Reactions and Properties: Functional Group Chemistry Report - BTEC
VerifiedAdded on 2023/01/09
|6
|1563
|1
Report
AI Summary
This report delves into the intricacies of functional group chemistry, focusing on the structure, reactions, and properties of aldehydes, ketones, carboxylic acids, acyl chlorides, acid anhydrides, amides, and halogenoalkanes. The report explores key reactions such as nucleophilic addition, oxidation, reduction, esterification, and substitution and elimination reactions. It provides detailed explanations of reaction mechanisms, including the addition of aldehydes and ketones with HCN and 2,4-dinitrophenylhydrazine, substitution of halogenoalkanes with NaOH, and elimination reactions. Furthermore, the report illustrates the synthesis of ethyl ethanoate and N-ethylacetamide, starting from ethanol, ethanal, chloroethane, and ethanoyl chloride. The content is enriched with equations and diagrams to enhance understanding and is supported by relevant references.
FUNCTIONAL GROUP CHEMISTRY FOR DESIGNER MOLECULES
SECTION 1
ALDEHYDES AND KETONES
Aldehydes are organic compounds whose functional group is comprised of a single oxygen
atom bonded onto a carbon atom. The structural formula of the aldehyde functional group is –
CHO and a general structural formulae of aldehyde is RCHO such as ethanal CH3CHO. Most
aldehydes are derived from dehydration of corresponding carboxylic acid. On the other hand,
ketones are organic compounds whose formula can be generally be represented as R’OR loke
(CH3)2CO. R’ and R represent carbon chains attached to the functional.
Reactions of Aldehydes and Ketones
Nucleophile Addition
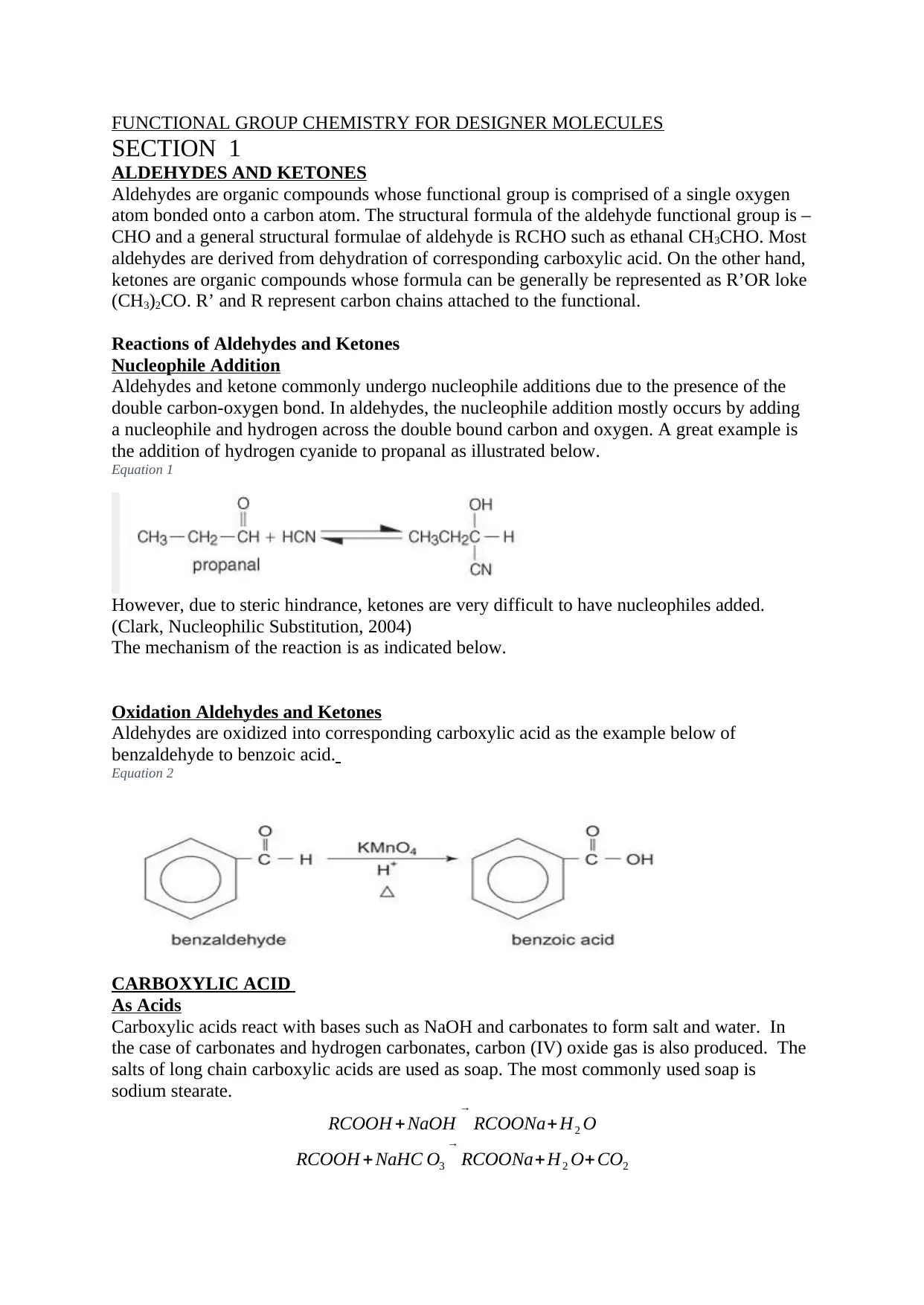
Aldehydes and ketone commonly undergo nucleophile additions due to the presence of the
double carbon-oxygen bond. In aldehydes, the nucleophile addition mostly occurs by adding
a nucleophile and hydrogen across the double bound carbon and oxygen. A great example is
the addition of hydrogen cyanide to propanal as illustrated below.
Equation 1
However, due to steric hindrance, ketones are very difficult to have nucleophiles added.
(Clark, Nucleophilic Substitution, 2004)
The mechanism of the reaction is as indicated below.
Oxidation Aldehydes and Ketones
Aldehydes are oxidized into corresponding carboxylic acid as the example below of
benzaldehyde to benzoic acid.
Equation 2
CARBOXYLIC ACID
As Acids
Carboxylic acids react with bases such as NaOH and carbonates to form salt and water. In
the case of carbonates and hydrogen carbonates, carbon (IV) oxide gas is also produced. The
salts of long chain carboxylic acids are used as soap. The most commonly used soap is
sodium stearate.
RCOOH + NaOH →
RCOONa+H2 O
RCOOH + NaHC O3
→
RCOONa+ H2 O+ CO2
SECTION 1
ALDEHYDES AND KETONES
Aldehydes are organic compounds whose functional group is comprised of a single oxygen
atom bonded onto a carbon atom. The structural formula of the aldehyde functional group is –
CHO and a general structural formulae of aldehyde is RCHO such as ethanal CH3CHO. Most
aldehydes are derived from dehydration of corresponding carboxylic acid. On the other hand,
ketones are organic compounds whose formula can be generally be represented as R’OR loke
(CH3)2CO. R’ and R represent carbon chains attached to the functional.
Reactions of Aldehydes and Ketones
Nucleophile Addition
Aldehydes and ketone commonly undergo nucleophile additions due to the presence of the
double carbon-oxygen bond. In aldehydes, the nucleophile addition mostly occurs by adding
a nucleophile and hydrogen across the double bound carbon and oxygen. A great example is
the addition of hydrogen cyanide to propanal as illustrated below.
Equation 1
However, due to steric hindrance, ketones are very difficult to have nucleophiles added.
(Clark, Nucleophilic Substitution, 2004)
The mechanism of the reaction is as indicated below.
Oxidation Aldehydes and Ketones
Aldehydes are oxidized into corresponding carboxylic acid as the example below of
benzaldehyde to benzoic acid.
Equation 2
CARBOXYLIC ACID
As Acids
Carboxylic acids react with bases such as NaOH and carbonates to form salt and water. In
the case of carbonates and hydrogen carbonates, carbon (IV) oxide gas is also produced. The
salts of long chain carboxylic acids are used as soap. The most commonly used soap is
sodium stearate.
RCOOH + NaOH →
RCOONa+H2 O
RCOOH + NaHC O3
→
RCOONa+ H2 O+ CO2
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Electrophilic Substitution
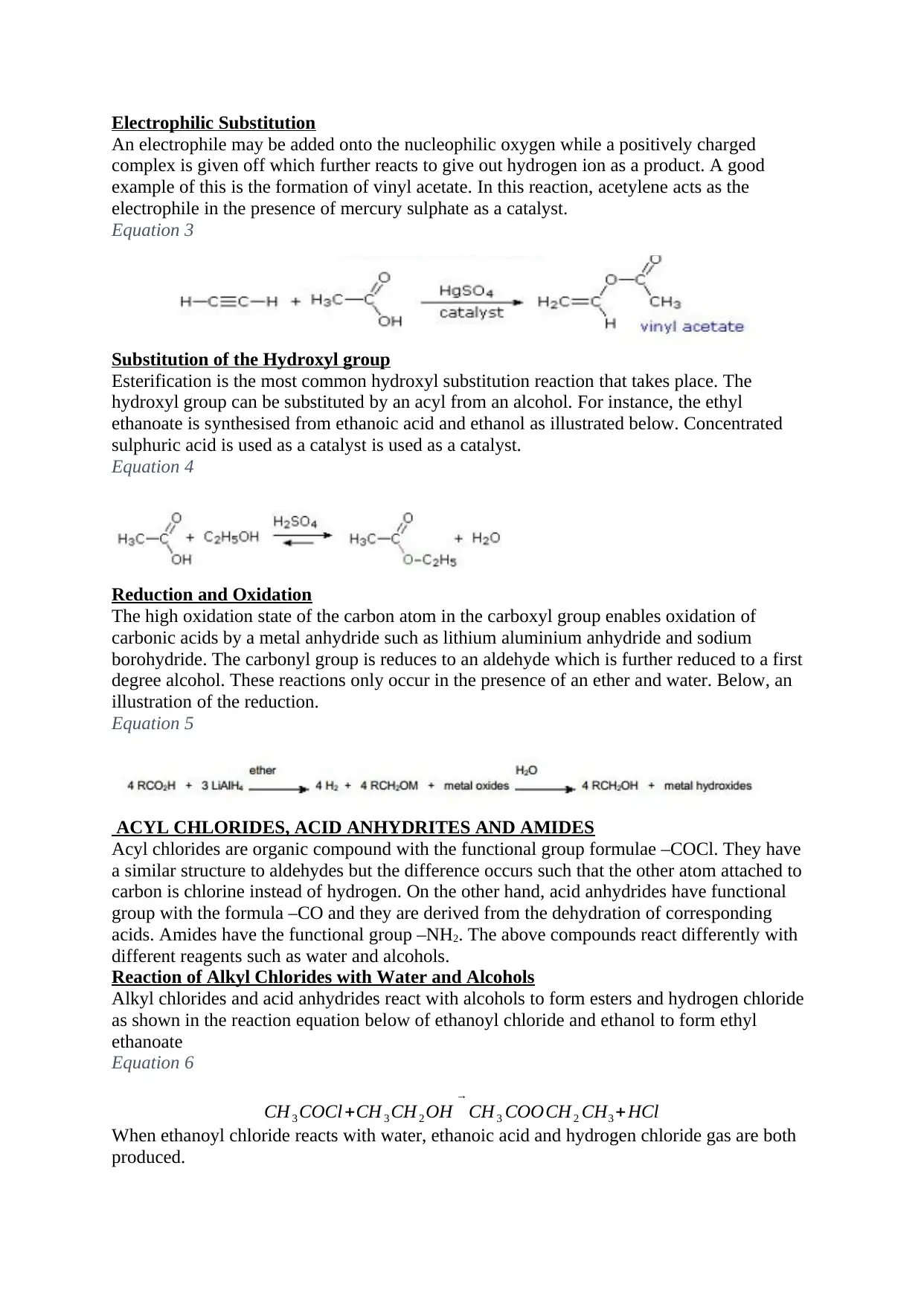
An electrophile may be added onto the nucleophilic oxygen while a positively charged
complex is given off which further reacts to give out hydrogen ion as a product. A good
example of this is the formation of vinyl acetate. In this reaction, acetylene acts as the
electrophile in the presence of mercury sulphate as a catalyst.
Equation 3
Substitution of the Hydroxyl group
Esterification is the most common hydroxyl substitution reaction that takes place. The
hydroxyl group can be substituted by an acyl from an alcohol. For instance, the ethyl
ethanoate is synthesised from ethanoic acid and ethanol as illustrated below. Concentrated
sulphuric acid is used as a catalyst is used as a catalyst.
Equation 4
Reduction and Oxidation
The high oxidation state of the carbon atom in the carboxyl group enables oxidation of
carbonic acids by a metal anhydride such as lithium aluminium anhydride and sodium
borohydride. The carbonyl group is reduces to an aldehyde which is further reduced to a first
degree alcohol. These reactions only occur in the presence of an ether and water. Below, an
illustration of the reduction.
Equation 5
ACYL CHLORIDES, ACID ANHYDRITES AND AMIDES
Acyl chlorides are organic compound with the functional group formulae –COCl. They have
a similar structure to aldehydes but the difference occurs such that the other atom attached to
carbon is chlorine instead of hydrogen. On the other hand, acid anhydrides have functional
group with the formula –CO and they are derived from the dehydration of corresponding
acids. Amides have the functional group –NH2. The above compounds react differently with
different reagents such as water and alcohols.
Reaction of Alkyl Chlorides with Water and Alcohols
Alkyl chlorides and acid anhydrides react with alcohols to form esters and hydrogen chloride
as shown in the reaction equation below of ethanoyl chloride and ethanol to form ethyl
ethanoate
Equation 6
CH 3 COCl +CH 3 CH 2 OH →
CH 3 COOCH 2 CH3 + HCl
When ethanoyl chloride reacts with water, ethanoic acid and hydrogen chloride gas are both
produced.
An electrophile may be added onto the nucleophilic oxygen while a positively charged
complex is given off which further reacts to give out hydrogen ion as a product. A good
example of this is the formation of vinyl acetate. In this reaction, acetylene acts as the
electrophile in the presence of mercury sulphate as a catalyst.
Equation 3
Substitution of the Hydroxyl group
Esterification is the most common hydroxyl substitution reaction that takes place. The
hydroxyl group can be substituted by an acyl from an alcohol. For instance, the ethyl
ethanoate is synthesised from ethanoic acid and ethanol as illustrated below. Concentrated
sulphuric acid is used as a catalyst is used as a catalyst.
Equation 4
Reduction and Oxidation
The high oxidation state of the carbon atom in the carboxyl group enables oxidation of
carbonic acids by a metal anhydride such as lithium aluminium anhydride and sodium
borohydride. The carbonyl group is reduces to an aldehyde which is further reduced to a first
degree alcohol. These reactions only occur in the presence of an ether and water. Below, an
illustration of the reduction.
Equation 5
ACYL CHLORIDES, ACID ANHYDRITES AND AMIDES
Acyl chlorides are organic compound with the functional group formulae –COCl. They have
a similar structure to aldehydes but the difference occurs such that the other atom attached to
carbon is chlorine instead of hydrogen. On the other hand, acid anhydrides have functional
group with the formula –CO and they are derived from the dehydration of corresponding
acids. Amides have the functional group –NH2. The above compounds react differently with
different reagents such as water and alcohols.
Reaction of Alkyl Chlorides with Water and Alcohols
Alkyl chlorides and acid anhydrides react with alcohols to form esters and hydrogen chloride
as shown in the reaction equation below of ethanoyl chloride and ethanol to form ethyl
ethanoate
Equation 6
CH 3 COCl +CH 3 CH 2 OH →
CH 3 COOCH 2 CH3 + HCl
When ethanoyl chloride reacts with water, ethanoic acid and hydrogen chloride gas are both
produced.

Equation 7
CH 3 COCl + H2 O →
CH 3 COOH + HCl
The reaction of acyl chlorides with phenols is an important process in the pharmaceutical
industry as it is used to produce acetylsalicyic acid commonly referred to as aspirin. Here, 2-
hydroxybenzoic acid (salicylic acid) is reacted with ethanoyl chloride. However, for this
process acid anhydrides are preferred to the acyl chlorides.
Acid anhydrides react with water to form their corresponding acids. They also react with
ammonia to give out amides.
SECTION 2
Addition of Aldehyde or Ketone with HCN
RCHO + HCN ↔
R ( CH ) OHCN
The CN- nucleophile attacks the partially positive carbon atom and bonds to it. This leaves a
very negatively charged oxygen atom singly bound to the carbon atom. This oxygen attracts a
hydrogen from the HCN which bonds to it forming an –OH group on the aldehyde or ketone.
Figure 1Addition of Aldehyde with HCN
Addition-Elimination of Aldehyde Or Ketone With 2,4-Dinitrophenylhydrazine
In the figures below showing the mechanism, X stands for the dinitrophenyl ring. In the first
stage of the reaction, the 2,4-Dinitrophenylhydrazine adds itself to the double carbon oxygen
bond. This comprises the addition reaction.
The oxygen atom detaches from the aldehyde or ketone and attaches to the two hydrogen
atoms on the hydrazine and form water as a leaving group. This is the elimination stage.
For this process to take place a strong base, in this case 2,4-Dinitrophenylhydrazine,
is essential.
CH 3 COCl + H2 O →
CH 3 COOH + HCl
The reaction of acyl chlorides with phenols is an important process in the pharmaceutical
industry as it is used to produce acetylsalicyic acid commonly referred to as aspirin. Here, 2-
hydroxybenzoic acid (salicylic acid) is reacted with ethanoyl chloride. However, for this
process acid anhydrides are preferred to the acyl chlorides.
Acid anhydrides react with water to form their corresponding acids. They also react with
ammonia to give out amides.
SECTION 2
Addition of Aldehyde or Ketone with HCN
RCHO + HCN ↔
R ( CH ) OHCN
The CN- nucleophile attacks the partially positive carbon atom and bonds to it. This leaves a
very negatively charged oxygen atom singly bound to the carbon atom. This oxygen attracts a
hydrogen from the HCN which bonds to it forming an –OH group on the aldehyde or ketone.
Figure 1Addition of Aldehyde with HCN
Addition-Elimination of Aldehyde Or Ketone With 2,4-Dinitrophenylhydrazine
In the figures below showing the mechanism, X stands for the dinitrophenyl ring. In the first
stage of the reaction, the 2,4-Dinitrophenylhydrazine adds itself to the double carbon oxygen
bond. This comprises the addition reaction.
The oxygen atom detaches from the aldehyde or ketone and attaches to the two hydrogen
atoms on the hydrazine and form water as a leaving group. This is the elimination stage.
For this process to take place a strong base, in this case 2,4-Dinitrophenylhydrazine,
is essential.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Figure 2 Addition-Elimination Reaction with 2,4-dinitrophenylhydrazine
Comparison between the Addition Reaction and Addition-Elimination Reaction
For an addition-elimination reaction to take place, it is important for a strong base to be
present. It is also important that the atom bonded to the alpha carbon to easily form a leaving
group in the elimination stage.
Substitution of Halogenoalkane with Aqueous NaOH
Figure 3 Substitution of Halogenoalkane with Aqueous NaOH
Elimination of Halogenoalkane with Ethanolic NaOH
A highly concentrated alkali hydroxide of potassium or sodium when reacted with a
Halogenoalkane leads to the deprotonating of the alpha carbon while eradicating the bromine
halogen atom from the molecule. The bromine atom leaves as bromide ion in solution. For
this reaction to take place, heat must be added to the reactants.
Comparison between the Addition Reaction and Addition-Elimination Reaction
For an addition-elimination reaction to take place, it is important for a strong base to be
present. It is also important that the atom bonded to the alpha carbon to easily form a leaving
group in the elimination stage.
Substitution of Halogenoalkane with Aqueous NaOH
Figure 3 Substitution of Halogenoalkane with Aqueous NaOH
Elimination of Halogenoalkane with Ethanolic NaOH
A highly concentrated alkali hydroxide of potassium or sodium when reacted with a
Halogenoalkane leads to the deprotonating of the alpha carbon while eradicating the bromine
halogen atom from the molecule. The bromine atom leaves as bromide ion in solution. For
this reaction to take place, heat must be added to the reactants.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Figure 4Elimination of Halogenoalkane with Ethanolic NaOH
Comparison between substitution and elimination reaction of halogenoalkane with NaOH
Substitution reaction is predominant in primary halogenoalkanes while elimination is
dominant in the tertiary halogenoalkanes.
In the elimination reaction, the OH- ion acts as a base deprotonating the carbon atom and
forms a water molecule leading to a series of electron transfer within the halogenoalkane
molecule. This consequently causes the break off of bromide ion from the molecule. On the
other hand, in the substitution reaction, OH- ion acts as a nucleophile that attaches itself to the
partially positive carbon atom attached to the bromine. This leads to the loss of the bromine
atom as bromide ion as the OH- ion replaces it in the structure.
A substitution reaction leads to the formation of an alcohol while an elimination reaction
forms an unsaturated acyl compound that has been dehalogenated.
In the elimination reaction, heat a highly concentrated alkali hydroxide solution of pure
ethanol is required. However, in the substitution reaction, the alkali hydroxide (sodium or
potassium) is in a water solution with a relatively low concentration and no heat is applied for
the reaction to occur.
SECTION 3
Synthesis of ethyl ethanoate (CH3CO2CH2CH3), starting from ethanol and ethanal
This process will involve a series of reactions that will commence with the oxidation of
ethanol to ethanoic acid. This will be carried out in the presence of a strong oxidising agent
such as potassium dichromate as the equation below
Equation 8
CH 3 COH + H2
+¿ , K Cr2 O7
→
CH 3 COOH + H2 O¿
The ethanoic acid formed is then reacted in the presence of concentrated sulphuric acid to
form ethyl ethanoate.
Equation 9
CH 3 COOH +CH 3 CH 2 OH concentrated H2 SO4
↔
CH 3 COOCH 2 CH 3 +HCl
Synthesis of N-ethylacetamide (CH3CONHCH2CH3), starting from chloroethane and
ethanoyl chloride
Comparison between substitution and elimination reaction of halogenoalkane with NaOH
Substitution reaction is predominant in primary halogenoalkanes while elimination is
dominant in the tertiary halogenoalkanes.
In the elimination reaction, the OH- ion acts as a base deprotonating the carbon atom and
forms a water molecule leading to a series of electron transfer within the halogenoalkane
molecule. This consequently causes the break off of bromide ion from the molecule. On the
other hand, in the substitution reaction, OH- ion acts as a nucleophile that attaches itself to the
partially positive carbon atom attached to the bromine. This leads to the loss of the bromine
atom as bromide ion as the OH- ion replaces it in the structure.
A substitution reaction leads to the formation of an alcohol while an elimination reaction
forms an unsaturated acyl compound that has been dehalogenated.
In the elimination reaction, heat a highly concentrated alkali hydroxide solution of pure
ethanol is required. However, in the substitution reaction, the alkali hydroxide (sodium or
potassium) is in a water solution with a relatively low concentration and no heat is applied for
the reaction to occur.
SECTION 3
Synthesis of ethyl ethanoate (CH3CO2CH2CH3), starting from ethanol and ethanal
This process will involve a series of reactions that will commence with the oxidation of
ethanol to ethanoic acid. This will be carried out in the presence of a strong oxidising agent
such as potassium dichromate as the equation below
Equation 8
CH 3 COH + H2
+¿ , K Cr2 O7
→
CH 3 COOH + H2 O¿
The ethanoic acid formed is then reacted in the presence of concentrated sulphuric acid to
form ethyl ethanoate.
Equation 9
CH 3 COOH +CH 3 CH 2 OH concentrated H2 SO4
↔
CH 3 COOCH 2 CH 3 +HCl
Synthesis of N-ethylacetamide (CH3CONHCH2CH3), starting from chloroethane and
ethanoyl chloride

The chloroethane is converted to ethylamine through a substitution reaction.
Equation 10
NH 3+CH 3 CH2 Cl ↔
CH 3 CH 2 NH 2 + HCl
The ethylamine is then reacted with ethanoyl chloride to form N-ethylacetamide.
Equation 11
CH 3 CH 2 NH 2 +CH3 C H2 OCl↔
CH 3 C H2 ONHC H2 CH3 + HCl
References
Clark, J. (2004). Nucleophilic Substitution. Retrieved from Chemguide:
https://www.chemguide.co.uk/mechanisms/nucsub/whatis.html
Clark, J. (2015). The Reaction of Acyl Chloride with Water, Alcohols and Phenols. Retrieved
from Chemguide.co.uk:
https://www.chemguide.co.uk/organicprops/acylchlorides/oxygen.html
Clarke, J. (2000). Elimination Versus Substitution in Halogenoalkanes. Retrieved from
chemguide.co.uk.
Houghton Mifflin Harcourt. (2016). Reaction of Aldehydes and Ketones. Retrieved from
Cliffs Notes: http://www.cliffsnotes.com
Equation 10
NH 3+CH 3 CH2 Cl ↔
CH 3 CH 2 NH 2 + HCl
The ethylamine is then reacted with ethanoyl chloride to form N-ethylacetamide.
Equation 11
CH 3 CH 2 NH 2 +CH3 C H2 OCl↔
CH 3 C H2 ONHC H2 CH3 + HCl
References
Clark, J. (2004). Nucleophilic Substitution. Retrieved from Chemguide:
https://www.chemguide.co.uk/mechanisms/nucsub/whatis.html
Clark, J. (2015). The Reaction of Acyl Chloride with Water, Alcohols and Phenols. Retrieved
from Chemguide.co.uk:
https://www.chemguide.co.uk/organicprops/acylchlorides/oxygen.html
Clarke, J. (2000). Elimination Versus Substitution in Halogenoalkanes. Retrieved from
chemguide.co.uk.
Houghton Mifflin Harcourt. (2016). Reaction of Aldehydes and Ketones. Retrieved from
Cliffs Notes: http://www.cliffsnotes.com
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 6
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





