Measuring Heat Energy of Fuels
Added on 2023-06-15
17 Pages2560 Words334 Views
MEASURING HEAT ENERGY OF FUELS 1
MEASURING HEAT ENERGY OF FUELS
By Name
Course
Instructor
Institution
Location
Date
MEASURING HEAT ENERGY OF FUELS
By Name
Course
Instructor
Institution
Location
Date

MEASURING HEAT ENERGY OF FUELS 2
ABSTRACT
There are numerous chemical reactions that are visible in our surrounding for example as
combustion. Combustion is like an oxidation reaction since oxygen is allowed to burn and in the
process, it combines with the substance being burnt. The substance will hence get oxidized in the
process while the oxygen is reduced to a different substance. For instance, combustion of
methanol leads to the formation of water vapor and carbon dioxide gas as in the below results.
All combustion reactions are exothermic reaction since it releases a large amount of energy
during the reaction. This energy change which occurs during the reaction is known as the
enthalpy of combustion.
INTRODUCTION
Enthalpy is best understood on the two terms;
• TEMPERATURE
Measures the kinetic energies of molecules present in a substance Independent of the
amount of substance present
• HEAT
A measure of the total energy of a substance, it depends on the amount of substance
present. For instance.A bucket full of water at 500C would have the same temperature as
a 250ml beaker of water at the same temperature, but the heat content of the bucket
would be bigger. And a bucket of oil and a bucket of water at 50ºC would have the same
temperature, but the heat content of the bucket of oil would be bigger.
ABSTRACT
There are numerous chemical reactions that are visible in our surrounding for example as
combustion. Combustion is like an oxidation reaction since oxygen is allowed to burn and in the
process, it combines with the substance being burnt. The substance will hence get oxidized in the
process while the oxygen is reduced to a different substance. For instance, combustion of
methanol leads to the formation of water vapor and carbon dioxide gas as in the below results.
All combustion reactions are exothermic reaction since it releases a large amount of energy
during the reaction. This energy change which occurs during the reaction is known as the
enthalpy of combustion.
INTRODUCTION
Enthalpy is best understood on the two terms;
• TEMPERATURE
Measures the kinetic energies of molecules present in a substance Independent of the
amount of substance present
• HEAT
A measure of the total energy of a substance, it depends on the amount of substance
present. For instance.A bucket full of water at 500C would have the same temperature as
a 250ml beaker of water at the same temperature, but the heat content of the bucket
would be bigger. And a bucket of oil and a bucket of water at 50ºC would have the same
temperature, but the heat content of the bucket of oil would be bigger.

MEASURING HEAT ENERGY OF FUELS 3
Therefore we can define enthalpy of combustion as the amount of heat released by a complete
combustion (burning between the organic compound and oxygen) of one mole of a substance.
Combustion is always exothermic i.e. the enthalpy for the combustion reaction is negative
(ΔHcombustion is negative). The heat of combustion is defined as a positive value i.e. the heat of
combustion = - ΔHcombustion. The heat of combustion can be measured experimentally (Tremaine,
2012).
The molar enthalpy change can always be calculated (Sato, 2014). A simple method to calculate
the enthalpy change of a reaction is to measure the temperature change caused by the reaction.
ΔH = m cp
ΔT.........................................................................................................1
ΔH = the enthalpy of the reaction
m = the mass of the sample which changes the temperature
Cp = the heat capacity of the substance which changes temperature, the heat capacity measures
how much energy is required to change the temperature of 1 g of the substance by 1 oC, the heat
capacity differs from substance to substance, water=4.2 J/g oC or ethanol=2.4 J/g oC
ΔT = temperature
This will give the energy in kJ released during the reaction to calculate the molar
enthalpy change, kJ mol-1, divide by the number of moles used in the reaction (Wohlfarth, 2016).
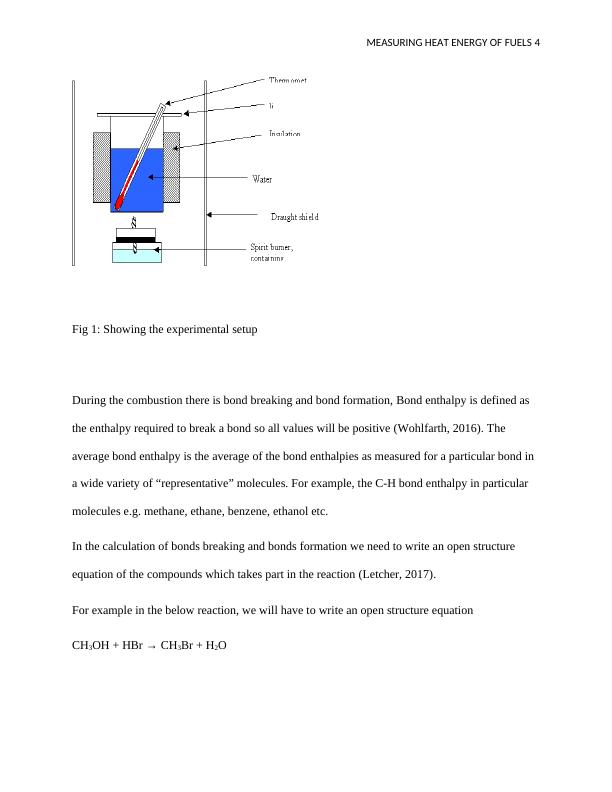
The experimental setup can be shown in the diagram below;
Therefore we can define enthalpy of combustion as the amount of heat released by a complete
combustion (burning between the organic compound and oxygen) of one mole of a substance.
Combustion is always exothermic i.e. the enthalpy for the combustion reaction is negative
(ΔHcombustion is negative). The heat of combustion is defined as a positive value i.e. the heat of
combustion = - ΔHcombustion. The heat of combustion can be measured experimentally (Tremaine,
2012).
The molar enthalpy change can always be calculated (Sato, 2014). A simple method to calculate
the enthalpy change of a reaction is to measure the temperature change caused by the reaction.
ΔH = m cp
ΔT.........................................................................................................1
ΔH = the enthalpy of the reaction
m = the mass of the sample which changes the temperature
Cp = the heat capacity of the substance which changes temperature, the heat capacity measures
how much energy is required to change the temperature of 1 g of the substance by 1 oC, the heat
capacity differs from substance to substance, water=4.2 J/g oC or ethanol=2.4 J/g oC
ΔT = temperature
This will give the energy in kJ released during the reaction to calculate the molar
enthalpy change, kJ mol-1, divide by the number of moles used in the reaction (Wohlfarth, 2016).
The experimental setup can be shown in the diagram below;

MEASURING HEAT ENERGY OF FUELS 4
Fig 1: Showing the experimental setup
During the combustion there is bond breaking and bond formation, Bond enthalpy is defined as
the enthalpy required to break a bond so all values will be positive (Wohlfarth, 2016). The
average bond enthalpy is the average of the bond enthalpies as measured for a particular bond in
a wide variety of “representative” molecules. For example, the C-H bond enthalpy in particular
molecules e.g. methane, ethane, benzene, ethanol etc.
In the calculation of bonds breaking and bonds formation we need to write an open structure
equation of the compounds which takes part in the reaction (Letcher, 2017).
For example in the below reaction, we will have to write an open structure equation
CH3OH + HBr → CH3Br + H2O
Fig 1: Showing the experimental setup
During the combustion there is bond breaking and bond formation, Bond enthalpy is defined as
the enthalpy required to break a bond so all values will be positive (Wohlfarth, 2016). The
average bond enthalpy is the average of the bond enthalpies as measured for a particular bond in
a wide variety of “representative” molecules. For example, the C-H bond enthalpy in particular
molecules e.g. methane, ethane, benzene, ethanol etc.
In the calculation of bonds breaking and bonds formation we need to write an open structure
equation of the compounds which takes part in the reaction (Letcher, 2017).
For example in the below reaction, we will have to write an open structure equation
CH3OH + HBr → CH3Br + H2O
End of preview
Want to access all the pages? Upload your documents or become a member.
Related Documents
Thermochemical Equation for Combustion of Octane and Bond Enthalpylg...
|6
|1517
|352
Assignment on Chemical Changelg...
|7
|1661
|343
Enthalpy of Combustion in Different Alcohol Chains and Brancheslg...
|14
|3827
|499
Standard Enthalpy of Combustion of Ethanollg...
|6
|1084
|102
CHANGES IN ENTHALPY DOWN THE ALCOHOL GROUP WITH INCREASING CARBON ATOMS CHAINS Introduction Alcoholslg...
|13
|3734
|80
Calculations Temperature of cold water PDFlg...
|6
|501
|59
