Food as Energy – Fuel for Life: Chemistry Essentials Scenario-Based Problem
Added on 2023-06-10
10 Pages3067 Words450 Views
MODULE 3 SCENARIO-BASED PROBLEM
FOOD AS ENERGY – FUEL FOR LIFE
Not only do we use energy for transportation, heating, lights, and countless devices but we also need
it to power our bodies. The food we eat provides energy for metabolism and physical activity. Aerobic
respiration involves oxidation of the nutrient molecule to produce carbon dioxide and water. It is the
same overall reaction as combustion, but proceeds by a very different pathway. The chemical energy
released is harnessed to produce adenosine triphosphate (ATP), commonly described as the body’s
energy “currency”. One molecule of palmitic acid for example makes about 130 molecules of ATP.
Recently, controversy has erupted over whether fat or sugar is the worst culprit in the obesity
epidemic. It is a complex story, but one property of nutrients that can be well determined is their
energy content.
In the first part of this scenario, your task is to assess which has a higher energy content, sugar or fat.
These generic classes of food will be represented by sucrose (table sugar) and palmitic acid, the most
abundant saturated fatty acid in food. You will use concepts of physical chemistry to examine aspects
of the energy content of food, how much oxygen is required for respiration, how that oxygen is
transported, and the chemical kinetic factors that determine how readily reactions occur.
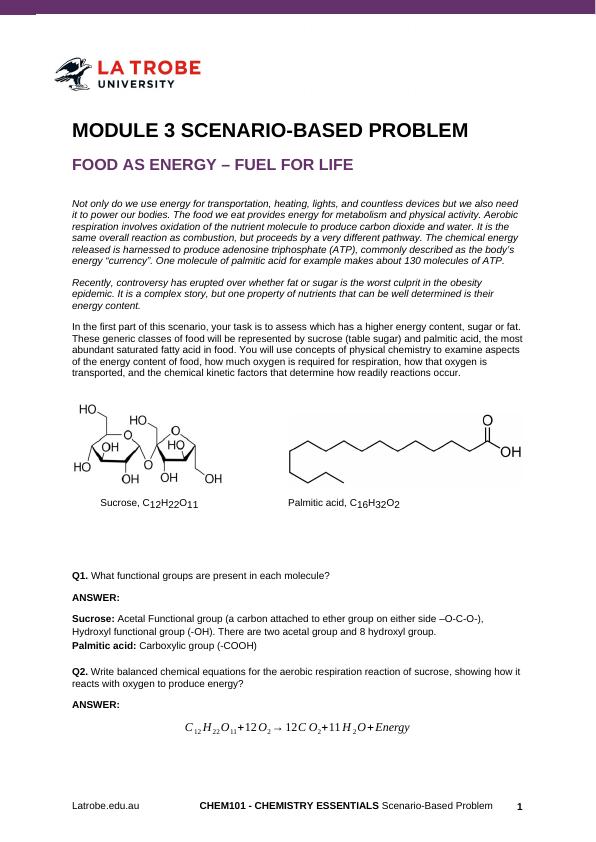
Sucrose, C12H22O11 Palmitic acid, C16H32O2
Q1. What functional groups are present in each molecule?
ANSWER:
Sucrose: Acetal Functional group (a carbon attached to ether group on either side –O-C-O-),
Hydroxyl functional group (-OH). There are two acetal group and 8 hydroxyl group.
Palmitic acid: Carboxylic group (-COOH)
Q2. Write balanced chemical equations for the aerobic respiration reaction of sucrose, showing how it
reacts with oxygen to produce energy?
ANSWER:
C12 H22 O11+12 O2 → 12C O2+11 H 2 O+ Energy
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 1
FOOD AS ENERGY – FUEL FOR LIFE
Not only do we use energy for transportation, heating, lights, and countless devices but we also need
it to power our bodies. The food we eat provides energy for metabolism and physical activity. Aerobic
respiration involves oxidation of the nutrient molecule to produce carbon dioxide and water. It is the
same overall reaction as combustion, but proceeds by a very different pathway. The chemical energy
released is harnessed to produce adenosine triphosphate (ATP), commonly described as the body’s
energy “currency”. One molecule of palmitic acid for example makes about 130 molecules of ATP.
Recently, controversy has erupted over whether fat or sugar is the worst culprit in the obesity
epidemic. It is a complex story, but one property of nutrients that can be well determined is their
energy content.
In the first part of this scenario, your task is to assess which has a higher energy content, sugar or fat.
These generic classes of food will be represented by sucrose (table sugar) and palmitic acid, the most
abundant saturated fatty acid in food. You will use concepts of physical chemistry to examine aspects
of the energy content of food, how much oxygen is required for respiration, how that oxygen is
transported, and the chemical kinetic factors that determine how readily reactions occur.
Sucrose, C12H22O11 Palmitic acid, C16H32O2
Q1. What functional groups are present in each molecule?
ANSWER:
Sucrose: Acetal Functional group (a carbon attached to ether group on either side –O-C-O-),
Hydroxyl functional group (-OH). There are two acetal group and 8 hydroxyl group.
Palmitic acid: Carboxylic group (-COOH)
Q2. Write balanced chemical equations for the aerobic respiration reaction of sucrose, showing how it
reacts with oxygen to produce energy?
ANSWER:
C12 H22 O11+12 O2 → 12C O2+11 H 2 O+ Energy
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 1
Energy
It would be very difficult to directly measure the energy released in a respiration reaction that
proceeds via a complex biochemical pathway. However, thermodynamic enthalpy data is available for
an equivalent overall reaction that proceeds via combustion instead. Happily, the net enthalpy change
from reactants to products is the same regardless of how the reaction occurs (this is a central
postulate of thermodynamics called “Hess’s Law).
Q3. The molar enthalpy of combustion of sucrose, rHo = is -5645 kJ mol-1.
What term (one word) is used to describe a chemical reaction with a negative change in enthalpy?
ANSWER:
Negative change in enthalpy corresponds to the exothermic reaction i.e energy is released during the
reaction.
Q4. Calculate the molecular weights of both sucrose and palmitic acid, by summing the contributions
from C, H and O atoms and enter your data in the table below. (Useful data: relative atomic masses C
12.01, H 1.01, O 16.00).
ANSWER:
Element Sucrose Palmitic acid
C 12 ×12.01=144.12 16 ×12.01=192.16
H 22 ×1.01=22.22 32 ×1.01=32.32
O 11×16.00=176 2 ×16.00=32
Molecular weight (Total) 342.34 gram/mole 256.48 gram/mole
Q5. Calculate the number of moles in 1.00 g of sucrose and of palmitic acid. Enter your answers in
the table below. (Hint: n = m/MW).
ANSWER:
Sucrose
We know that molar mass of sucrose = 342.34 gram/mole
342.34 gram of sucrose will contain 1 mole of sucrose
1.00 gram of sucrose will contain 1
342.34 moleof sucrose
Therefore 1.00 gram of sucrose will contain 0.00292 mole of sucrose.
Palmitic acid
We know that molar mass of palmitic acid = 256.48 gram/mole
256.48 gram of palmitic acid will contain 1 mole of palmitic acid
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 2
It would be very difficult to directly measure the energy released in a respiration reaction that
proceeds via a complex biochemical pathway. However, thermodynamic enthalpy data is available for
an equivalent overall reaction that proceeds via combustion instead. Happily, the net enthalpy change
from reactants to products is the same regardless of how the reaction occurs (this is a central
postulate of thermodynamics called “Hess’s Law).
Q3. The molar enthalpy of combustion of sucrose, rHo = is -5645 kJ mol-1.
What term (one word) is used to describe a chemical reaction with a negative change in enthalpy?
ANSWER:
Negative change in enthalpy corresponds to the exothermic reaction i.e energy is released during the
reaction.
Q4. Calculate the molecular weights of both sucrose and palmitic acid, by summing the contributions
from C, H and O atoms and enter your data in the table below. (Useful data: relative atomic masses C
12.01, H 1.01, O 16.00).
ANSWER:
Element Sucrose Palmitic acid
C 12 ×12.01=144.12 16 ×12.01=192.16
H 22 ×1.01=22.22 32 ×1.01=32.32
O 11×16.00=176 2 ×16.00=32
Molecular weight (Total) 342.34 gram/mole 256.48 gram/mole
Q5. Calculate the number of moles in 1.00 g of sucrose and of palmitic acid. Enter your answers in
the table below. (Hint: n = m/MW).
ANSWER:
Sucrose
We know that molar mass of sucrose = 342.34 gram/mole
342.34 gram of sucrose will contain 1 mole of sucrose
1.00 gram of sucrose will contain 1
342.34 moleof sucrose
Therefore 1.00 gram of sucrose will contain 0.00292 mole of sucrose.
Palmitic acid
We know that molar mass of palmitic acid = 256.48 gram/mole
256.48 gram of palmitic acid will contain 1 mole of palmitic acid
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 2

1.00 gram of palmitic acid will contain 1
256.48 moleof palmitic acid
Therefore 1.00 gram of palmitic acid will contain 0.00389 mole of palmitic acid.
Q6. Calculate the energy released by respiration of 1.00 g of sucrose and of palmitic acid. Enter your
answers in the table below. (hint: use the number of mol from the previous Q, along with the molar
enthalpy of combustion)
Answer:
Sucrose
C12 H22 O11+12 O2 → 12C O2+11 H 2 O+energy ∆ H=−5645 kJ /mol
As we did calculation in question 5, that 1.00 gram of sucrose contains 0.00292 mole
According to the balanced reaction, enthalpy change is the release in energy i.e
1 mole of sucrose produces 5645 kJ of energy
So, 0.00292 mole of sucrose will produce 5645 ×0.00292 kJ of energy
Therefore energy released by respiration of 1.00 gram of sucrose will be 16.483 kJ
Palmitic acid
C16 H32 O2 +23 O2 → 16 C O2 +16 H2 O+ energy ∆ H =−9978 kJ /mol
As we did calculation in question 5, that 1.00 gram of palmitic acid contains 0.00389 mole
According to the balanced reaction, enthalpy change is the release in energy i.e
1 mole of palmitic acid produces 9978 kJ of energy
So, 0.00292 mole of palmitic acid will produce 9978 × 0.00389 kJof energy
Therefore energy released by respiration of 1.00 gram of palmitic acid will be 38.814 kJ
Q7. Based on the energy released in respiration per gram, which is the more potent fuel source, sugar
or fat? Is this consistent with the notion that each new CO bond made via oxidation releases energy?
ANSWER:
From the above calculation in question 6, we see that for the same of amount of sucrose (sugar) and
palmitic acid (fat), respiration of palmitic acid produces more amount of energy than sucrose (more
than double).
Therefore, we can say that fat is more potent fuel source than sugar.
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 3
256.48 moleof palmitic acid
Therefore 1.00 gram of palmitic acid will contain 0.00389 mole of palmitic acid.
Q6. Calculate the energy released by respiration of 1.00 g of sucrose and of palmitic acid. Enter your
answers in the table below. (hint: use the number of mol from the previous Q, along with the molar
enthalpy of combustion)
Answer:
Sucrose
C12 H22 O11+12 O2 → 12C O2+11 H 2 O+energy ∆ H=−5645 kJ /mol
As we did calculation in question 5, that 1.00 gram of sucrose contains 0.00292 mole
According to the balanced reaction, enthalpy change is the release in energy i.e
1 mole of sucrose produces 5645 kJ of energy
So, 0.00292 mole of sucrose will produce 5645 ×0.00292 kJ of energy
Therefore energy released by respiration of 1.00 gram of sucrose will be 16.483 kJ
Palmitic acid
C16 H32 O2 +23 O2 → 16 C O2 +16 H2 O+ energy ∆ H =−9978 kJ /mol
As we did calculation in question 5, that 1.00 gram of palmitic acid contains 0.00389 mole
According to the balanced reaction, enthalpy change is the release in energy i.e
1 mole of palmitic acid produces 9978 kJ of energy
So, 0.00292 mole of palmitic acid will produce 9978 × 0.00389 kJof energy
Therefore energy released by respiration of 1.00 gram of palmitic acid will be 38.814 kJ
Q7. Based on the energy released in respiration per gram, which is the more potent fuel source, sugar
or fat? Is this consistent with the notion that each new CO bond made via oxidation releases energy?
ANSWER:
From the above calculation in question 6, we see that for the same of amount of sucrose (sugar) and
palmitic acid (fat), respiration of palmitic acid produces more amount of energy than sucrose (more
than double).
Therefore, we can say that fat is more potent fuel source than sugar.
Latrobe.edu.au CHEM101 - CHEMISTRY ESSENTIALS Scenario-Based Problem 3

End of preview
Want to access all the pages? Upload your documents or become a member.
Related Documents
Carbohydrates Macromoleculelg...
|4
|675
|77
Module 2 Scenario-Based Problem: Fixing dis-functional organic chemical datalg...
|7
|797
|148
Biology Study Materiallg...
|9
|872
|56
