Marketing of Classic and Specialist Cars: BMW Case Study Analysis
VerifiedAdded on 2021/01/03
|13
|3201
|99
Homework Assignment
AI Summary
This assignment is a research ethics application for a project titled "Marketing of classic and specialist cars," focusing on BMW as a case study. The application, submitted to the Ethics Panel at Plymouth Marjon University, includes investigator information, a checklist assessing ethical considerations, and details on data collection methods. The research involves human participants, specifically students, businessmen, and others, and utilizes qualitative and quantitative research methods to analyze how companies can promote their products and services effectively. The data will be collected through questionnaires and analysed by inspecting and examining the collected data. The application addresses risk levels, scholarly review, and participant consent, ensuring compliance with ethical guidelines. The project aims to understand how companies can attract different customer segments towards various cars. The research will be conducted at Plymouth Marjon University, and it has undergone scholarly review by a departmental colleague.

Ethics Application Code (provided by Ethics Panel upon submission):
Please submit your completed application to ethicspanel@marjon.ac.uk. Applications should be
saved as ONE pdf/word document, including all required documentation. All applicants should
complete Question 16 (signatures) as appropriate.
All forms and templates, and application deadlines, are provided via the staff intranet and PGR
Dashboard.
Please refer to the Guidance documents for Initial Research Ethics Checklist and Ethics Review
Protocol when completing this form.
.Title of Research Project
.”Marketing of classic and specialist cars”. A case study of BMW.
.Investigator Information
Principle investigator (or student’s name)
Name:
Department:
Institutional email:
Are co-investigators involved? (if student application, insert supervisor’s name)
If YES, please provide the names and institutional contact details of co-investigators, describe the
decision-making processes for collaborative research studies and if Terms of Reference exist, attach
them to the application.
1
Please submit your completed application to ethicspanel@marjon.ac.uk. Applications should be
saved as ONE pdf/word document, including all required documentation. All applicants should
complete Question 16 (signatures) as appropriate.
All forms and templates, and application deadlines, are provided via the staff intranet and PGR
Dashboard.
Please refer to the Guidance documents for Initial Research Ethics Checklist and Ethics Review
Protocol when completing this form.
.Title of Research Project
.”Marketing of classic and specialist cars”. A case study of BMW.
.Investigator Information
Principle investigator (or student’s name)
Name:
Department:
Institutional email:
Are co-investigators involved? (if student application, insert supervisor’s name)
If YES, please provide the names and institutional contact details of co-investigators, describe the
decision-making processes for collaborative research studies and if Terms of Reference exist, attach
them to the application.
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
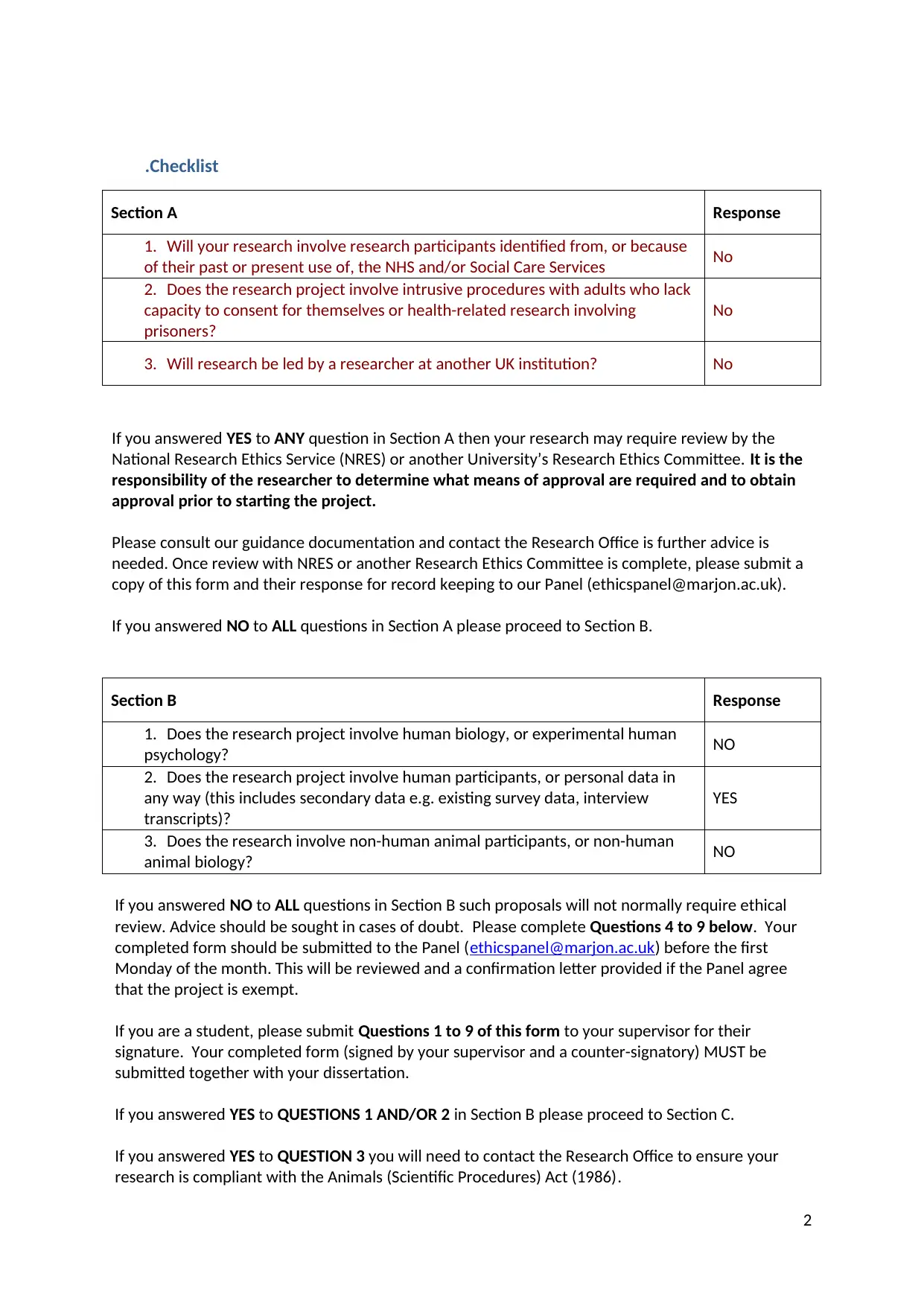
.Checklist
Section A Response
1. Will your research involve research participants identified from, or because
of their past or present use of, the NHS and/or Social Care Services No
2. Does the research project involve intrusive procedures with adults who lack
capacity to consent for themselves or health-related research involving
prisoners?
No
3. Will research be led by a researcher at another UK institution? No
If you answered YES to ANY question in Section A then your research may require review by the
National Research Ethics Service (NRES) or another University’s Research Ethics Committee. It is the
responsibility of the researcher to determine what means of approval are required and to obtain
approval prior to starting the project.
Please consult our guidance documentation and contact the Research Office is further advice is
needed. Once review with NRES or another Research Ethics Committee is complete, please submit a
copy of this form and their response for record keeping to our Panel (ethicspanel@marjon.ac.uk).
If you answered NO to ALL questions in Section A please proceed to Section B.
Section B Response
1. Does the research project involve human biology, or experimental human
psychology? NO
2. Does the research project involve human participants, or personal data in
any way (this includes secondary data e.g. existing survey data, interview
transcripts)?
YES
3. Does the research involve non-human animal participants, or non-human
animal biology? NO
If you answered NO to ALL questions in Section B such proposals will not normally require ethical
review. Advice should be sought in cases of doubt. Please complete Questions 4 to 9 below. Your
completed form should be submitted to the Panel (ethicspanel@marjon.ac.uk) before the first
Monday of the month. This will be reviewed and a confirmation letter provided if the Panel agree
that the project is exempt.
If you are a student, please submit Questions 1 to 9 of this form to your supervisor for their
signature. Your completed form (signed by your supervisor and a counter-signatory) MUST be
submitted together with your dissertation.
If you answered YES to QUESTIONS 1 AND/OR 2 in Section B please proceed to Section C.
If you answered YES to QUESTION 3 you will need to contact the Research Office to ensure your
research is compliant with the Animals (Scientific Procedures) Act (1986).
2
Section A Response
1. Will your research involve research participants identified from, or because
of their past or present use of, the NHS and/or Social Care Services No
2. Does the research project involve intrusive procedures with adults who lack
capacity to consent for themselves or health-related research involving
prisoners?
No
3. Will research be led by a researcher at another UK institution? No
If you answered YES to ANY question in Section A then your research may require review by the
National Research Ethics Service (NRES) or another University’s Research Ethics Committee. It is the
responsibility of the researcher to determine what means of approval are required and to obtain
approval prior to starting the project.
Please consult our guidance documentation and contact the Research Office is further advice is
needed. Once review with NRES or another Research Ethics Committee is complete, please submit a
copy of this form and their response for record keeping to our Panel (ethicspanel@marjon.ac.uk).
If you answered NO to ALL questions in Section A please proceed to Section B.
Section B Response
1. Does the research project involve human biology, or experimental human
psychology? NO
2. Does the research project involve human participants, or personal data in
any way (this includes secondary data e.g. existing survey data, interview
transcripts)?
YES
3. Does the research involve non-human animal participants, or non-human
animal biology? NO
If you answered NO to ALL questions in Section B such proposals will not normally require ethical
review. Advice should be sought in cases of doubt. Please complete Questions 4 to 9 below. Your
completed form should be submitted to the Panel (ethicspanel@marjon.ac.uk) before the first
Monday of the month. This will be reviewed and a confirmation letter provided if the Panel agree
that the project is exempt.
If you are a student, please submit Questions 1 to 9 of this form to your supervisor for their
signature. Your completed form (signed by your supervisor and a counter-signatory) MUST be
submitted together with your dissertation.
If you answered YES to QUESTIONS 1 AND/OR 2 in Section B please proceed to Section C.
If you answered YES to QUESTION 3 you will need to contact the Research Office to ensure your
research is compliant with the Animals (Scientific Procedures) Act (1986).
2
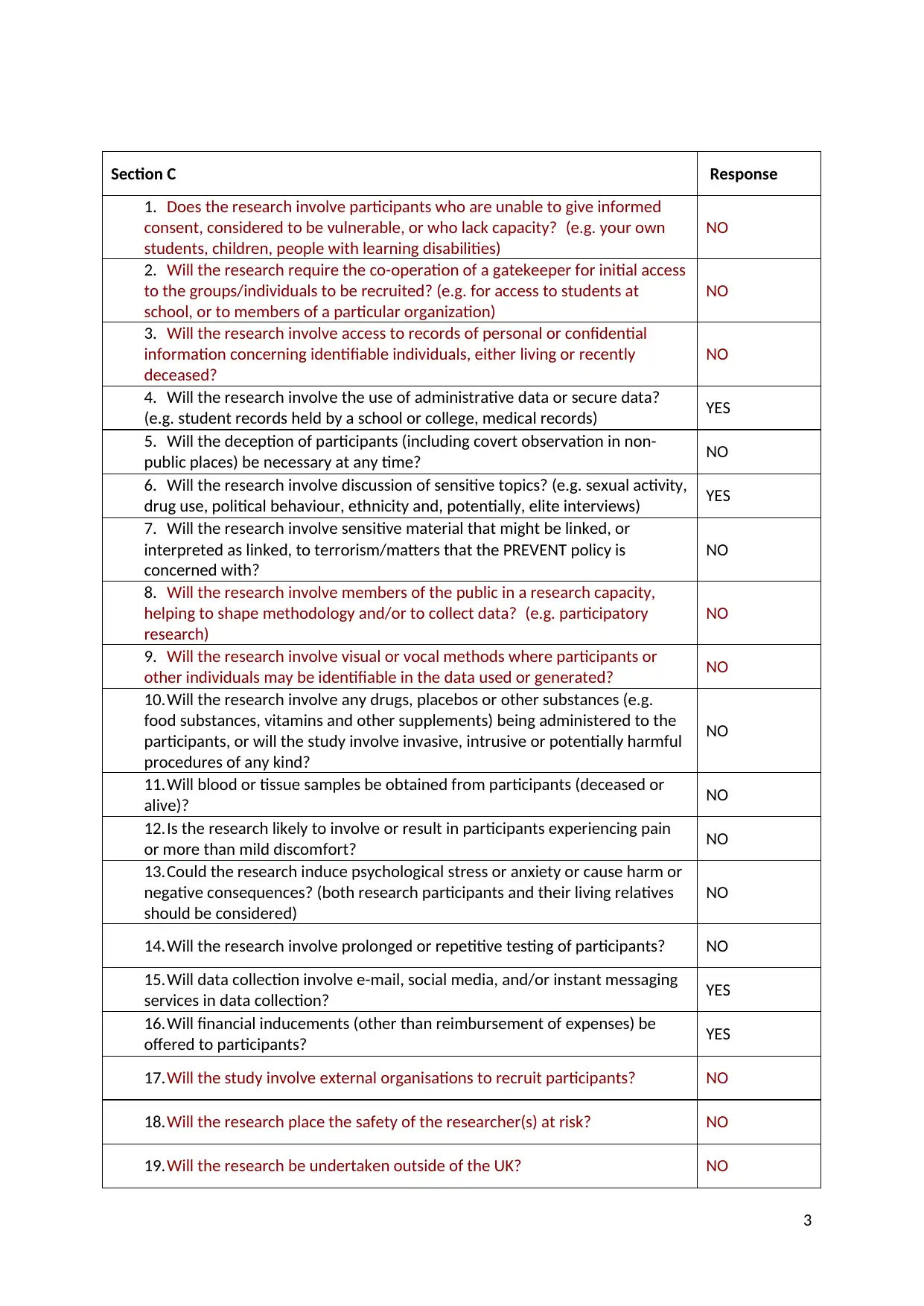
Section C Response
1. Does the research involve participants who are unable to give informed
consent, considered to be vulnerable, or who lack capacity? (e.g. your own
students, children, people with learning disabilities)
NO
2. Will the research require the co-operation of a gatekeeper for initial access
to the groups/individuals to be recruited? (e.g. for access to students at
school, or to members of a particular organization)
NO
3. Will the research involve access to records of personal or confidential
information concerning identifiable individuals, either living or recently
deceased?
NO
4. Will the research involve the use of administrative data or secure data?
(e.g. student records held by a school or college, medical records) YES
5. Will the deception of participants (including covert observation in non-
public places) be necessary at any time? NO
6. Will the research involve discussion of sensitive topics? (e.g. sexual activity,
drug use, political behaviour, ethnicity and, potentially, elite interviews) YES
7. Will the research involve sensitive material that might be linked, or
interpreted as linked, to terrorism/matters that the PREVENT policy is
concerned with?
NO
8. Will the research involve members of the public in a research capacity,
helping to shape methodology and/or to collect data? (e.g. participatory
research)
NO
9. Will the research involve visual or vocal methods where participants or
other individuals may be identifiable in the data used or generated? NO
10.Will the research involve any drugs, placebos or other substances (e.g.
food substances, vitamins and other supplements) being administered to the
participants, or will the study involve invasive, intrusive or potentially harmful
procedures of any kind?
NO
11.Will blood or tissue samples be obtained from participants (deceased or
alive)? NO
12.Is the research likely to involve or result in participants experiencing pain
or more than mild discomfort? NO
13.Could the research induce psychological stress or anxiety or cause harm or
negative consequences? (both research participants and their living relatives
should be considered)
NO
14.Will the research involve prolonged or repetitive testing of participants? NO
15.Will data collection involve e-mail, social media, and/or instant messaging
services in data collection? YES
16.Will financial inducements (other than reimbursement of expenses) be
offered to participants? YES
17.Will the study involve external organisations to recruit participants? NO
18.Will the research place the safety of the researcher(s) at risk? NO
19.Will the research be undertaken outside of the UK? NO
3
1. Does the research involve participants who are unable to give informed
consent, considered to be vulnerable, or who lack capacity? (e.g. your own
students, children, people with learning disabilities)
NO
2. Will the research require the co-operation of a gatekeeper for initial access
to the groups/individuals to be recruited? (e.g. for access to students at
school, or to members of a particular organization)
NO
3. Will the research involve access to records of personal or confidential
information concerning identifiable individuals, either living or recently
deceased?
NO
4. Will the research involve the use of administrative data or secure data?
(e.g. student records held by a school or college, medical records) YES
5. Will the deception of participants (including covert observation in non-
public places) be necessary at any time? NO
6. Will the research involve discussion of sensitive topics? (e.g. sexual activity,
drug use, political behaviour, ethnicity and, potentially, elite interviews) YES
7. Will the research involve sensitive material that might be linked, or
interpreted as linked, to terrorism/matters that the PREVENT policy is
concerned with?
NO
8. Will the research involve members of the public in a research capacity,
helping to shape methodology and/or to collect data? (e.g. participatory
research)
NO
9. Will the research involve visual or vocal methods where participants or
other individuals may be identifiable in the data used or generated? NO
10.Will the research involve any drugs, placebos or other substances (e.g.
food substances, vitamins and other supplements) being administered to the
participants, or will the study involve invasive, intrusive or potentially harmful
procedures of any kind?
NO
11.Will blood or tissue samples be obtained from participants (deceased or
alive)? NO
12.Is the research likely to involve or result in participants experiencing pain
or more than mild discomfort? NO
13.Could the research induce psychological stress or anxiety or cause harm or
negative consequences? (both research participants and their living relatives
should be considered)
NO
14.Will the research involve prolonged or repetitive testing of participants? NO
15.Will data collection involve e-mail, social media, and/or instant messaging
services in data collection? YES
16.Will financial inducements (other than reimbursement of expenses) be
offered to participants? YES
17.Will the study involve external organisations to recruit participants? NO
18.Will the research place the safety of the researcher(s) at risk? NO
19.Will the research be undertaken outside of the UK? NO
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
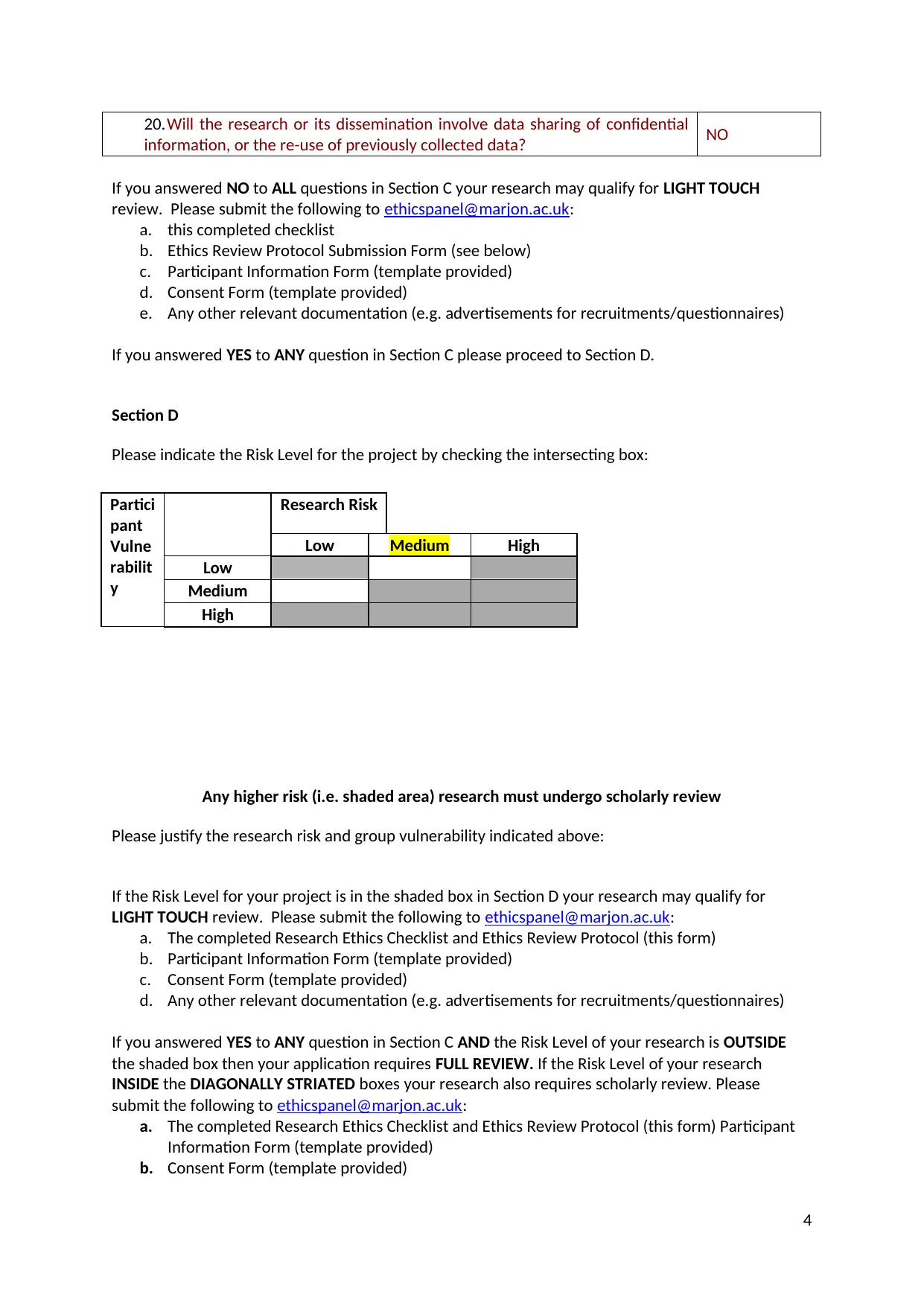
20.Will the research or its dissemination involve data sharing of confidential
information, or the re-use of previously collected data? NO
If you answered NO to ALL questions in Section C your research may qualify for LIGHT TOUCH
review. Please submit the following to ethicspanel@marjon.ac.uk:
a. this completed checklist
b. Ethics Review Protocol Submission Form (see below)
c. Participant Information Form (template provided)
d. Consent Form (template provided)
e. Any other relevant documentation (e.g. advertisements for recruitments/questionnaires)
If you answered YES to ANY question in Section C please proceed to Section D.
Section D
Please indicate the Risk Level for the project by checking the intersecting box:
Partici
pant
Vulne
rabilit
y
Research Risk
Low Medium High
Low
Medium
High
Any higher risk (i.e. shaded area) research must undergo scholarly review
Please justify the research risk and group vulnerability indicated above:
If the Risk Level for your project is in the shaded box in Section D your research may qualify for
LIGHT TOUCH review. Please submit the following to ethicspanel@marjon.ac.uk:
a. The completed Research Ethics Checklist and Ethics Review Protocol (this form)
b. Participant Information Form (template provided)
c. Consent Form (template provided)
d. Any other relevant documentation (e.g. advertisements for recruitments/questionnaires)
If you answered YES to ANY question in Section C AND the Risk Level of your research is OUTSIDE
the shaded box then your application requires FULL REVIEW. If the Risk Level of your research
INSIDE the DIAGONALLY STRIATED boxes your research also requires scholarly review. Please
submit the following to ethicspanel@marjon.ac.uk:
a. The completed Research Ethics Checklist and Ethics Review Protocol (this form) Participant
Information Form (template provided)
b. Consent Form (template provided)
4
information, or the re-use of previously collected data? NO
If you answered NO to ALL questions in Section C your research may qualify for LIGHT TOUCH
review. Please submit the following to ethicspanel@marjon.ac.uk:
a. this completed checklist
b. Ethics Review Protocol Submission Form (see below)
c. Participant Information Form (template provided)
d. Consent Form (template provided)
e. Any other relevant documentation (e.g. advertisements for recruitments/questionnaires)
If you answered YES to ANY question in Section C please proceed to Section D.
Section D
Please indicate the Risk Level for the project by checking the intersecting box:
Partici
pant
Vulne
rabilit
y
Research Risk
Low Medium High
Low
Medium
High
Any higher risk (i.e. shaded area) research must undergo scholarly review
Please justify the research risk and group vulnerability indicated above:
If the Risk Level for your project is in the shaded box in Section D your research may qualify for
LIGHT TOUCH review. Please submit the following to ethicspanel@marjon.ac.uk:
a. The completed Research Ethics Checklist and Ethics Review Protocol (this form)
b. Participant Information Form (template provided)
c. Consent Form (template provided)
d. Any other relevant documentation (e.g. advertisements for recruitments/questionnaires)
If you answered YES to ANY question in Section C AND the Risk Level of your research is OUTSIDE
the shaded box then your application requires FULL REVIEW. If the Risk Level of your research
INSIDE the DIAGONALLY STRIATED boxes your research also requires scholarly review. Please
submit the following to ethicspanel@marjon.ac.uk:
a. The completed Research Ethics Checklist and Ethics Review Protocol (this form) Participant
Information Form (template provided)
b. Consent Form (template provided)
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

c. A copy of the outcome of your scholarly review (this could be the review itself, or a message
indicating the outcome)
d. Any other relevant documentation (e.g. advertisements for recruitments/questionnaires)
5
indicating the outcome)
d. Any other relevant documentation (e.g. advertisements for recruitments/questionnaires)
5

.Other Organisations and Research Ethics Panel Approval(s)
a. Where will the research be conducted?
Plymouth Marjon University
If your research involves another institution or site please specify below:
Hospital specify site(s)
School/College/University specify site(s)
Community within the SW specify site(s)
International specify site(s)
Other specify site(s)
b. Have you received approval from the other institution to conduct this research? If
yes, please attach a copy of the approval to your application
c. Have any other Research Ethics Panel approved this project?
If yes, please attach a copy of the approval to your application
It is the responsibility of the research to determine what means of approval are required and to
obtain approval prior to starting the project
.Project Start and End Dates
Estimated start date for the component of this project that involves human participants or derived
data: 20.01.2019
Estimated completion date of involvement of human participants or their derived data for this
project : 25.04.2019
.Scholarly Review
Has the proposed research undergone scholarly review?
If YES, please indicate the type of review and provide a copy of the review:
Review by Departmental Colleague
Review by Plymouth Marjon University Colleague
Review by Department Head/Dean
Review by External Colleague
Review by Funding Agency
6
a. Where will the research be conducted?
Plymouth Marjon University
If your research involves another institution or site please specify below:
Hospital specify site(s)
School/College/University specify site(s)
Community within the SW specify site(s)
International specify site(s)
Other specify site(s)
b. Have you received approval from the other institution to conduct this research? If
yes, please attach a copy of the approval to your application
c. Have any other Research Ethics Panel approved this project?
If yes, please attach a copy of the approval to your application
It is the responsibility of the research to determine what means of approval are required and to
obtain approval prior to starting the project
.Project Start and End Dates
Estimated start date for the component of this project that involves human participants or derived
data: 20.01.2019
Estimated completion date of involvement of human participants or their derived data for this
project : 25.04.2019
.Scholarly Review
Has the proposed research undergone scholarly review?
If YES, please indicate the type of review and provide a copy of the review:
Review by Departmental Colleague
Review by Plymouth Marjon University Colleague
Review by Department Head/Dean
Review by External Colleague
Review by Funding Agency
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Any research posing greater than minimal risk (Section 11 below) requires scholarly review. Attach
a copy of your scholarly review, or any correspondence indicating the outcome of that review.
.Rationale
Describe your proposed research using, wherever possible, language understandable for a non-
specialist reader. You can, and should, include references as appropriate.
The importance of this research is to analyse the concept of marketing through which company can
easily promote variety of products and services among customers. With this study, it is possible for
respective company is to gain attention of different class people towards variety of cars.
.Methods
Describe where and how data will be collected and how they will be analysed.
The data will collected and gathered through ask questions to each and every respondents which
they are selected. This can be analysed by inspecting, examining and analysing data and information.
Include as appendices all questionnaires, interview guides, standard operating procedures and/or
other instruments to be used in data collection
7
a copy of your scholarly review, or any correspondence indicating the outcome of that review.
.Rationale
Describe your proposed research using, wherever possible, language understandable for a non-
specialist reader. You can, and should, include references as appropriate.
The importance of this research is to analyse the concept of marketing through which company can
easily promote variety of products and services among customers. With this study, it is possible for
respective company is to gain attention of different class people towards variety of cars.
.Methods
Describe where and how data will be collected and how they will be analysed.
The data will collected and gathered through ask questions to each and every respondents which
they are selected. This can be analysed by inspecting, examining and analysing data and information.
Include as appendices all questionnaires, interview guides, standard operating procedures and/or
other instruments to be used in data collection
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

.Participants and/or Data
a. Describe the participants to be recruited, or the individuals about whom personally
identifiable information will be collected.
The participants which are selected by researcher are students, businessmen and many
more.
b. Describe the types of data that will be collected and if appropriate how confidentiality will
be maintained.
There are mainly two types of research method that includes qualitative and
quantitative which assist in collecting and gathering accurate data. In this, an
investigator can use qualitative method which can assist in providing relevant
information regarding specific topic.
c. Does the proposed research involve participants who have a pre-existing relationship with
any of the investigators (e.g. instructor-student, clinician-patient, minister-congregant)?
If YES, please explain the relationships and how power differentials (actual or perceived) will
be managed.
No
d. Does the proposed research involve extraction or collection of personally identifiable
information about the participant (e.g. medical records)?
If YES, please explain how consent from the individuals or authorisation from the data
custodian will be obtained.
No
e. Does the proposed research result in products (physical or intellectual) that are
commercialisable ?
No
If YES, please explain how ownership will be negotiated and communicated to participants.
8
a. Describe the participants to be recruited, or the individuals about whom personally
identifiable information will be collected.
The participants which are selected by researcher are students, businessmen and many
more.
b. Describe the types of data that will be collected and if appropriate how confidentiality will
be maintained.
There are mainly two types of research method that includes qualitative and
quantitative which assist in collecting and gathering accurate data. In this, an
investigator can use qualitative method which can assist in providing relevant
information regarding specific topic.
c. Does the proposed research involve participants who have a pre-existing relationship with
any of the investigators (e.g. instructor-student, clinician-patient, minister-congregant)?
If YES, please explain the relationships and how power differentials (actual or perceived) will
be managed.
No
d. Does the proposed research involve extraction or collection of personally identifiable
information about the participant (e.g. medical records)?
If YES, please explain how consent from the individuals or authorisation from the data
custodian will be obtained.
No
e. Does the proposed research result in products (physical or intellectual) that are
commercialisable ?
No
If YES, please explain how ownership will be negotiated and communicated to participants.
8

.Experience of Investigators with this type of research
a. Please provide a brief description of previous experience with this type of research,
including data collection techniques, by the research team. If there has not been previous
experience, please describe how the researchers will be prepared.
I feel good while developing this research report and analyse new topic which are helpful in
gaining knowledge and information in better manner.
b. For projects that will involve community members (e.g. peer researchers) in the collection
and/or analysis of data, please describe their status within the research team (e.g. are they
considered employees, volunteers or participants) and what kind of training they will
receive?
The peer member are those who help researcher in order to complete specific research
report and analyse each topic in proper manner. Peers, employees and voluntaries are such
individual who are collect and gather all relevant data and information which are appropriate for
the researcher.
.Compensation
Will participants receive compensation for participation?
Financial
In-kind
Other
If YES, please provide details and justifications for the amount or the value of the compensation
offered and how will compensation be affected if participants chose to withdraw.
9
a. Please provide a brief description of previous experience with this type of research,
including data collection techniques, by the research team. If there has not been previous
experience, please describe how the researchers will be prepared.
I feel good while developing this research report and analyse new topic which are helpful in
gaining knowledge and information in better manner.
b. For projects that will involve community members (e.g. peer researchers) in the collection
and/or analysis of data, please describe their status within the research team (e.g. are they
considered employees, volunteers or participants) and what kind of training they will
receive?
The peer member are those who help researcher in order to complete specific research
report and analyse each topic in proper manner. Peers, employees and voluntaries are such
individual who are collect and gather all relevant data and information which are appropriate for
the researcher.
.Compensation
Will participants receive compensation for participation?
Financial
In-kind
Other
If YES, please provide details and justifications for the amount or the value of the compensation
offered and how will compensation be affected if participants chose to withdraw.
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

.Possible Risks
a. Please indicate all potential risks to participants as individuals or as a member of a
community that may arise from the research:
Physical (e.g. any bodily contact or administration of any substance):
Psychological/emotional (e.g. feeling uncomfortable or embarrassed:
Social (e.g. loss of status, privacy or reputation):
Legal (e.g. apprehension or arrest):
b. Please briefly describe each of the risks noted in 11(a), justify the indicated in Section D,
and outline the steps that will be taken to manage and/or minimise risks.
Sometime, physical risk is occurs when researcher are choose dangerous place or
location where they are conduct investigation. This will create risk for them and harm their
body.
.Possible Benefits
Describe any potential direct benefits to participants from their involvement in the
project as a result of this research. If there are potential direct benefits to the community, the
scientific/scholarly community or society as a result of this research, please also describe
these here.
They are gain benefits from participants who are involve in this research report and
analyse data and information in better manner. If they are select knowledgeable data and
information so this will assist them in developing accurate research project in effective way.
.Consent Process
a. Describe the process that will be used to obtain informed consent and explain how it will
be recorded.
The researcher use informed consent which assist them in collecting and gathering
appropriate data and information regarding research topic. This will assist in recording and storing
effective data which are helpful in analysing accurate point of an investigation. They are fill
email and send letters which are provided to them in order to examine their view point.
Please include as appendices any documents (e.g. consent documents, information letters,
email scripts) to be used in the consent process.
10
a. Please indicate all potential risks to participants as individuals or as a member of a
community that may arise from the research:
Physical (e.g. any bodily contact or administration of any substance):
Psychological/emotional (e.g. feeling uncomfortable or embarrassed:
Social (e.g. loss of status, privacy or reputation):
Legal (e.g. apprehension or arrest):
b. Please briefly describe each of the risks noted in 11(a), justify the indicated in Section D,
and outline the steps that will be taken to manage and/or minimise risks.
Sometime, physical risk is occurs when researcher are choose dangerous place or
location where they are conduct investigation. This will create risk for them and harm their
body.
.Possible Benefits
Describe any potential direct benefits to participants from their involvement in the
project as a result of this research. If there are potential direct benefits to the community, the
scientific/scholarly community or society as a result of this research, please also describe
these here.
They are gain benefits from participants who are involve in this research report and
analyse data and information in better manner. If they are select knowledgeable data and
information so this will assist them in developing accurate research project in effective way.
.Consent Process
a. Describe the process that will be used to obtain informed consent and explain how it will
be recorded.
The researcher use informed consent which assist them in collecting and gathering
appropriate data and information regarding research topic. This will assist in recording and storing
effective data which are helpful in analysing accurate point of an investigation. They are fill
email and send letters which are provided to them in order to examine their view point.
Please include as appendices any documents (e.g. consent documents, information letters,
email scripts) to be used in the consent process.
10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

b. Where applicable, please describe how participants will be informed of their right to
withdraw from the project and outline the procedures that will be followed to allow them
to exercise this right including timelines for withdrawal of data.
In this research report, participants are allow to withdraw data and information which
are given by them. This will assist in developing and making appropriate research project in
order to analyse or examine respondents view points and opinions effectively.
11
withdraw from the project and outline the procedures that will be followed to allow them
to exercise this right including timelines for withdrawal of data.
In this research report, participants are allow to withdraw data and information which
are given by them. This will assist in developing and making appropriate research project in
order to analyse or examine respondents view points and opinions effectively.
11

.Data Management
a. Will electronic data be stored in a place other than the Plymouth Marjon University secure
network drive?
b. Will electronic data be transferred between institutions?
c. Will a data sharing agreement be required?
d. Will data be retained for future research?
e. Will the data be disposed of securely?
If YES to any of the above questions, please provide an outline of data management processes for
your research and how this will be communicated to participants.
12
a. Will electronic data be stored in a place other than the Plymouth Marjon University secure
network drive?
b. Will electronic data be transferred between institutions?
c. Will a data sharing agreement be required?
d. Will data be retained for future research?
e. Will the data be disposed of securely?
If YES to any of the above questions, please provide an outline of data management processes for
your research and how this will be communicated to participants.
12
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 13
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





