Investigating Heat and Mass Transfer in the Dissolution of Particles
VerifiedAdded on 2023/06/12
|4
|522
|57
Report
AI Summary
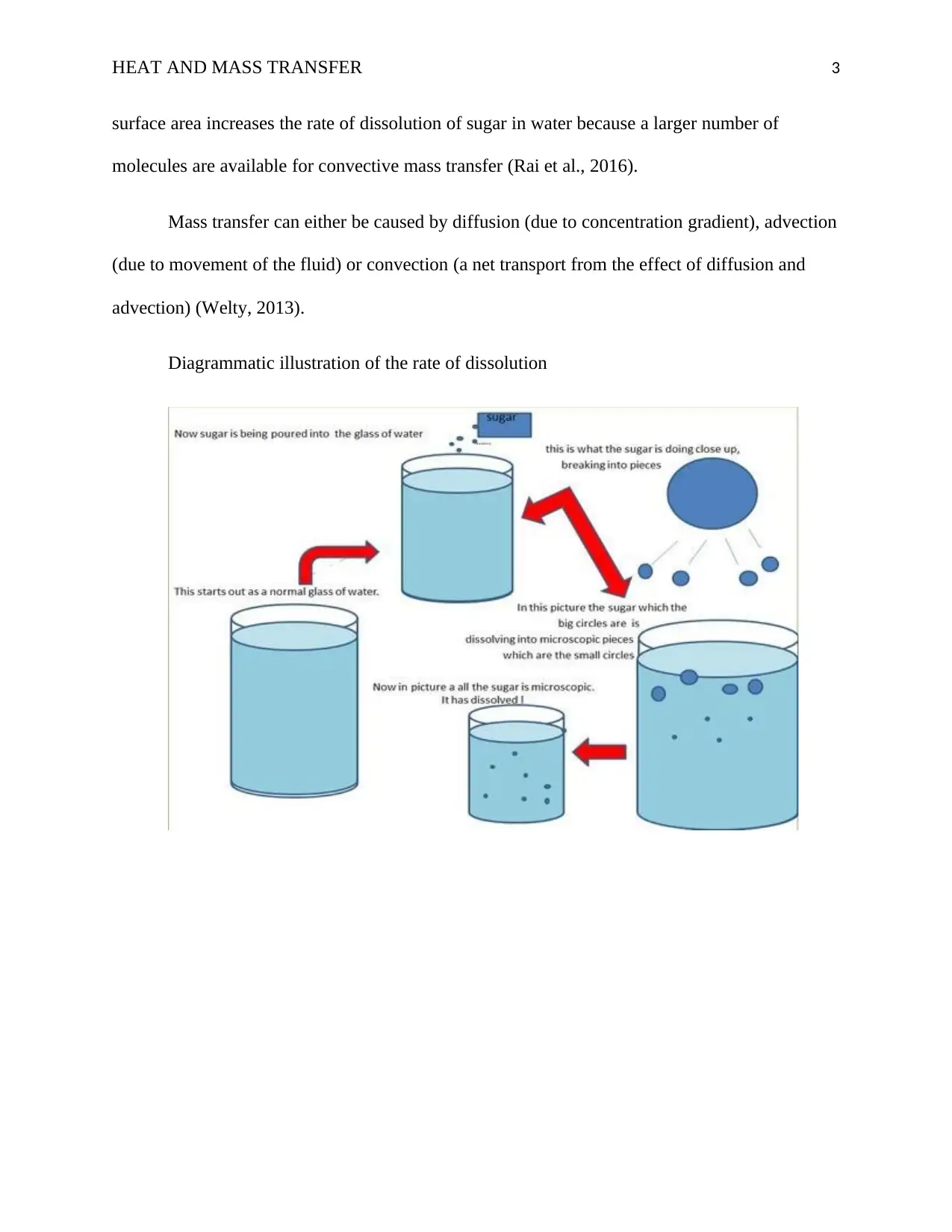
This report delves into the principles of heat and mass transfer concerning the dissolution of particles, emphasizing the role of temperature, stirring, and particle size in influencing dissolution rates. It explains that the dissolution rate of a solute in a solvent increases with temperature due to the enhanced kinetic energy of solvent molecules, and stirring accelerates dissolution by introducing convective mass transfer, as opposed to the slower molecular diffusion described by Fick's law. The report also highlights that the size of sugar particles affects the dissolution rate, with larger particles dissolving more slowly due to reduced convective mass transfer, while increased surface area accelerates dissolution. The document references key literature to support its explanations of diffusion, advection, and convection in mass transfer.
1 out of 4






![[object Object]](/_next/static/media/star-bottom.7253800d.svg)