Latrobe University CHEM101: Scenario-Based Problem Food as Energy
VerifiedAdded on 2023/06/10
|10
|3067
|450
Homework Assignment
AI Summary
This chemistry assignment, part of Latrobe University's CHEM101 course, focuses on food as a source of energy. The scenario-based problem examines the energy content of sucrose (sugar) and palmitic acid (fat) through the lens of aerobic respiration. Students are tasked with balancing chemical equations, calculating molar masses, determining energy released per gram, and comparing the potency of sugar versus fat as fuel sources. The assignment further investigates oxygen requirements for daily energy quotas, utilizing the ideal gas equation to calculate oxygen volume. Additionally, it explores oxygen transport via hemoglobin, examining equilibrium reactions and the impact of oxygen pressure. The problem set reinforces concepts of enthalpy, stoichiometry, and chemical kinetics, providing a comprehensive understanding of energy metabolism and related chemical principles.
MODULE 3 SCENARIO-BASED PROBLEM
FOOD AS ENERGY – FUEL FOR LIFE
Not only do we use energy for transportation, heating, lights, and countless devices but we also need
it to power our bodies. The food we eat provides energy for metabolism and physical activity. Aerobic
respiration involves oxidation of the nutrient molecule to produce carbon dioxide and water. It is the
same overall reaction as combustion, but proceeds by a very different pathway. The chemical energy
released is harnessed to produce adenosine triphosphate (ATP), commonly described as the body’s
energy “currency”. One molecule of palmitic acid for example makes about 130 molecules of ATP.
Recently, controversy has erupted over whether fat or sugar is the worst culprit in the obesity
epidemic. It is a complex story, but one property of nutrients that can be well determined is their
energy content.
In the first part of this scenario, your task is to assess which has a higher energy content, sugar or fat.
These generic classes of food will be represented by sucrose (table sugar) and palmitic acid, the most
abundant saturated fatty acid in food. You will use concepts of physical chemistry to examine aspects
of the energy content of food, how much oxygen is required for respiration, how that oxygen is
transported, and the chemical kinetic factors that determine how readily reactions occur.
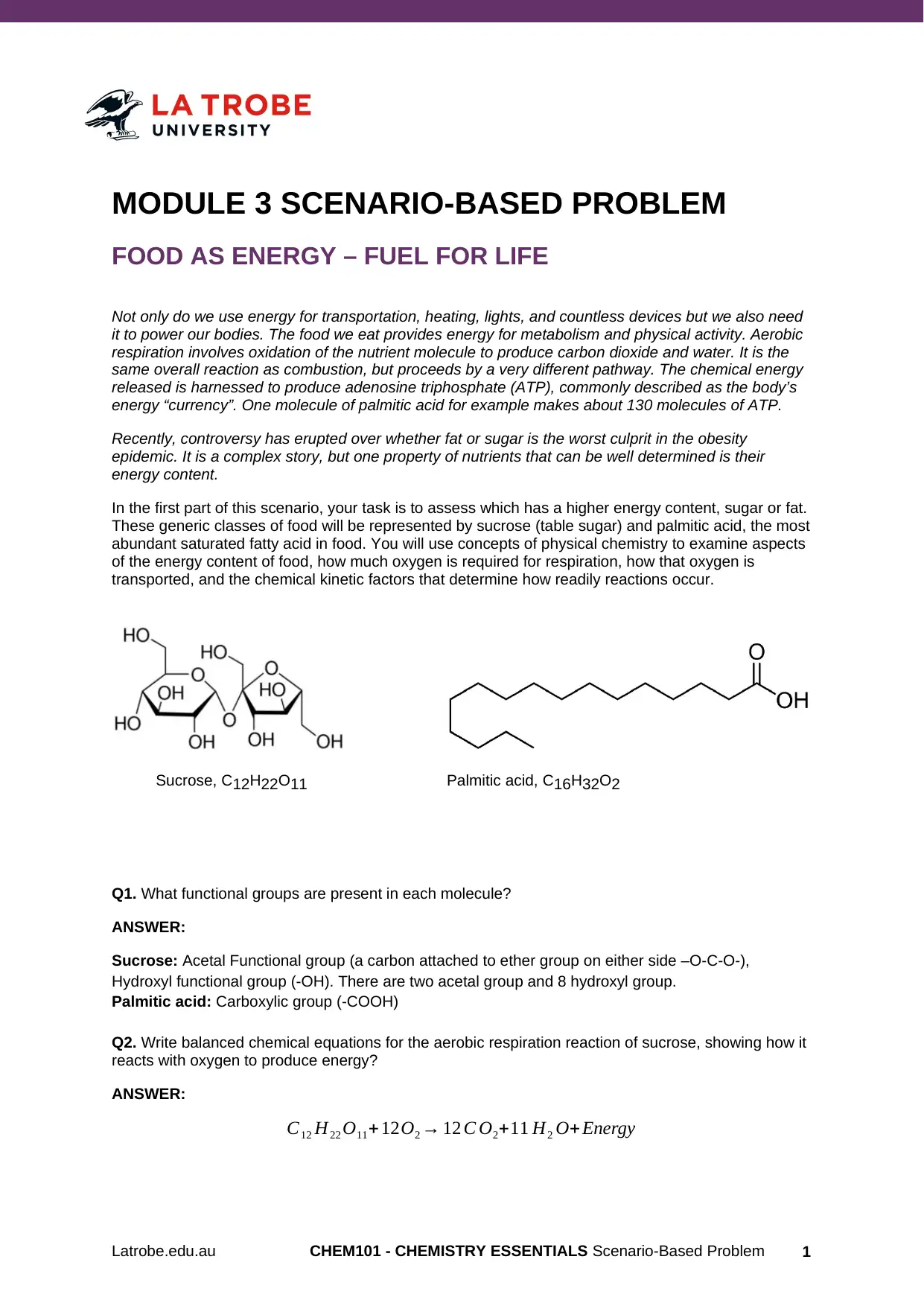
Sucrose, C12H22O11 Palmitic acid, C16H32O2
Q1. What functional groups are present in each molecule?
ANSWER:
Sucrose: Acetal Functional group (a carbon attached to ether group on either side –O-C-O-),
Hydroxyl functional group (-OH). There are two acetal group and 8 hydroxyl group.
Palmitic acid: Carboxylic group (-COOH)
Q2. Write balanced chemical equations for the aerobic respiration reaction of sucrose, showing how it
reacts with oxygen to produce energy?
ANSWER:
C12 H22 O11+ 12O2 → 12 C O2+11 H2 O+ Energy
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 1
FOOD AS ENERGY – FUEL FOR LIFE
Not only do we use energy for transportation, heating, lights, and countless devices but we also need
it to power our bodies. The food we eat provides energy for metabolism and physical activity. Aerobic
respiration involves oxidation of the nutrient molecule to produce carbon dioxide and water. It is the
same overall reaction as combustion, but proceeds by a very different pathway. The chemical energy
released is harnessed to produce adenosine triphosphate (ATP), commonly described as the body’s
energy “currency”. One molecule of palmitic acid for example makes about 130 molecules of ATP.
Recently, controversy has erupted over whether fat or sugar is the worst culprit in the obesity
epidemic. It is a complex story, but one property of nutrients that can be well determined is their
energy content.
In the first part of this scenario, your task is to assess which has a higher energy content, sugar or fat.
These generic classes of food will be represented by sucrose (table sugar) and palmitic acid, the most
abundant saturated fatty acid in food. You will use concepts of physical chemistry to examine aspects
of the energy content of food, how much oxygen is required for respiration, how that oxygen is
transported, and the chemical kinetic factors that determine how readily reactions occur.
Sucrose, C12H22O11 Palmitic acid, C16H32O2
Q1. What functional groups are present in each molecule?
ANSWER:
Sucrose: Acetal Functional group (a carbon attached to ether group on either side –O-C-O-),
Hydroxyl functional group (-OH). There are two acetal group and 8 hydroxyl group.
Palmitic acid: Carboxylic group (-COOH)
Q2. Write balanced chemical equations for the aerobic respiration reaction of sucrose, showing how it
reacts with oxygen to produce energy?
ANSWER:
C12 H22 O11+ 12O2 → 12 C O2+11 H2 O+ Energy
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Energy
It would be very difficult to directly measure the energy released in a respiration reaction that
proceeds via a complex biochemical pathway. However, thermodynamic enthalpy data is available for
an equivalent overall reaction that proceeds via combustion instead. Happily, the net enthalpy change
from reactants to products is the same regardless of how the reaction occurs (this is a central
postulate of thermodynamics called “Hess’s Law).
Q3. The molar enthalpy of combustion of sucrose, rHo = is -5645 kJ mol-1.
What term (one word) is used to describe a chemical reaction with a negative change in enthalpy?
ANSWER:
Negative change in enthalpy corresponds to the exothermic reaction i.e energy is released during the
reaction.
Q4. Calculate the molecular weights of both sucrose and palmitic acid, by summing the contributions
from C, H and O atoms and enter your data in the table below. (Useful data: relative atomic masses C
12.01, H 1.01, O 16.00).
ANSWER:
Element Sucrose Palmitic acid
C 12 ×12.01=144.12 16 ×12.01=192.16
H 22 ×1.01=22.22 32 ×1.01=32.32
O 11 ×16.00=176 2 ×16.00=32
Molecular weight (Total) 342.34 gram/mole 256.48 gram/mole
Q5. Calculate the number of moles in 1.00 g of sucrose and of palmitic acid. Enter your answers in
the table below. (Hint: n = m/MW).
ANSWER:
Sucrose
We know that molar mass of sucrose = 342.34 gram/mole
342.34 gram of sucrose will contain 1 mole of sucrose
1.00 gram of sucrose will contain 1
342.34 moleof sucrose
Therefore 1.00 gram of sucrose will contain 0.00292 mole of sucrose.
Palmitic acid
We know that molar mass of palmitic acid = 256.48 gram/mole
256.48 gram of palmitic acid will contain 1 mole of palmitic acid
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 2
It would be very difficult to directly measure the energy released in a respiration reaction that
proceeds via a complex biochemical pathway. However, thermodynamic enthalpy data is available for
an equivalent overall reaction that proceeds via combustion instead. Happily, the net enthalpy change
from reactants to products is the same regardless of how the reaction occurs (this is a central
postulate of thermodynamics called “Hess’s Law).
Q3. The molar enthalpy of combustion of sucrose, rHo = is -5645 kJ mol-1.
What term (one word) is used to describe a chemical reaction with a negative change in enthalpy?
ANSWER:
Negative change in enthalpy corresponds to the exothermic reaction i.e energy is released during the
reaction.
Q4. Calculate the molecular weights of both sucrose and palmitic acid, by summing the contributions
from C, H and O atoms and enter your data in the table below. (Useful data: relative atomic masses C
12.01, H 1.01, O 16.00).
ANSWER:
Element Sucrose Palmitic acid
C 12 ×12.01=144.12 16 ×12.01=192.16
H 22 ×1.01=22.22 32 ×1.01=32.32
O 11 ×16.00=176 2 ×16.00=32
Molecular weight (Total) 342.34 gram/mole 256.48 gram/mole
Q5. Calculate the number of moles in 1.00 g of sucrose and of palmitic acid. Enter your answers in
the table below. (Hint: n = m/MW).
ANSWER:
Sucrose
We know that molar mass of sucrose = 342.34 gram/mole
342.34 gram of sucrose will contain 1 mole of sucrose
1.00 gram of sucrose will contain 1
342.34 moleof sucrose
Therefore 1.00 gram of sucrose will contain 0.00292 mole of sucrose.
Palmitic acid
We know that molar mass of palmitic acid = 256.48 gram/mole
256.48 gram of palmitic acid will contain 1 mole of palmitic acid
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 2

1.00 gram of palmitic acid will contain 1
256.48 moleof palmitic acid
Therefore 1.00 gram of palmitic acid will contain 0.00389 mole of palmitic acid.
Q6. Calculate the energy released by respiration of 1.00 g of sucrose and of palmitic acid. Enter your
answers in the table below. (hint: use the number of mol from the previous Q, along with the molar
enthalpy of combustion)
Answer:
Sucrose
C12 H22 O11+ 12O2 → 12 C O2+11 H2 O+ energy ∆ H =−5645 kJ /mol
As we did calculation in question 5, that 1.00 gram of sucrose contains 0.00292 mole
According to the balanced reaction, enthalpy change is the release in energy i.e
1 mole of sucrose produces 5645 kJ of energy
So, 0.00292 mole of sucrose will produce 5645 ×0.00292 kJof energy
Therefore energy released by respiration of 1.00 gram of sucrose will be 16.483 kJ
Palmitic acid
C16 H32 O2 +23 O2 → 16 C O2+ 16 H2 O+energy ∆ H=−9978 kJ /mol
As we did calculation in question 5, that 1.00 gram of palmitic acid contains 0.00389 mole
According to the balanced reaction, enthalpy change is the release in energy i.e
1 mole of palmitic acid produces 9978 kJ of energy
So, 0.00292 mole of palmitic acid will produce 9978 × 0.00389kJ of energy
Therefore energy released by respiration of 1.00 gram of palmitic acid will be 38.814 kJ
Q7. Based on the energy released in respiration per gram, which is the more potent fuel source, sugar
or fat? Is this consistent with the notion that each new CO bond made via oxidation releases energy?
ANSWER:
From the above calculation in question 6, we see that for the same of amount of sucrose (sugar) and
palmitic acid (fat), respiration of palmitic acid produces more amount of energy than sucrose (more
than double).
Therefore, we can say that fat is more potent fuel source than sugar.
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 3
256.48 moleof palmitic acid
Therefore 1.00 gram of palmitic acid will contain 0.00389 mole of palmitic acid.
Q6. Calculate the energy released by respiration of 1.00 g of sucrose and of palmitic acid. Enter your
answers in the table below. (hint: use the number of mol from the previous Q, along with the molar
enthalpy of combustion)
Answer:
Sucrose
C12 H22 O11+ 12O2 → 12 C O2+11 H2 O+ energy ∆ H =−5645 kJ /mol
As we did calculation in question 5, that 1.00 gram of sucrose contains 0.00292 mole
According to the balanced reaction, enthalpy change is the release in energy i.e
1 mole of sucrose produces 5645 kJ of energy
So, 0.00292 mole of sucrose will produce 5645 ×0.00292 kJof energy
Therefore energy released by respiration of 1.00 gram of sucrose will be 16.483 kJ
Palmitic acid
C16 H32 O2 +23 O2 → 16 C O2+ 16 H2 O+energy ∆ H=−9978 kJ /mol
As we did calculation in question 5, that 1.00 gram of palmitic acid contains 0.00389 mole
According to the balanced reaction, enthalpy change is the release in energy i.e
1 mole of palmitic acid produces 9978 kJ of energy
So, 0.00292 mole of palmitic acid will produce 9978 × 0.00389kJ of energy
Therefore energy released by respiration of 1.00 gram of palmitic acid will be 38.814 kJ
Q7. Based on the energy released in respiration per gram, which is the more potent fuel source, sugar
or fat? Is this consistent with the notion that each new CO bond made via oxidation releases energy?
ANSWER:
From the above calculation in question 6, we see that for the same of amount of sucrose (sugar) and
palmitic acid (fat), respiration of palmitic acid produces more amount of energy than sucrose (more
than double).
Therefore, we can say that fat is more potent fuel source than sugar.
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Balanced chemical reaction comparison shows that more number of CO2 (i.e 16 molecule of CO2) is
produced in case of palmitic acid than the CO2 produced in the case of sucrose.
C16 H32 O2 +23 O2 → 16 C O2+ 16 H2 O+energy ∆ H=−9978 kJ
mol (Palmitic acid)
C12 H22 O11+12O2 → 12 C O2+11 H2 O+ energy ∆ H =−5645 kJ
mol ( Sucrose)
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 4
produced in case of palmitic acid than the CO2 produced in the case of sucrose.
C16 H32 O2 +23 O2 → 16 C O2+ 16 H2 O+energy ∆ H=−9978 kJ
mol (Palmitic acid)
C12 H22 O11+12O2 → 12 C O2+11 H2 O+ energy ∆ H =−5645 kJ
mol ( Sucrose)
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
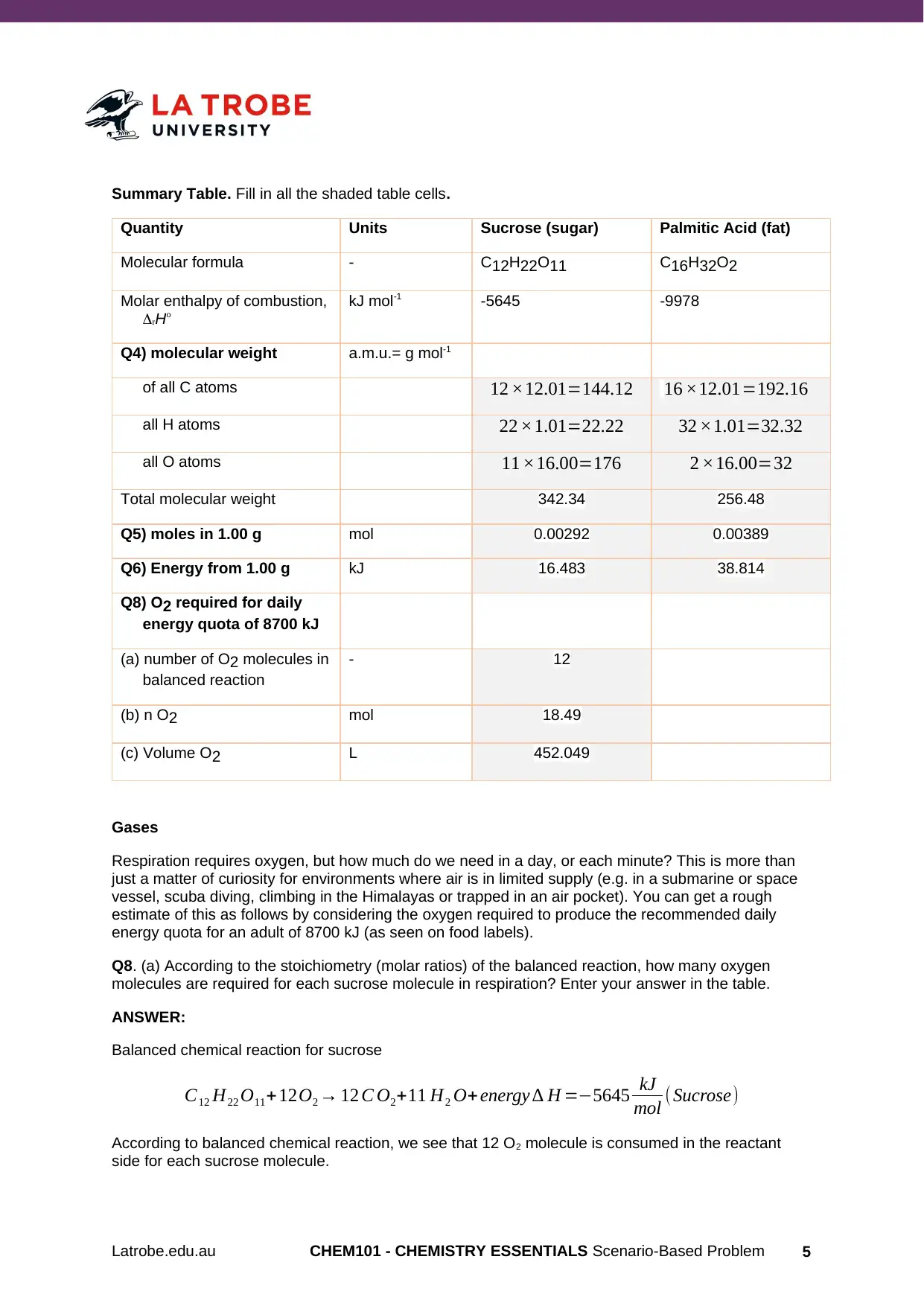
Summary Table. Fill in all the shaded table cells.
Quantity Units Sucrose (sugar) Palmitic Acid (fat)
Molecular formula - C12H22O11 C16H32O2
Molar enthalpy of combustion,
rHo
kJ mol-1 -5645 -9978
Q4) molecular weight a.m.u.= g mol-1
of all C atoms 12 ×12.01=144.12 16 ×12.01=192.16
all H atoms 22 ×1.01=22.22 32 ×1.01=32.32
all O atoms 11 ×16.00=176 2 ×16.00=32
Total molecular weight 342.34 256.48
Q5) moles in 1.00 g mol 0.00292 0.00389
Q6) Energy from 1.00 g kJ 16.483 38.814
Q8) O2 required for daily
energy quota of 8700 kJ
(a) number of O2 molecules in
balanced reaction
- 12
(b) n O2 mol 18.49
(c) Volume O2 L 452.049
Gases
Respiration requires oxygen, but how much do we need in a day, or each minute? This is more than
just a matter of curiosity for environments where air is in limited supply (e.g. in a submarine or space
vessel, scuba diving, climbing in the Himalayas or trapped in an air pocket). You can get a rough
estimate of this as follows by considering the oxygen required to produce the recommended daily
energy quota for an adult of 8700 kJ (as seen on food labels).
Q8. (a) According to the stoichiometry (molar ratios) of the balanced reaction, how many oxygen
molecules are required for each sucrose molecule in respiration? Enter your answer in the table.
ANSWER:
Balanced chemical reaction for sucrose
C12 H22 O11+12O2 → 12 C O2+11 H2 O+ energy ∆ H =−5645 kJ
mol ( Sucrose)
According to balanced chemical reaction, we see that 12 O2 molecule is consumed in the reactant
side for each sucrose molecule.
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 5
Quantity Units Sucrose (sugar) Palmitic Acid (fat)
Molecular formula - C12H22O11 C16H32O2
Molar enthalpy of combustion,
rHo
kJ mol-1 -5645 -9978
Q4) molecular weight a.m.u.= g mol-1
of all C atoms 12 ×12.01=144.12 16 ×12.01=192.16
all H atoms 22 ×1.01=22.22 32 ×1.01=32.32
all O atoms 11 ×16.00=176 2 ×16.00=32
Total molecular weight 342.34 256.48
Q5) moles in 1.00 g mol 0.00292 0.00389
Q6) Energy from 1.00 g kJ 16.483 38.814
Q8) O2 required for daily
energy quota of 8700 kJ
(a) number of O2 molecules in
balanced reaction
- 12
(b) n O2 mol 18.49
(c) Volume O2 L 452.049
Gases
Respiration requires oxygen, but how much do we need in a day, or each minute? This is more than
just a matter of curiosity for environments where air is in limited supply (e.g. in a submarine or space
vessel, scuba diving, climbing in the Himalayas or trapped in an air pocket). You can get a rough
estimate of this as follows by considering the oxygen required to produce the recommended daily
energy quota for an adult of 8700 kJ (as seen on food labels).
Q8. (a) According to the stoichiometry (molar ratios) of the balanced reaction, how many oxygen
molecules are required for each sucrose molecule in respiration? Enter your answer in the table.
ANSWER:
Balanced chemical reaction for sucrose
C12 H22 O11+12O2 → 12 C O2+11 H2 O+ energy ∆ H =−5645 kJ
mol ( Sucrose)
According to balanced chemical reaction, we see that 12 O2 molecule is consumed in the reactant
side for each sucrose molecule.
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 5

(b) How many mole of O2 are required to generate 8700 kJ of energy from sucrose?
(The appendix provides examples of this type of calculation.)
Answer:
C12 H22 O11+12O2 → 12 C O2+11 H2 O+ energy ∆ H =−5645 kJ
mol ( Sucrose)
From the stoichiometry relation, we see that 1 mole of sucrose and 12 moles of O2 is required to
produce 5645 kJ of energy.
We can rewrite the statement as,
5645 kJ of energy is released when 12 moles of O2 is consumed in the reactant side.
So, 8700 kJ of energy is released when 12
5645 ×8700 moles of O2 is consumed.
Therefore, 18.49 mole of O2 is required to produce 8700 kJ of energy.
(c) Use the ideal gas equation to calculate the corresponding volume of oxygen gas at standard
atmospheric temperature and pressure (SATP) of 25 ºC and 100 kPa (1 bar)? Enter your answer in
the table.
ANSWER:
Total moles of O2 required, n=18.49
Temperature, T= 25 ℃= ( 25+273.15 ) K=298.15 K
Pressure, P= 100kPa= 1 atm
Gas constant, R= 0.082 Latm K-1mol-1
Applying ideal gas equation, we have
PV =nRT
Plugging the given values, we have
1 atm ×V =18.49 mole 0.082 L . atm . K−1 mol−1 × 298.15 K
Unknown volume (V) can be found from the above equation.
V =18.49 × 0.082× 298.15=452.049 Litres
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 6
(The appendix provides examples of this type of calculation.)
Answer:
C12 H22 O11+12O2 → 12 C O2+11 H2 O+ energy ∆ H =−5645 kJ
mol ( Sucrose)
From the stoichiometry relation, we see that 1 mole of sucrose and 12 moles of O2 is required to
produce 5645 kJ of energy.
We can rewrite the statement as,
5645 kJ of energy is released when 12 moles of O2 is consumed in the reactant side.
So, 8700 kJ of energy is released when 12
5645 ×8700 moles of O2 is consumed.
Therefore, 18.49 mole of O2 is required to produce 8700 kJ of energy.
(c) Use the ideal gas equation to calculate the corresponding volume of oxygen gas at standard
atmospheric temperature and pressure (SATP) of 25 ºC and 100 kPa (1 bar)? Enter your answer in
the table.
ANSWER:
Total moles of O2 required, n=18.49
Temperature, T= 25 ℃= ( 25+273.15 ) K=298.15 K
Pressure, P= 100kPa= 1 atm
Gas constant, R= 0.082 Latm K-1mol-1
Applying ideal gas equation, we have
PV =nRT
Plugging the given values, we have
1 atm ×V =18.49 mole 0.082 L . atm . K−1 mol−1 × 298.15 K
Unknown volume (V) can be found from the above equation.
V =18.49 × 0.082× 298.15=452.049 Litres
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
(d) State at least two limitations/assumptions in this method of estimation.
ANSWER:
Two assumption/limitations of ideal gas
1. Gas particles i.e oxygen are assumed to be of negligible volume.
2. Intermolecular interaction is assumed to be negligible.
Extra material for fitness fanatics… (not assessed)
One measure of fitness is VO2 max, the volume of oxygen that an athlete can use during exercise. It
is measured in millilitres per kilogram of body weight per minute (mL kg-1 min-1). A moderately fit
person may have a VO2 max value of 40, an elite athlete 80. For comparison, the daily VO2 value
from the previous question can be converted into these units as follows:
VO2 resting ( mL kg-1 min−1)=VO2(L day-1 )×
( 1000(mL/L )
24×60 (min day-1 )(60 kg ) )
By what factor does O2 (or energy) consumption increase during exercise?
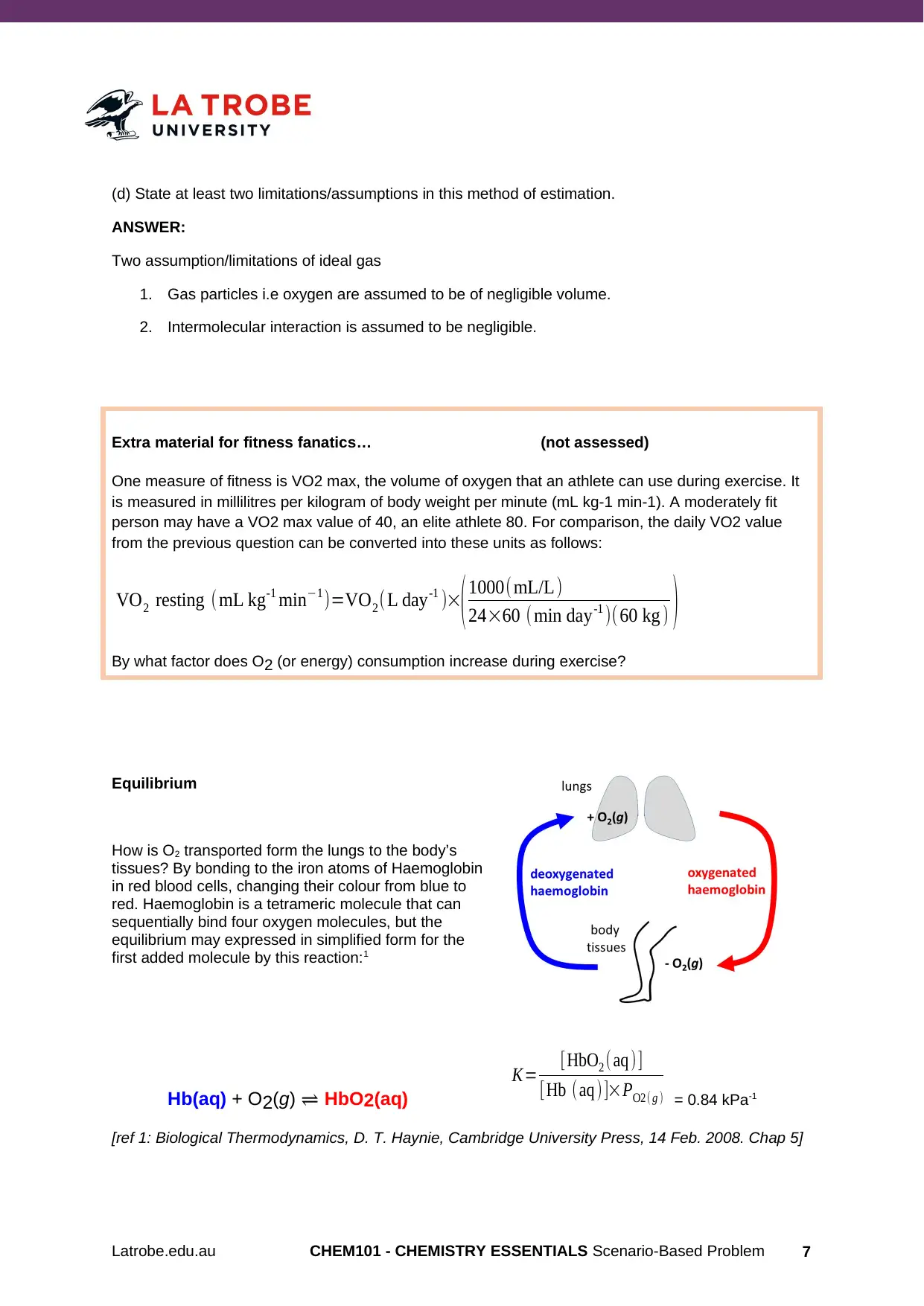
Equilibrium
How is O2 transported form the lungs to the body’s
tissues? By bonding to the iron atoms of Haemoglobin
in red blood cells, changing their colour from blue to
red. Haemoglobin is a tetrameric molecule that can
sequentially bind four oxygen molecules, but the
equilibrium may expressed in simplified form for the
first added molecule by this reaction:1
Hb(aq) + O2(g) ⇌ HbO2(aq)
K= [HbO2 ( aq )]
[Hb ( aq )]×PO2 ( g ) = 0.84 kPa-1
[ref 1: Biological Thermodynamics, D. T. Haynie, Cambridge University Press, 14 Feb. 2008. Chap 5]
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 7
ANSWER:
Two assumption/limitations of ideal gas
1. Gas particles i.e oxygen are assumed to be of negligible volume.
2. Intermolecular interaction is assumed to be negligible.
Extra material for fitness fanatics… (not assessed)
One measure of fitness is VO2 max, the volume of oxygen that an athlete can use during exercise. It
is measured in millilitres per kilogram of body weight per minute (mL kg-1 min-1). A moderately fit
person may have a VO2 max value of 40, an elite athlete 80. For comparison, the daily VO2 value
from the previous question can be converted into these units as follows:
VO2 resting ( mL kg-1 min−1)=VO2(L day-1 )×
( 1000(mL/L )
24×60 (min day-1 )(60 kg ) )
By what factor does O2 (or energy) consumption increase during exercise?
Equilibrium
How is O2 transported form the lungs to the body’s
tissues? By bonding to the iron atoms of Haemoglobin
in red blood cells, changing their colour from blue to
red. Haemoglobin is a tetrameric molecule that can
sequentially bind four oxygen molecules, but the
equilibrium may expressed in simplified form for the
first added molecule by this reaction:1
Hb(aq) + O2(g) ⇌ HbO2(aq)
K= [HbO2 ( aq )]
[Hb ( aq )]×PO2 ( g ) = 0.84 kPa-1
[ref 1: Biological Thermodynamics, D. T. Haynie, Cambridge University Press, 14 Feb. 2008. Chap 5]
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
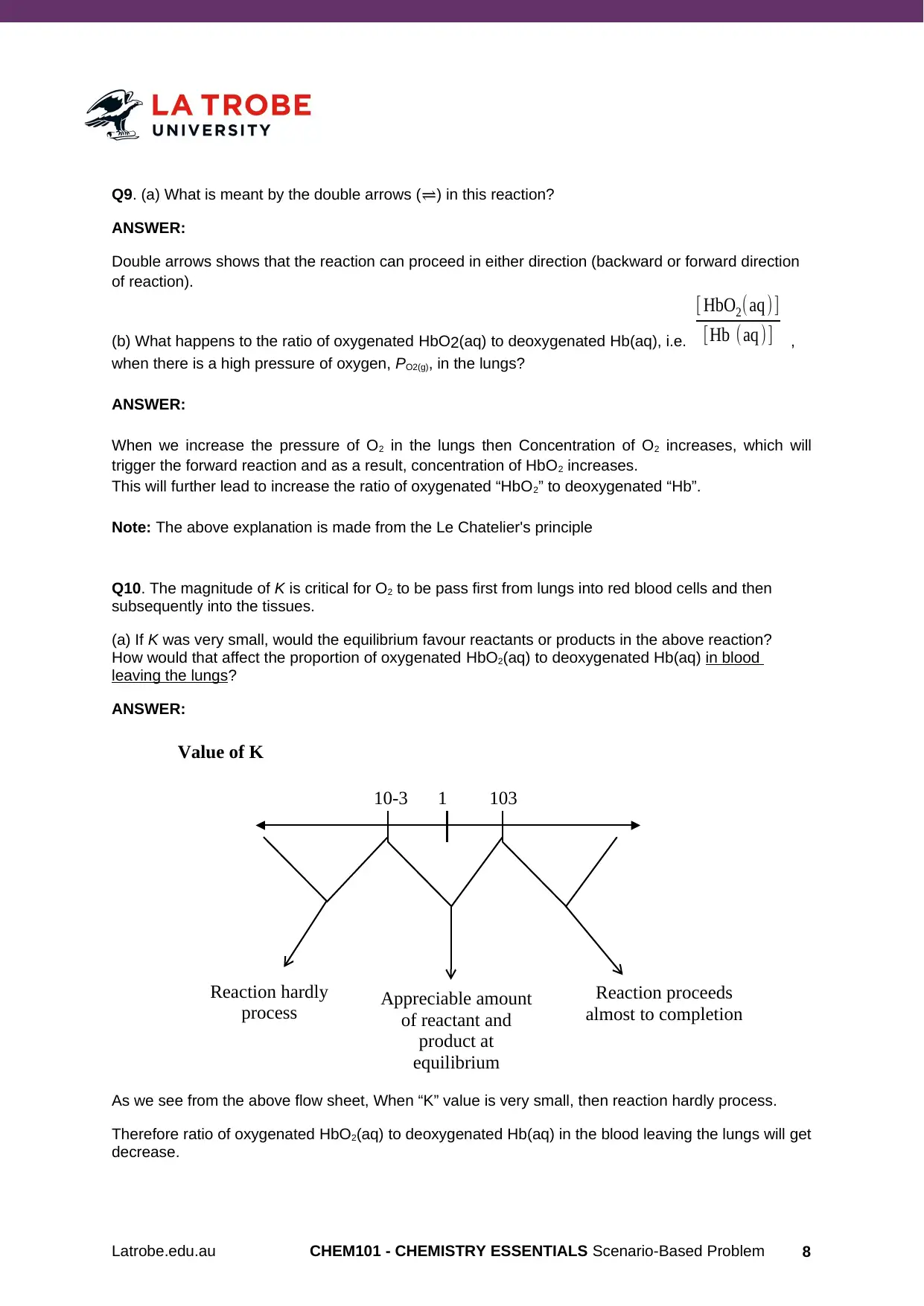
10310-3 1
Reaction hardly
process Appreciable amount
of reactant and
product at
equilibrium
Reaction proceeds
almost to completion
Value of K
Q9. (a) What is meant by the double arrows (⇌) in this reaction?
ANSWER:
Double arrows shows that the reaction can proceed in either direction (backward or forward direction
of reaction).
(b) What happens to the ratio of oxygenated HbO2(aq) to deoxygenated Hb(aq), i.e.
[ HbO2(aq ) ]
[Hb (aq )] ,
when there is a high pressure of oxygen, PO2(g), in the lungs?
ANSWER:
When we increase the pressure of O2 in the lungs then Concentration of O2 increases, which will
trigger the forward reaction and as a result, concentration of HbO2 increases.
This will further lead to increase the ratio of oxygenated “HbO2” to deoxygenated “Hb”.
Note: The above explanation is made from the Le Chatelier's principle
Q10. The magnitude of K is critical for O2 to be pass first from lungs into red blood cells and then
subsequently into the tissues.
(a) If K was very small, would the equilibrium favour reactants or products in the above reaction?
How would that affect the proportion of oxygenated HbO2(aq) to deoxygenated Hb(aq) in blood
leaving the lungs?
ANSWER:
As we see from the above flow sheet, When “K” value is very small, then reaction hardly process.
Therefore ratio of oxygenated HbO2(aq) to deoxygenated Hb(aq) in the blood leaving the lungs will get
decrease.
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 8
Reaction hardly
process Appreciable amount
of reactant and
product at
equilibrium
Reaction proceeds
almost to completion
Value of K
Q9. (a) What is meant by the double arrows (⇌) in this reaction?
ANSWER:
Double arrows shows that the reaction can proceed in either direction (backward or forward direction
of reaction).
(b) What happens to the ratio of oxygenated HbO2(aq) to deoxygenated Hb(aq), i.e.
[ HbO2(aq ) ]
[Hb (aq )] ,
when there is a high pressure of oxygen, PO2(g), in the lungs?
ANSWER:
When we increase the pressure of O2 in the lungs then Concentration of O2 increases, which will
trigger the forward reaction and as a result, concentration of HbO2 increases.
This will further lead to increase the ratio of oxygenated “HbO2” to deoxygenated “Hb”.
Note: The above explanation is made from the Le Chatelier's principle
Q10. The magnitude of K is critical for O2 to be pass first from lungs into red blood cells and then
subsequently into the tissues.
(a) If K was very small, would the equilibrium favour reactants or products in the above reaction?
How would that affect the proportion of oxygenated HbO2(aq) to deoxygenated Hb(aq) in blood
leaving the lungs?
ANSWER:
As we see from the above flow sheet, When “K” value is very small, then reaction hardly process.
Therefore ratio of oxygenated HbO2(aq) to deoxygenated Hb(aq) in the blood leaving the lungs will get
decrease.
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 8

(b) Now consider the situation in the tissues of the body that rely on diffusion of oxygen from the
bloodstream. If K was very large, how would that affect the amount of oxygen passing from blood into
the tissues?
(hint: Consider whether the forward or reverse reaction is required now)
ANSWER:
Larger value of K will lead to forward reaction and there will be increased concentration of oxygenated
haemoglobin. The oxygen will pass in much higher rate when the K value is large.
Q11. The equilibrium constant, K, for binding of haemoglobin for carbon monoxide, CO, is about 200-
300 times greater than for O2. Explain using chemical equilibrium principles why this makes
breathing CO hazardous.
ANSWER:
Oxygen in lungs bind with haemoglobin as
Hb ( aq ) + 4 O2 ( aq ) ⇌ Hb ( O2 ) 4 ( aq ) ( 1)
Binding of carbon monoxide to haemoglobin is around 300 times stronger than oxygen, which takes
place as follows
Hb ( aq ) + 4 CO ( aq ) ⇌ Hb ( CO )4 ( aq ) (2)
If we inhale carbon monoxide, then the first reaction will reversed
During to strong binding nature of carbondioxide, equilibrium position will tries to remain towards right
in equation 2. As a result, there will be no enough oxygenated haemoglobin will be the lungs and
blood vessels to maintain life processes and person may result to death.
Kinetics.
Broadly speaking, the factors that influence chemical reaction rates may be summarised as follows.
Chemical nature of reactants (Is the chemical reaction thermodynamically “downhill” so that it
can occur spontaneously at all?)
Reactant Concentration (or pressure for a gas)
Physical state of reactants (e.g. phase, or surface area of solids)
Presence of catalyst
Temperature
Q12. For each of the following observations, identify which one these five factors is most important,
and briefly explain how/why that is the case.
(a) The ability to sustain aerobic exercise is limited by the amount of haemoglobin in the blood.
ANSWER:
Reactant concentration i.e amount of haemoglobin is most important in the blood to circulate the life
processes. Anaemia is the most common disease found when the deficiency of haemoglobin occurs
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 9
bloodstream. If K was very large, how would that affect the amount of oxygen passing from blood into
the tissues?
(hint: Consider whether the forward or reverse reaction is required now)
ANSWER:
Larger value of K will lead to forward reaction and there will be increased concentration of oxygenated
haemoglobin. The oxygen will pass in much higher rate when the K value is large.
Q11. The equilibrium constant, K, for binding of haemoglobin for carbon monoxide, CO, is about 200-
300 times greater than for O2. Explain using chemical equilibrium principles why this makes
breathing CO hazardous.
ANSWER:
Oxygen in lungs bind with haemoglobin as
Hb ( aq ) + 4 O2 ( aq ) ⇌ Hb ( O2 ) 4 ( aq ) ( 1)
Binding of carbon monoxide to haemoglobin is around 300 times stronger than oxygen, which takes
place as follows
Hb ( aq ) + 4 CO ( aq ) ⇌ Hb ( CO )4 ( aq ) (2)
If we inhale carbon monoxide, then the first reaction will reversed
During to strong binding nature of carbondioxide, equilibrium position will tries to remain towards right
in equation 2. As a result, there will be no enough oxygenated haemoglobin will be the lungs and
blood vessels to maintain life processes and person may result to death.
Kinetics.
Broadly speaking, the factors that influence chemical reaction rates may be summarised as follows.
Chemical nature of reactants (Is the chemical reaction thermodynamically “downhill” so that it
can occur spontaneously at all?)
Reactant Concentration (or pressure for a gas)
Physical state of reactants (e.g. phase, or surface area of solids)
Presence of catalyst
Temperature
Q12. For each of the following observations, identify which one these five factors is most important,
and briefly explain how/why that is the case.
(a) The ability to sustain aerobic exercise is limited by the amount of haemoglobin in the blood.
ANSWER:
Reactant concentration i.e amount of haemoglobin is most important in the blood to circulate the life
processes. Anaemia is the most common disease found when the deficiency of haemoglobin occurs
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

in our body. Haemoglobin helps to transport oxygen to the parts of the body for maintaining life
processes.
(b) Non-starch polysaccharides are large complex carbohydrates, and an important source of dietary
fibre. Their chemical energy content is similar to other carbohydrates like sucrose. Why is it that some
bacteria can process these molecules but they do not undergo respiration via by human digestion?
ANSWER:
Breakdown of complex molecule like non starch polysaccharides are not possible inside our body.
However, It can be fermented into short chain fatty acids, Which may take a long time to degrade and
supply energy. In our regular human digestion, food molecules breakdown in a cycle of day and
supply energy.
(c) Potato is made edible by cooking it.
ANSWER:
Starch is a polysaccharides, which is found in potato, mainly considered as rich resources of starch.
Due to its complex nature of molecule, it is very difficult to digest. Therefore, proper cooking is
required before taking into our body.
(d) Digestion is enhanced by chewing our food.
ANSWER:
Digestion can be enhanced by chewing our food in various ways
1. Mechanically breakdown of larger pieces of food into smaller ones leads to increased surface
of the food particles. Therefore, action of enzyme can takes place on entire surface of food.
2. Prolonged chewing of food will helps in proper contact of food particle with the saliva for a
long time, which helps in digestion. Saliva is an enzyme which secreted from the near mouth
part.
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 10
processes.
(b) Non-starch polysaccharides are large complex carbohydrates, and an important source of dietary
fibre. Their chemical energy content is similar to other carbohydrates like sucrose. Why is it that some
bacteria can process these molecules but they do not undergo respiration via by human digestion?
ANSWER:
Breakdown of complex molecule like non starch polysaccharides are not possible inside our body.
However, It can be fermented into short chain fatty acids, Which may take a long time to degrade and
supply energy. In our regular human digestion, food molecules breakdown in a cycle of day and
supply energy.
(c) Potato is made edible by cooking it.
ANSWER:
Starch is a polysaccharides, which is found in potato, mainly considered as rich resources of starch.
Due to its complex nature of molecule, it is very difficult to digest. Therefore, proper cooking is
required before taking into our body.
(d) Digestion is enhanced by chewing our food.
ANSWER:
Digestion can be enhanced by chewing our food in various ways
1. Mechanically breakdown of larger pieces of food into smaller ones leads to increased surface
of the food particles. Therefore, action of enzyme can takes place on entire surface of food.
2. Prolonged chewing of food will helps in proper contact of food particle with the saliva for a
long time, which helps in digestion. Saliva is an enzyme which secreted from the near mouth
part.
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 10
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
![Analysis of Carbohydrates in Human Body: Biology Report, [Date]](/_next/image/?url=https%3A%2F%2Fdesklib.com%2Fmedia%2Fimages%2Fnh%2F7b5ea273413144b985b8efdebb047dc4.jpg&w=256&q=75)

