in
order to be equal.
Enthalpy and entropy Reaction change
Question 1 (a)
(i) The enthalpy and entropy of the reaction Change in respect to temperature. This is
determined using the second law of the thermodynamics which illustrates “total
entropy of an isolated system can never decrease over time”. Therefore, the
accumulative entropy cannot vary in perfect scenario where the reaction is in a steady
state of equality. This cannot be continuous process since the reaction is endothermic
therefore it ends up losing heat from the surrounding. When 1-bitene and trans-2-
butene reacts the temperature will increase in the beaker. Therefore, you can easily
compute the enthalpy and entropy of the reaction can be solved using Gibbs free
energy question: ∆G = ∆H - T∆S. Where ∆G, is the highest amount of the job that a
reaction can do for the environments. Ultimately mobile energy is same difference
between the thermal energy of the entropy change during the reaction and the entropy
change during the reaction. Since heat energy change to is termed as chemical
bonding and entropy is related to the problem of the system. Therefore, in calculation
the net ionic reaction of the entropy. The H is the amount of heat in the beaker so the
expected sign will be positive since heat is being absorbed from the surrounding to
the beaker during the reaction hence we refer this kind of reaction as endothermic
reaction. The magnitude of T∆S in the reaction at equilibrium given the maximum
time in minutes is illustrated in the figure below.
(ii) The reaction is at equilibrium, and it will not proceed to the right product side in
order to be equal.
Physical chemistry 1
(b)The enthalpy of vaporization of Asl3 is termed latent heat of the evaporation
This is calculated as ∆ H =T × L. But T must be in degrees Celsius therefore we convert -
7803 +-273=-853 degrees Celsius
∆ H =−853 ×11.6
H= 853
11.6 =73.5 J mol−1 K−1
This is amount of heat required for the reaction to break bond molecule of the mount hence the
name latent heat of evaporation.
The entropy of vaporization is simply the elevation in entropy with respect to vaporization of a
liquid. This is always positive. basically is termed as ∆ S and mostly in transition stage the
perature
Trans 2-butene
1-butene
Time (min)
Equilibrium state
(b)The enthalpy of vaporization of Asl3 is termed latent heat of the evaporation
This is calculated as ∆ H =T × L. But T must be in degrees Celsius therefore we convert -
7803 +-273=-853 degrees Celsius
∆ H =−853 ×11.6
H= 853
11.6 =73.5 J mol−1 K−1
This is amount of heat required for the reaction to break bond molecule of the mount hence the
name latent heat of evaporation.
The entropy of vaporization is simply the elevation in entropy with respect to vaporization of a
liquid. This is always positive. basically is termed as ∆ S and mostly in transition stage the
perature
Trans 2-butene
1-butene
Time (min)
Equilibrium state

Physical chemistry 1
evaporation both phases are at equilibrium so apparently the
different between theGibbs free energy is same ¿ zero .
∆ S= ∆ H
T ∆
Where ∆ H is the thermal or sometimes refer as latent heat of evaporation since it’s a
thermodynamic equation the simple T is taken as thermal heat or temperature measuring in
kelvin k. The latent heat of evaporation is then equal to the heat of vaporization divided by the
boiling point.
∆ S=73.5
0.6 =122.5 j/kg ∆
Therefore the entropy of evaporation at a normal standard pressure is equal 122.5 J mol−1 K−1
This values does not obey Trouton’s rule where most compounds ranges between 85 to 88
mol−1 K−1
Question 2.
A) What is the meaning of the standard electrode potential in this case? It refers to the
amount of work done per valence which is present from the reverse reaction so that it
drives the reaction. It is important to interpret the cell process in terms of temporary
reaction, in addition of oxygen in half reaction and removal of oxygen in another half
reaction.
The following shows the behavior of the two graphs: endothermic and exothermic
reaction.
An exothermic reaction
evaporation both phases are at equilibrium so apparently the
different between theGibbs free energy is same ¿ zero .
∆ S= ∆ H
T ∆
Where ∆ H is the thermal or sometimes refer as latent heat of evaporation since it’s a
thermodynamic equation the simple T is taken as thermal heat or temperature measuring in
kelvin k. The latent heat of evaporation is then equal to the heat of vaporization divided by the
boiling point.
∆ S=73.5
0.6 =122.5 j/kg ∆
Therefore the entropy of evaporation at a normal standard pressure is equal 122.5 J mol−1 K−1
This values does not obey Trouton’s rule where most compounds ranges between 85 to 88
mol−1 K−1
Question 2.
A) What is the meaning of the standard electrode potential in this case? It refers to the
amount of work done per valence which is present from the reverse reaction so that it
drives the reaction. It is important to interpret the cell process in terms of temporary
reaction, in addition of oxygen in half reaction and removal of oxygen in another half
reaction.
The following shows the behavior of the two graphs: endothermic and exothermic
reaction.
An exothermic reaction
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
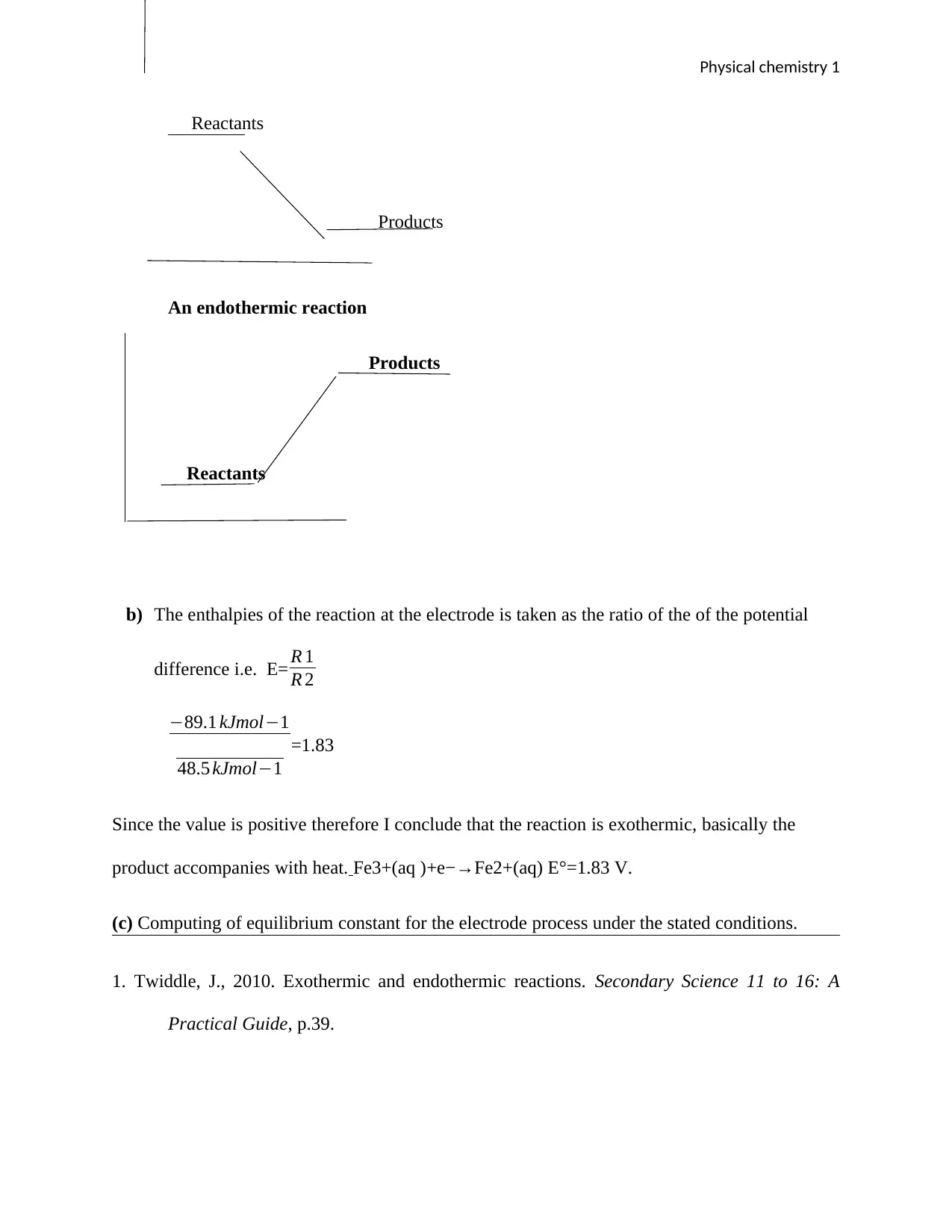
Physical chemistry 1
Reactants
Products
An endothermic reaction
Products
Reactants
b) The enthalpies of the reaction at the electrode is taken as the ratio of the of the potential
difference i.e. E= R 1
R 2
−89.1 kJmol−1
48.5 kJmol−1
=1.83
Since the value is positive therefore I conclude that the reaction is exothermic, basically the
product accompanies with heat. Fe3+(aq )+e−→Fe2+(aq) E°=1.83 V.
(c) Computing of equilibrium constant for the electrode process under the stated conditions.
1. Twiddle, J., 2010. Exothermic and endothermic reactions. Secondary Science 11 to 16: A
Practical Guide, p.39.
Reactants
Products
An endothermic reaction
Products
Reactants
b) The enthalpies of the reaction at the electrode is taken as the ratio of the of the potential
difference i.e. E= R 1
R 2
−89.1 kJmol−1
48.5 kJmol−1
=1.83
Since the value is positive therefore I conclude that the reaction is exothermic, basically the
product accompanies with heat. Fe3+(aq )+e−→Fe2+(aq) E°=1.83 V.
(c) Computing of equilibrium constant for the electrode process under the stated conditions.
1. Twiddle, J., 2010. Exothermic and endothermic reactions. Secondary Science 11 to 16: A
Practical Guide, p.39.

Physical chemistry 1
Solubility equilibrium happens when a chemical substance (solid) is in equilibrium with a
solution of the same chemical substance (Rahimpour et al. 2012, Pg. 496- 512). It happens
where there is an equilibrium between the undissolved salt and the dissolved salt.
Apply Le Chatelier’s principle to the above reaction, clearly identify how changing the
temperature, pressure and using catalyst would affect
The equilibrium of the reaction: The principle states that if changes are made to a reaction at
equilibrium, the equilibrium will move in position proportional to the change until a new
equilibrium is attained. Lowering temperature favors an exothermic reaction and therefore the
equilibrium will shift to the right. Raising temperature move shift the equilibrium leftward. A
rise in temperature in gases increases the rate of reaction shifting it leftward. The same case with
the introduction of a catalyst which shifts the reaction leftward.
The rate of the reaction: Lowering the temperature favors formation of products but reduces
the rate of reaction. An increase in pressure and introduction of a catalyst will speed up the rate
of reaction.
Ferric solution + electron –ferrous solution + electron
Give us
ΔG°= (0.77 V) time 1,83 v=-0.45
Then solve the last expression for ΔG° for the overall half-reaction,
Which is taken as ΔG° = F [(−1.83 Volte’s) + (−0.77)] = F (0.23 Volte’s)
Solubility equilibrium happens when a chemical substance (solid) is in equilibrium with a
solution of the same chemical substance (Rahimpour et al. 2012, Pg. 496- 512). It happens
where there is an equilibrium between the undissolved salt and the dissolved salt.
Apply Le Chatelier’s principle to the above reaction, clearly identify how changing the
temperature, pressure and using catalyst would affect
The equilibrium of the reaction: The principle states that if changes are made to a reaction at
equilibrium, the equilibrium will move in position proportional to the change until a new
equilibrium is attained. Lowering temperature favors an exothermic reaction and therefore the
equilibrium will shift to the right. Raising temperature move shift the equilibrium leftward. A
rise in temperature in gases increases the rate of reaction shifting it leftward. The same case with
the introduction of a catalyst which shifts the reaction leftward.
The rate of the reaction: Lowering the temperature favors formation of products but reduces
the rate of reaction. An increase in pressure and introduction of a catalyst will speed up the rate
of reaction.
Ferric solution + electron –ferrous solution + electron
Give us
ΔG°= (0.77 V) time 1,83 v=-0.45
Then solve the last expression for ΔG° for the overall half-reaction,
Which is taken as ΔG° = F [(−1.83 Volte’s) + (−0.77)] = F (0.23 Volte’s)

Physical chemistry 1
All the three electron at the anode is (n = 3) and gets in the overall reaction. Therefore, you
substitute to the equation 1 which will be
And solve for E° gives the following:
ΔG°F (0.43 V) E°=−n FE node cell=−(4)(F) (E anode cell) =−1.13 V3=−1.043 V
(d) Calculating of enthalpy change of the electrode reaction and the rate of alteration of the cell
potential with temperature.
When calculating the entropy change of the n=3 and n=2 ions of iron in the system. We apply the
second law of entropy of universe as it required by the spontaneity
∆ H°f,298 of the n=3 is -205.8 kJ per moles so ∆H according to the information given above
reaction (i.e. the system) is -571.6 kJ. We know: ∆ H°(surroundings) = - ∆ H°(system) and: ∆S
(of environment) = ∆ H node (in the environment) / Temperature = - ∆ H node (system)
Temperature = + 500.6 x 10 3 / 298 = + 1920 J per mole
Therefore, the total entropy change, change of the universe = change of environment +
environment change = - 4067 + 1920 = + 1597 J per mole
Therefore, there is an overall elevation in the accumulation entropy of the environment,
according to the expectation from the second equation.
(e) It’s clear that the entropy increases because the reaction gives heat to the surrounding also the
standard electric potential of n=3 and n=2 involves combination of both positively charged ions.
Hence its referred to as exothermic. Following the second law of thermodynamics and second
law of entropy for I deal exothermic reaction must increase its entropy.
All the three electron at the anode is (n = 3) and gets in the overall reaction. Therefore, you
substitute to the equation 1 which will be
And solve for E° gives the following:
ΔG°F (0.43 V) E°=−n FE node cell=−(4)(F) (E anode cell) =−1.13 V3=−1.043 V
(d) Calculating of enthalpy change of the electrode reaction and the rate of alteration of the cell
potential with temperature.
When calculating the entropy change of the n=3 and n=2 ions of iron in the system. We apply the
second law of entropy of universe as it required by the spontaneity
∆ H°f,298 of the n=3 is -205.8 kJ per moles so ∆H according to the information given above
reaction (i.e. the system) is -571.6 kJ. We know: ∆ H°(surroundings) = - ∆ H°(system) and: ∆S
(of environment) = ∆ H node (in the environment) / Temperature = - ∆ H node (system)
Temperature = + 500.6 x 10 3 / 298 = + 1920 J per mole
Therefore, the total entropy change, change of the universe = change of environment +
environment change = - 4067 + 1920 = + 1597 J per mole
Therefore, there is an overall elevation in the accumulation entropy of the environment,
according to the expectation from the second equation.
(e) It’s clear that the entropy increases because the reaction gives heat to the surrounding also the
standard electric potential of n=3 and n=2 involves combination of both positively charged ions.
Hence its referred to as exothermic. Following the second law of thermodynamics and second
law of entropy for I deal exothermic reaction must increase its entropy.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Physical chemistry 1
Question3. kinetic reaction
(a) A change in “B” while “A” being held constant leads to a double change in the rate of
reaction. A change in “A” does not affect the rate of reaction.
Overall Reaction Order
1
2
3 Increasing rate of reaction
Determine k, Rate Constant (including units)
Rate = Change B/Change t = K (B)
0.02/0.01= 2-s
Take for example A and B is the reactant in this case therefore we can draw the energy detailed
based on the reaction.
2. Rahimpour, M.R., Dehnavi, M.R., Allahgholipour, F., Iranshahi, D. and Jokar, S.M., 2012.
Assessment and comparison of different catalytic coupling exothermic and endothermic
reactions: a review. Applied Energy, 99, pp.496-512.
Question3. kinetic reaction
(a) A change in “B” while “A” being held constant leads to a double change in the rate of
reaction. A change in “A” does not affect the rate of reaction.
Overall Reaction Order
1
2
3 Increasing rate of reaction
Determine k, Rate Constant (including units)
Rate = Change B/Change t = K (B)
0.02/0.01= 2-s
Take for example A and B is the reactant in this case therefore we can draw the energy detailed
based on the reaction.
2. Rahimpour, M.R., Dehnavi, M.R., Allahgholipour, F., Iranshahi, D. and Jokar, S.M., 2012.
Assessment and comparison of different catalytic coupling exothermic and endothermic
reactions: a review. Applied Energy, 99, pp.496-512.
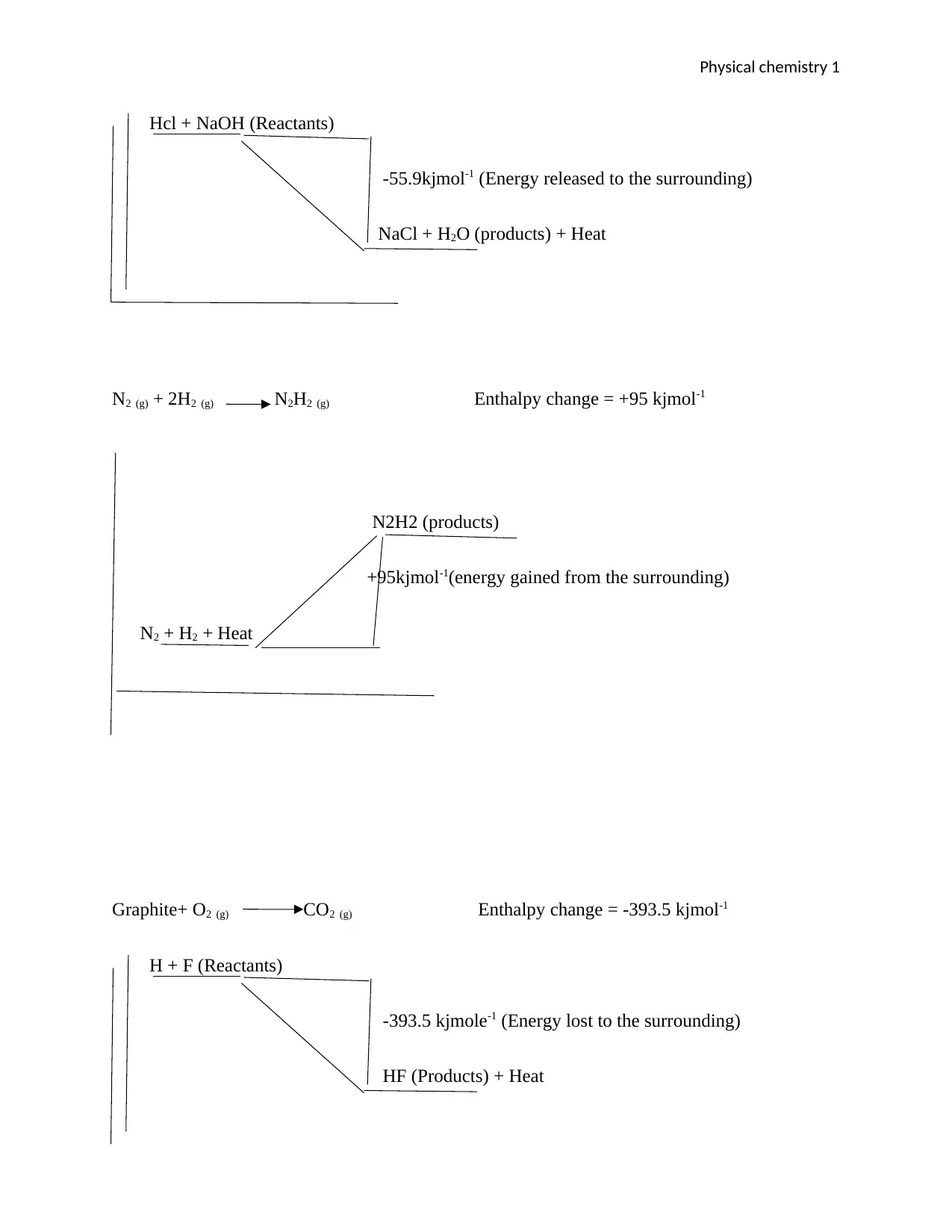
Physical chemistry 1
Hcl + NaOH (Reactants)
-55.9kjmol-1 (Energy released to the surrounding)
NaCl + H2O (products) + Heat
N2 (g) + 2H2 (g) N2H2 (g) Enthalpy change = +95 kjmol-1
N2H2 (products)
+95kjmol-1(energy gained from the surrounding)
N2 + H2 + Heat
Graphite+ O2 (g) CO2 (g) Enthalpy change = -393.5 kjmol-1
H + F (Reactants)
-393.5 kjmole-1 (Energy lost to the surrounding)
HF (Products) + Heat
Hcl + NaOH (Reactants)
-55.9kjmol-1 (Energy released to the surrounding)
NaCl + H2O (products) + Heat
N2 (g) + 2H2 (g) N2H2 (g) Enthalpy change = +95 kjmol-1
N2H2 (products)
+95kjmol-1(energy gained from the surrounding)
N2 + H2 + Heat
Graphite+ O2 (g) CO2 (g) Enthalpy change = -393.5 kjmol-1
H + F (Reactants)
-393.5 kjmole-1 (Energy lost to the surrounding)
HF (Products) + Heat

Physical chemistry 1
Estimation of the activation enthalpy, entropy and free energy in methanol. Based on the
values of kinetic movement such active entropy, activated of enthalpy, activated free-
energy, and r process is constant, for a series of nucleus that gets attraction to solution, are
computed basing on both a solution-phase translational entropy model and an perfect gas
stage changing entropy model. The product obtains from the aqueous stage in translation
entropy model are in good agreement with the practice values, while the high range of the
estimation of the activation of mobile energy from a perfect gas stage translational entropy.
For instance:
Moles of water and carbon dioxide = 0.06 moles
Mass of water = 0.06 x 18 = 1.08g, Mass of CO2 = 0.06 X 44 = 2.64 g
Total mass= 3.72 g
Specific heat of water and CO2=0.846 + 4.2 =5.046
Enthalpy change = 3.72 x 5.046 x 22
= + 412.964 kjmole-1
b) The entropy of activation in this case simply suggests a reaction of the two parameters
along the enthalpy of the activation which is basically obtained from the temperature
dependence of the reaction, the information is analyzed using the eyring law.
Question 4
graph of the aspartate of decarboxylase catalysis where the L-alanine is converted to L-aspartate
L-alanine + CO2, then the initial rate for different concentrations of L-aspirate can be followed
Estimation of the activation enthalpy, entropy and free energy in methanol. Based on the
values of kinetic movement such active entropy, activated of enthalpy, activated free-
energy, and r process is constant, for a series of nucleus that gets attraction to solution, are
computed basing on both a solution-phase translational entropy model and an perfect gas
stage changing entropy model. The product obtains from the aqueous stage in translation
entropy model are in good agreement with the practice values, while the high range of the
estimation of the activation of mobile energy from a perfect gas stage translational entropy.
For instance:
Moles of water and carbon dioxide = 0.06 moles
Mass of water = 0.06 x 18 = 1.08g, Mass of CO2 = 0.06 X 44 = 2.64 g
Total mass= 3.72 g
Specific heat of water and CO2=0.846 + 4.2 =5.046
Enthalpy change = 3.72 x 5.046 x 22
= + 412.964 kjmole-1
b) The entropy of activation in this case simply suggests a reaction of the two parameters
along the enthalpy of the activation which is basically obtained from the temperature
dependence of the reaction, the information is analyzed using the eyring law.
Question 4
graph of the aspartate of decarboxylase catalysis where the L-alanine is converted to L-aspartate
L-alanine + CO2, then the initial rate for different concentrations of L-aspirate can be followed
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Physical chemistry 1
by monitoring the volume of CO2 produced. The results of a series of such experiments are given
below
[L-aspartate]/ mol dm-3 Initial rate / mol CO2 min-1
20 14
30 19
40 23
50 28
100 42
200 53
(a)
0 2 4 6 8 10 12
0
2
4
6
8
10
12
a graph of aspartate vs inti ti al rate
Series1 Series2 Series3
The graphs of this kind give a curve for the aspartate because of high concentration of the
enzyme while the initial rate remains fairly constant (k). In aspartate graph increase slowly from
by monitoring the volume of CO2 produced. The results of a series of such experiments are given
below
[L-aspartate]/ mol dm-3 Initial rate / mol CO2 min-1
20 14
30 19
40 23
50 28
100 42
200 53
(a)
0 2 4 6 8 10 12
0
2
4
6
8
10
12
a graph of aspartate vs inti ti al rate
Series1 Series2 Series3
The graphs of this kind give a curve for the aspartate because of high concentration of the
enzyme while the initial rate remains fairly constant (k). In aspartate graph increase slowly from

Physical chemistry 1
zero to about 50 L-aspartate]/ mol dm. This because the enzyme is inactive due to some factors
such as temperature and nutrient media that are necessary. Also the environment is a major fact,
during the first line, the enzymes are adapting the new condition. Instant rise of the graph
indicates that every condition is now perfect. Therefore, the amount of carbon v oxide produced
is elevated in accordance to the amount of work done. The initial rate lacks inhibitor that is the
reason why is fairly constant. The factors can be:
Temperature: an increase in temperature increases the rate of reaction due to an increase in high
energy collisions (Twiddle 2010, Pg. 39).
Pressure: pressure increases the rate of reaction in gases but no effect on reaction involving
liquids and solids.
Catalyst: Presence of a catalyst increases the rate of reaction
Nature of reactants: Gases generally react faster than solids and liquids. Solids are the less
reactive among the three states of matter.
Surface area: Smaller particles react faster than larger particles
(b) If we add an inhibitor it is found that the maximum initial rate of reaction value remains
the same but the Michaelis constant changes. What does this tell you about the inhibition
mechanism? In addition of inhibitor to the enzyme there will be no change since
enzymes are specific in nature not all inhibitor will affect the enzyme hence a graph of
the enzyme shows no change. Apparently this inhibitor is not for this enzyme. But for
the initial rate there will be rise of the graph indicating the inhibitors has acted as catalyst.
zero to about 50 L-aspartate]/ mol dm. This because the enzyme is inactive due to some factors
such as temperature and nutrient media that are necessary. Also the environment is a major fact,
during the first line, the enzymes are adapting the new condition. Instant rise of the graph
indicates that every condition is now perfect. Therefore, the amount of carbon v oxide produced
is elevated in accordance to the amount of work done. The initial rate lacks inhibitor that is the
reason why is fairly constant. The factors can be:
Temperature: an increase in temperature increases the rate of reaction due to an increase in high
energy collisions (Twiddle 2010, Pg. 39).
Pressure: pressure increases the rate of reaction in gases but no effect on reaction involving
liquids and solids.
Catalyst: Presence of a catalyst increases the rate of reaction
Nature of reactants: Gases generally react faster than solids and liquids. Solids are the less
reactive among the three states of matter.
Surface area: Smaller particles react faster than larger particles
(b) If we add an inhibitor it is found that the maximum initial rate of reaction value remains
the same but the Michaelis constant changes. What does this tell you about the inhibition
mechanism? In addition of inhibitor to the enzyme there will be no change since
enzymes are specific in nature not all inhibitor will affect the enzyme hence a graph of
the enzyme shows no change. Apparently this inhibitor is not for this enzyme. But for
the initial rate there will be rise of the graph indicating the inhibitors has acted as catalyst.
1 out of 12
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
