Essential Chemistry Article 2022
Added on 2022-09-29
7 Pages1400 Words27 Views
Essential Chemistry 2
Boiling Temperatures of Compounds 2-
hexanone, 1-hexanol, 2-pentanone, 2-hexanol
Abstract: In organic chemistry, the melting and boiling points of organic compounds is a vital
property as it is used to offer other bodily and structural behaviours of the compounds. Liquids
boil while its gasoline stress equals the atmospheric strain. The kinetic electricity turns into
enough energy to break the bonds protecting the liquid particles together when boiling point is
reached. 2-hexanone, 2-pentanone and a couple of-hexanol are hydrocarbons having covalent
bonds. This article compares the boiling points of the above compares and the reason behind
their disparities.
Key words: Organic compounds. Hexanol. Hexanone. Pentanone. Boiling point
Table of Contents
Figure 1: Molecular structures of the four compounds.............................................................3
Table 1..............................................................................................................................................4
Introduction.....................................................................................................................................2
The boiling points...........................................................................................................................2
Conclusion........................................................................................................................................5
Reference.........................................................................................................................................6
1
Boiling Temperatures of Compounds 2-
hexanone, 1-hexanol, 2-pentanone, 2-hexanol
Abstract: In organic chemistry, the melting and boiling points of organic compounds is a vital
property as it is used to offer other bodily and structural behaviours of the compounds. Liquids
boil while its gasoline stress equals the atmospheric strain. The kinetic electricity turns into
enough energy to break the bonds protecting the liquid particles together when boiling point is
reached. 2-hexanone, 2-pentanone and a couple of-hexanol are hydrocarbons having covalent
bonds. This article compares the boiling points of the above compares and the reason behind
their disparities.
Key words: Organic compounds. Hexanol. Hexanone. Pentanone. Boiling point
Table of Contents
Figure 1: Molecular structures of the four compounds.............................................................3
Table 1..............................................................................................................................................4
Introduction.....................................................................................................................................2
The boiling points...........................................................................................................................2
Conclusion........................................................................................................................................5
Reference.........................................................................................................................................6
1

Essential Chemistry 2
Introduction
The differences between boiling points of different liquids can be explained using some
chemistry laws. A boiling point of a liquid is the steady temperature at which the liquid changes
it states from liquid to gases state without change in temperature. The heat energy is absorbed
and used to weaken the attraction forces between the liquid molecules causing the
intermolecular distance to increasing thus changing the state from liquid to gases (Ferris, 2018).
Boiling point temperatures of liquids is determined by masses of molecules and the strength of
intermolecular forces existing within the structures of the liquids and these forces include
various types of bonds such van der Waals forces and hydrogen bond (Spencer L. Seager, 2013).
Intermolecular forces are caused by bonding of molecules of substances and this forces give a
substance it’s physical characteristics. The boiling temperatures is directly proportional to the
molecular mass and strength of forces existing within the molecules of a liquid thus as the
forces and molecular mass goes up so does the boiling point temperatures.
The Boiling Points
These four compounds namely 1-hexanol, 2-hexanol, 2-hexanone and 2-pentanone have
different boiling point temperatures. Their boiling point temperatures are as shown in table 1
below. The boiling point temperatures of the compounds differ due to intermolecular forces
(S.K. Bhasin, 2013). There are various types of intermolecular forces including weak van der
Waals forces and hydrogen bonding. In our case, weak van der waals forces also termed as
weak London dispersion forces is evident in hydrocarbons whereby it exists between layers of
molecules while in hydrogen bonding dipolar force exists caused by hydrogen atoms (Pillon,
2016). As shown in table 1 below, 1-Hexanol (C6H14O) is first in table with a boiling temperature
of 157 oC, followed by 2-hexanol (C6H14O) having a boiling temperature of 137 oC. Their high
boiling is due to the high molecular mass caused by higher number of carbon atoms and in
2
Introduction
The differences between boiling points of different liquids can be explained using some
chemistry laws. A boiling point of a liquid is the steady temperature at which the liquid changes
it states from liquid to gases state without change in temperature. The heat energy is absorbed
and used to weaken the attraction forces between the liquid molecules causing the
intermolecular distance to increasing thus changing the state from liquid to gases (Ferris, 2018).
Boiling point temperatures of liquids is determined by masses of molecules and the strength of
intermolecular forces existing within the structures of the liquids and these forces include
various types of bonds such van der Waals forces and hydrogen bond (Spencer L. Seager, 2013).
Intermolecular forces are caused by bonding of molecules of substances and this forces give a
substance it’s physical characteristics. The boiling temperatures is directly proportional to the
molecular mass and strength of forces existing within the molecules of a liquid thus as the
forces and molecular mass goes up so does the boiling point temperatures.
The Boiling Points
These four compounds namely 1-hexanol, 2-hexanol, 2-hexanone and 2-pentanone have
different boiling point temperatures. Their boiling point temperatures are as shown in table 1
below. The boiling point temperatures of the compounds differ due to intermolecular forces
(S.K. Bhasin, 2013). There are various types of intermolecular forces including weak van der
Waals forces and hydrogen bonding. In our case, weak van der waals forces also termed as
weak London dispersion forces is evident in hydrocarbons whereby it exists between layers of
molecules while in hydrogen bonding dipolar force exists caused by hydrogen atoms (Pillon,
2016). As shown in table 1 below, 1-Hexanol (C6H14O) is first in table with a boiling temperature
of 157 oC, followed by 2-hexanol (C6H14O) having a boiling temperature of 137 oC. Their high
boiling is due to the high molecular mass caused by higher number of carbon atoms and in
2

Essential Chemistry 2
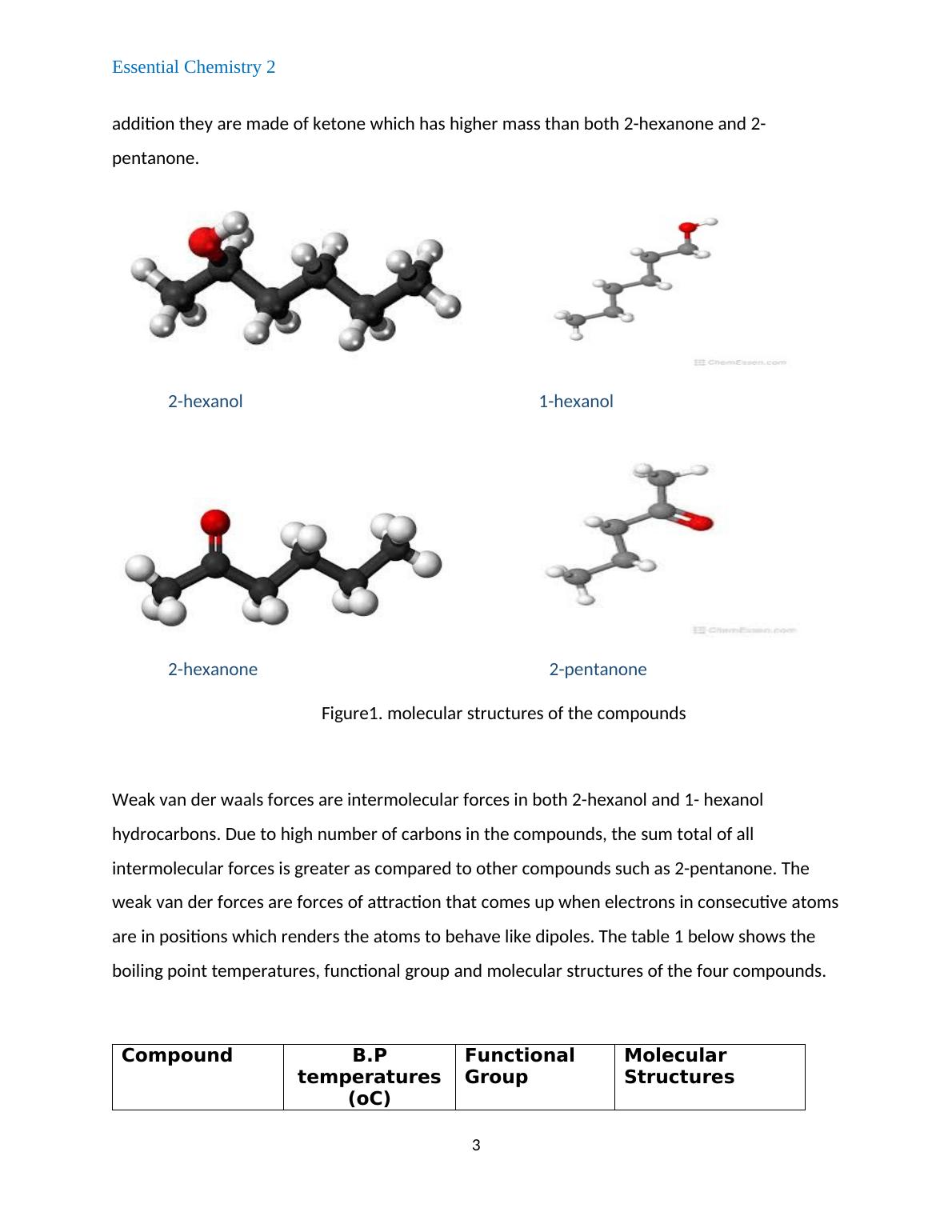
addition they are made of ketone which has higher mass than both 2-hexanone and 2-
pentanone.
2-hexanol 1-hexanol
2-hexanone 2-pentanone
Figure1. molecular structures of the compounds
Weak van der waals forces are intermolecular forces in both 2-hexanol and 1- hexanol
hydrocarbons. Due to high number of carbons in the compounds, the sum total of all
intermolecular forces is greater as compared to other compounds such as 2-pentanone. The
weak van der forces are forces of attraction that comes up when electrons in consecutive atoms
are in positions which renders the atoms to behave like dipoles. The table 1 below shows the
boiling point temperatures, functional group and molecular structures of the four compounds.
Compound B.P
temperatures
(oC)
Functional
Group
Molecular
Structures
3
addition they are made of ketone which has higher mass than both 2-hexanone and 2-
pentanone.
2-hexanol 1-hexanol
2-hexanone 2-pentanone
Figure1. molecular structures of the compounds
Weak van der waals forces are intermolecular forces in both 2-hexanol and 1- hexanol
hydrocarbons. Due to high number of carbons in the compounds, the sum total of all
intermolecular forces is greater as compared to other compounds such as 2-pentanone. The
weak van der forces are forces of attraction that comes up when electrons in consecutive atoms
are in positions which renders the atoms to behave like dipoles. The table 1 below shows the
boiling point temperatures, functional group and molecular structures of the four compounds.
Compound B.P
temperatures
(oC)
Functional
Group
Molecular
Structures
3
End of preview
Want to access all the pages? Upload your documents or become a member.
Related Documents
Boiling Temperatures of Compounds 2-hexanone, 1-hexanol, 2-pentanone, 2-hexanollg...
|5
|1371
|170
The Organic Chemistrylg...
|7
|1498
|818
Introduction On Chemistry - CHEM1010lg...
|4
|634
|132
Boiling Points of Organic Compoundslg...
|8
|1402
|17
Boiling Point and Intermolecular Forces in Aldehydes and Hydroxyl Group Compoundslg...
|8
|1622
|405
Boiling Points of Organic Compounds: Assignmentlg...
|7
|1307
|196
