Chemistry Lab: Enthalpy of Solution for Benzoic Acid Experiment
VerifiedAdded on 2023/01/19
|13
|3038
|84
Practical Assignment
AI Summary
This chemistry lab report details an experiment to determine the enthalpy of solution of benzoic acid in water. The report begins with the theoretical background, including the van't Hoff equation and the relationship between solubility and temperature. The experimental procedure involves saturating water with benzoic acid at various temperatures, followed by titration with NaOH to determine the concentration of benzoic acid at each temperature. The results section presents titration volumes, solubility calculations, and a graph of ln b against 1/T, used to determine the enthalpy change. The report also includes calculations of the Gibbs free energy and entropy of solution. Finally, the discussion questions address potential sources of error in the experiment, focusing on temperature control and the reversibility of the solubility process, providing insights into the accuracy and limitations of the experiment.
BJR2016V1
so
l
Name…………………………….. Date / /
Partner……………………………..
Enthalpy of solution of benzoic acid
1. Theory
Most substances exhibit a variation of solubility with temperature. Thermodynamically, this
variation of solubility is described by the van't Hoff equation:
d (ln K ) H
(1)
d(1 /T) R
where K is the equilibrium constant for the dissolution process, T is the absolute
temperature (kelvin) and H
sol is the enthalpy change for the solution process. For a
process such as:
solute(s) ⇌ solute(aq) (2)
where no additional particles (such as ions) are generated, the equilibrium
constant K at a given temperature can be approximated by the
molar (mol kg-1) solubility, b, of the solute (i.e the concentration of a
saturated solution at that temperature). (The relationship between K and b will
be more complex for ionic solutes, and must take into account electrostatic
interactions between ions.)
Substances for which H is positive (i.e. have endothermic heats of solution) will be
more soluble at higher temperatures, since d(ln b)/d(1/T) is negative. Similarly,
compounds that dissolve exothermically, with d(ln b)/d(1/T) positive, are more
soluble at lower temperatures.
Equation 1 can be integrated to give the more useful form:
lnK
H 1
lnb sol
R T constan
t
(3)
This equation can then be used to determine H
sol, by measuring solubility as a
function of temperature and plotting a graph of ln b against 1/T. The slope of the
graph gives a value of
H
sol since:
H
sol = R slope (4)
Molal solubilities (moles of solute / kg of solvent) are used because these are
temperature- independent concentration units: volume of solution varies with
temperature, but mass of solvent does not.
Heats of solution are dependent on the concentration of the dissolved species in
solution. In other words, the heat released (or absorbed) by dissolving one mole of
a solute is not a fixed quantity, unless it is dissolved in an infinite amount of water!
so
l
Name…………………………….. Date / /
Partner……………………………..
Enthalpy of solution of benzoic acid
1. Theory
Most substances exhibit a variation of solubility with temperature. Thermodynamically, this
variation of solubility is described by the van't Hoff equation:
d (ln K ) H
(1)
d(1 /T) R
where K is the equilibrium constant for the dissolution process, T is the absolute
temperature (kelvin) and H
sol is the enthalpy change for the solution process. For a
process such as:
solute(s) ⇌ solute(aq) (2)
where no additional particles (such as ions) are generated, the equilibrium
constant K at a given temperature can be approximated by the
molar (mol kg-1) solubility, b, of the solute (i.e the concentration of a
saturated solution at that temperature). (The relationship between K and b will
be more complex for ionic solutes, and must take into account electrostatic
interactions between ions.)
Substances for which H is positive (i.e. have endothermic heats of solution) will be
more soluble at higher temperatures, since d(ln b)/d(1/T) is negative. Similarly,
compounds that dissolve exothermically, with d(ln b)/d(1/T) positive, are more
soluble at lower temperatures.
Equation 1 can be integrated to give the more useful form:
lnK
H 1
lnb sol
R T constan
t
(3)
This equation can then be used to determine H
sol, by measuring solubility as a
function of temperature and plotting a graph of ln b against 1/T. The slope of the
graph gives a value of
H
sol since:
H
sol = R slope (4)
Molal solubilities (moles of solute / kg of solvent) are used because these are
temperature- independent concentration units: volume of solution varies with
temperature, but mass of solvent does not.
Heats of solution are dependent on the concentration of the dissolved species in
solution. In other words, the heat released (or absorbed) by dissolving one mole of
a solute is not a fixed quantity, unless it is dissolved in an infinite amount of water!
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

BJR2016V1
This is because an enthalpy of solution is really a sum of other enthalpies, e.g. the
enthalpy to separate one
This is because an enthalpy of solution is really a sum of other enthalpies, e.g. the
enthalpy to separate one
2Enthalpy of
solution
BJR2016V1
mole of the substance into individual molecules, which would be a fixed quantity,
and the enthalpy of the solvation process (interaction with water molecules), which
would depend on the relative amount of water present. The more dilute the final
solution, the greater the heat of solution.
As the solution approaches infinite dilution, H
sol approaches an asymptotic limit
(becomes constant), and it is this value that is usually given in the literature when an
enthalpy of solution is specified.
Risk Assessment – read before conducting experiment!
Substance/
Item
Use Primary
HAZARDS
Control
Measures (in
addition to
wearing safety
glasses, lab
coat and
closed in
shoes)
Residua
l Risk
Furth
er
Actio
n and
notes
benzoic acid
5 g
dissolved in
500 mL
water and
aliquots
taken
Irritating to eyes
L
2. Experimental Procedure
In this experiment you will determine the heat of solution of benzoic acid in water.
i. Saturate about 500 ml of distilled water with benzoic acid (5 g is sufficient), by
stirring for about ten minutes with water heated to 60 C. Divide the hot solution
between four 250-mL conical flasks and stopper each with a rubber bung. Place
one each of the conical flasks in constant temperature water baths at 10, 25, 35
and 45 C. The flasks will require about half an hour to attain thermal
equilibrium; they should be shaken occasionally during this period.
ii. While the flasks are thermally equilibrating, standardise (this means use
titrations to determine the exact concentration of) the 0.1 M NaOH solution
provided, using pre- standardised HCl solution (0.1070M) Titrate the NaOH from
a burette into 25.00 mL (pipetted!) aliquots of the HCl. This must be done
accurately! Use the standardised 0.1xxx M NaOH solution to prepare 0.04xxx M
NaOH (accurately dilute say 200 mL (burette) to 500 mL (volumetric flask)).
iii. After about half an hour, prepare to take two 25-mL samples from the conical
flask in the 10 C bath. These samples of solution must be collected without
drawing up any crystalline material from the saturated solution
into the pipette. This task can be made much easier by first filtering the
solution through a large filter paper (or lab paper) in a filter funnel into another
(dry!) flask, so as to remove all of the solid. Take a few crystals of the
solid and return them to the solution so as to keep it saturated,
and return this flask to the bath for five minutes to re-equilibrate
before withdrawing the two 25-mL samples. MEASURE AND RECORD THE
solution
BJR2016V1
mole of the substance into individual molecules, which would be a fixed quantity,
and the enthalpy of the solvation process (interaction with water molecules), which
would depend on the relative amount of water present. The more dilute the final
solution, the greater the heat of solution.
As the solution approaches infinite dilution, H
sol approaches an asymptotic limit
(becomes constant), and it is this value that is usually given in the literature when an
enthalpy of solution is specified.
Risk Assessment – read before conducting experiment!
Substance/
Item
Use Primary
HAZARDS
Control
Measures (in
addition to
wearing safety
glasses, lab
coat and
closed in
shoes)
Residua
l Risk
Furth
er
Actio
n and
notes
benzoic acid
5 g
dissolved in
500 mL
water and
aliquots
taken
Irritating to eyes
L
2. Experimental Procedure
In this experiment you will determine the heat of solution of benzoic acid in water.
i. Saturate about 500 ml of distilled water with benzoic acid (5 g is sufficient), by
stirring for about ten minutes with water heated to 60 C. Divide the hot solution
between four 250-mL conical flasks and stopper each with a rubber bung. Place
one each of the conical flasks in constant temperature water baths at 10, 25, 35
and 45 C. The flasks will require about half an hour to attain thermal
equilibrium; they should be shaken occasionally during this period.
ii. While the flasks are thermally equilibrating, standardise (this means use
titrations to determine the exact concentration of) the 0.1 M NaOH solution
provided, using pre- standardised HCl solution (0.1070M) Titrate the NaOH from
a burette into 25.00 mL (pipetted!) aliquots of the HCl. This must be done
accurately! Use the standardised 0.1xxx M NaOH solution to prepare 0.04xxx M
NaOH (accurately dilute say 200 mL (burette) to 500 mL (volumetric flask)).
iii. After about half an hour, prepare to take two 25-mL samples from the conical
flask in the 10 C bath. These samples of solution must be collected without
drawing up any crystalline material from the saturated solution
into the pipette. This task can be made much easier by first filtering the
solution through a large filter paper (or lab paper) in a filter funnel into another
(dry!) flask, so as to remove all of the solid. Take a few crystals of the
solid and return them to the solution so as to keep it saturated,
and return this flask to the bath for five minutes to re-equilibrate
before withdrawing the two 25-mL samples. MEASURE AND RECORD THE
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3Enthalpy of
solution
BJR2016V1
EXACT TEMPERATURE OF THE SOLUTION IN THE FLASK IN THE
BATH AT THE TIME THE SAMPLES ARE WITHDRAWN. Titrate these
samples with 0.04xxx M NaOH using phenolphthalein as the indicator. If
solution
BJR2016V1
EXACT TEMPERATURE OF THE SOLUTION IN THE FLASK IN THE
BATH AT THE TIME THE SAMPLES ARE WITHDRAWN. Titrate these
samples with 0.04xxx M NaOH using phenolphthalein as the indicator. If
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
4Enthalpy of
solution
BJR2016V1
reasonable agreement between the duplicate titrations is not obtained, repeat the
titration on a third sample.
iv. Carry out the same procedure with the other samples in the 25, 35 and 45 C
baths, again withdrawing the samples from the conical flasks while they remain in
the baths. Use a fresh filter paper, flask and funnel at each temperature. With
the two higher- temperature samples, preheat the pipette in the 45 C bath
before pipetting; this should prevent the saturated organic acid solution
crystallising in the pipette. (Note that although crystalline material must not be
withdrawn from the flask, if any crystals form through cooling in the pipette
after the solution is drawn up, these should be titrated along with the rest of
the solution; i.e. they should be rinsed from the pipette into the titration flask
with a small volume of deionised water.)
3. Results and Calculations
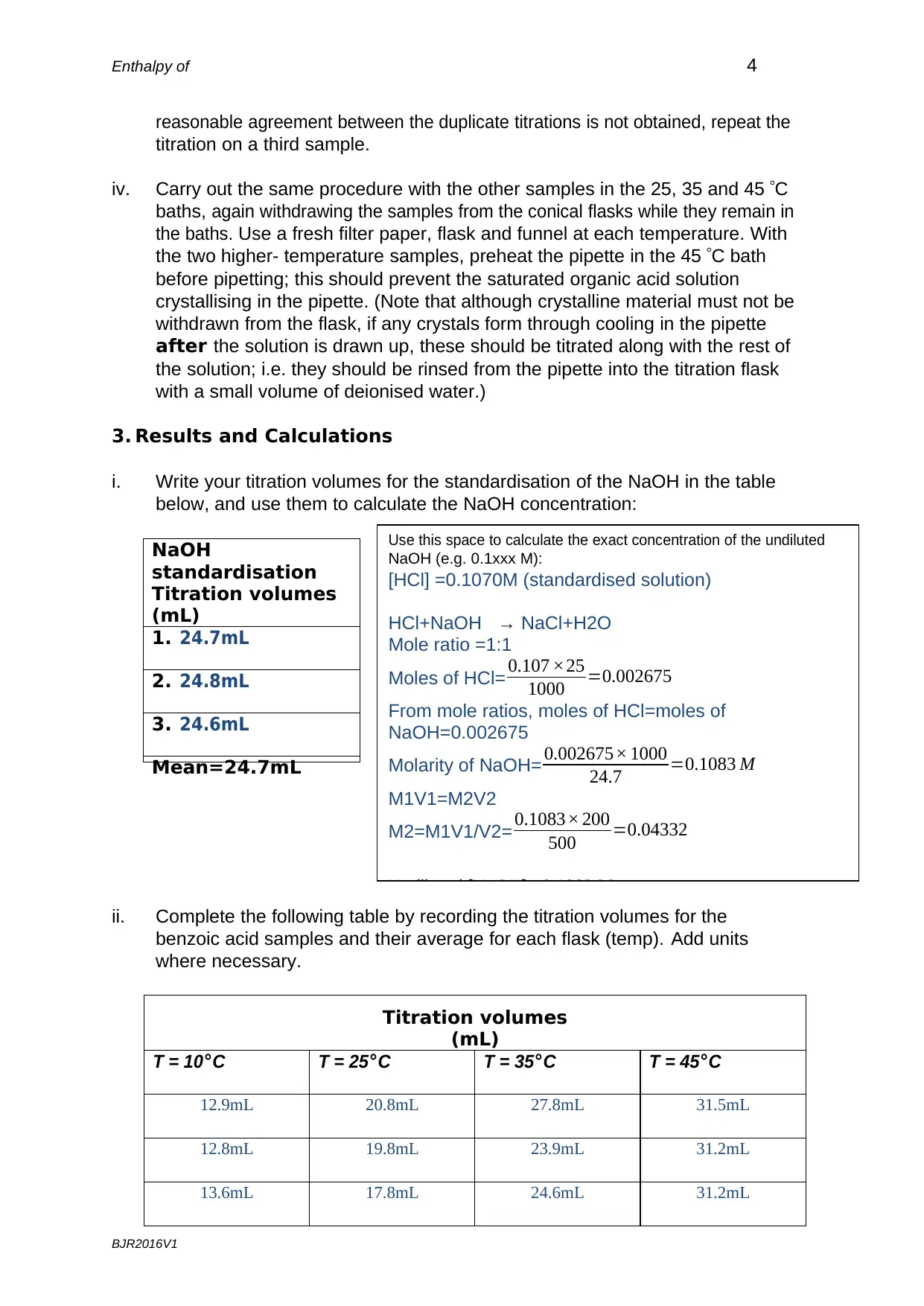
i. Write your titration volumes for the standardisation of the NaOH in the table
below, and use them to calculate the NaOH concentration:
ii. Complete the following table by recording the titration volumes for the
benzoic acid samples and their average for each flask (temp). Add units
where necessary.
Titration volumes
(mL)
T = 10°C T = 25°C T = 35°C T = 45°C
12.9mL 20.8mL 27.8mL 31.5mL
12.8mL 19.8mL 23.9mL 31.2mL
13.6mL 17.8mL 24.6mL 31.2mL
NaOH
standardisation
Titration volumes
(mL)
1. 24.7mL
2. 24.8mL
3. 24.6mL
Mean=24.7mL
Use this space to calculate the exact concentration of the undiluted
NaOH (e.g. 0.1xxx M):
[HCl] =0.1070M (standardised solution)
HCl+NaOH → NaCl+H2O
Mole ratio =1:1
Moles of HCl= 0.107 ×25
1000 =0.002675
From mole ratios, moles of HCl=moles of
NaOH=0.002675
Molarity of NaOH= 0.002675× 1000
24.7 =0.1083 M
M1V1=M2V2
M2=M1V1/V2= 0.1083× 200
500 =0.04332
Undiluted [NaOH] = 0.1083 M
solution
BJR2016V1
reasonable agreement between the duplicate titrations is not obtained, repeat the
titration on a third sample.
iv. Carry out the same procedure with the other samples in the 25, 35 and 45 C
baths, again withdrawing the samples from the conical flasks while they remain in
the baths. Use a fresh filter paper, flask and funnel at each temperature. With
the two higher- temperature samples, preheat the pipette in the 45 C bath
before pipetting; this should prevent the saturated organic acid solution
crystallising in the pipette. (Note that although crystalline material must not be
withdrawn from the flask, if any crystals form through cooling in the pipette
after the solution is drawn up, these should be titrated along with the rest of
the solution; i.e. they should be rinsed from the pipette into the titration flask
with a small volume of deionised water.)
3. Results and Calculations
i. Write your titration volumes for the standardisation of the NaOH in the table
below, and use them to calculate the NaOH concentration:
ii. Complete the following table by recording the titration volumes for the
benzoic acid samples and their average for each flask (temp). Add units
where necessary.
Titration volumes
(mL)
T = 10°C T = 25°C T = 35°C T = 45°C
12.9mL 20.8mL 27.8mL 31.5mL
12.8mL 19.8mL 23.9mL 31.2mL
13.6mL 17.8mL 24.6mL 31.2mL
NaOH
standardisation
Titration volumes
(mL)
1. 24.7mL
2. 24.8mL
3. 24.6mL
Mean=24.7mL
Use this space to calculate the exact concentration of the undiluted
NaOH (e.g. 0.1xxx M):
[HCl] =0.1070M (standardised solution)
HCl+NaOH → NaCl+H2O
Mole ratio =1:1
Moles of HCl= 0.107 ×25
1000 =0.002675
From mole ratios, moles of HCl=moles of
NaOH=0.002675
Molarity of NaOH= 0.002675× 1000
24.7 =0.1083 M
M1V1=M2V2
M2=M1V1/V2= 0.1083× 200
500 =0.04332
Undiluted [NaOH] = 0.1083 M

5Enthalpy of
solution
BJR2016V1
mean =
12.9+ 12.8
2 =12.85 mL
mean
=19.8mL
mean
=23.9mL
mean =
31.2+ 31.2
2 =31.2 mL
solution
BJR2016V1
mean =
12.9+ 12.8
2 =12.85 mL
mean
=19.8mL
mean
=23.9mL
mean =
31.2+ 31.2
2 =31.2 mL
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6Enthalpy of
solution
BJR2016V1
iii. From your mean titration figures, calculate the solubility (c) of the organic acid
in moles per litre at each of the four temperatures; then, using the table of
water densities given in Appendix A, calculate the molar solubility, b (ie the number
of moles of solute per kilogram of solvent), for each solution, assuming the solute
occupies zero volume. Record the values of c, b, ln b, temperature (K) and 1/T
in the table below, but use the space just below to show a sample calculation
of c and b for one of the solutions:
Reaction between sodium hydroxide and benzoic acid is as shown below
C6H5COOH(aq)+NaOH(aq) →C6H5COO-Na+(aq)+H2O(l)
The mole ration=1:1
At T = 10°C
Moles of NaOH=0.04332 × 12.85
1000 =5.56662×10−4 =moles of C 6 H 5 COOH (aq)
solubility in moles per litre=
moles
volume (litre) =5.56662 ×10−4 ×1000
25 =0.0222665 M
mass of solvent=volume×density= 25
1000 ×0.9997=0.024993 kg
molar solubility= moles
mass of solvent ( Kg) = 5.56662× 10−4
0.024993 =0.022273moles /kg of solvent
T = 25°C
Moles of NaOH=0.04332 × 19.8
1000 =8.57736 ×10−4=moles of C 6 H 5 COOH (aq)
solubility in moles per litre=
moles
volume (litre) = 8.57736× 10−4 × 1000
25 =0.034 3094 M
mass of solvent=volume ×density= 25
1000 ×0.9971=0. 024928 kg
molar solubility=
moles
mass of solvent ( Kg) = 8.57736 ×10−4
0.024928 =0. 034409 moles /kg of solvent
T = 35°C
Moles of NaOH= 0.04332 × 23.9
1000 =1.0353448× 10−3=moles of C 6 H 5COOH ( aq)
solubility in moles per litre=
moles
volume (litre) =1.0353448 ×10−3 × 1000
25 =0.0414 14 M
mass of solvent=volume×density= 25
1000 ×0.9941=0.024 8525 kg
molar solubility=
moles
mass of solvent (Kg)= 1.0353448× 10−3
0.024 8525 =0. 0416596 moles /kg of solvent
T = 45°C
Moles of NaOH=0.04332 × 31.2
1000 =1.35158× 10−3=moles of C 6 H 5COOH (aq)
solution
BJR2016V1
iii. From your mean titration figures, calculate the solubility (c) of the organic acid
in moles per litre at each of the four temperatures; then, using the table of
water densities given in Appendix A, calculate the molar solubility, b (ie the number
of moles of solute per kilogram of solvent), for each solution, assuming the solute
occupies zero volume. Record the values of c, b, ln b, temperature (K) and 1/T
in the table below, but use the space just below to show a sample calculation
of c and b for one of the solutions:
Reaction between sodium hydroxide and benzoic acid is as shown below
C6H5COOH(aq)+NaOH(aq) →C6H5COO-Na+(aq)+H2O(l)
The mole ration=1:1
At T = 10°C
Moles of NaOH=0.04332 × 12.85
1000 =5.56662×10−4 =moles of C 6 H 5 COOH (aq)
solubility in moles per litre=
moles
volume (litre) =5.56662 ×10−4 ×1000
25 =0.0222665 M
mass of solvent=volume×density= 25
1000 ×0.9997=0.024993 kg
molar solubility= moles
mass of solvent ( Kg) = 5.56662× 10−4
0.024993 =0.022273moles /kg of solvent
T = 25°C
Moles of NaOH=0.04332 × 19.8
1000 =8.57736 ×10−4=moles of C 6 H 5 COOH (aq)
solubility in moles per litre=
moles
volume (litre) = 8.57736× 10−4 × 1000
25 =0.034 3094 M
mass of solvent=volume ×density= 25
1000 ×0.9971=0. 024928 kg
molar solubility=
moles
mass of solvent ( Kg) = 8.57736 ×10−4
0.024928 =0. 034409 moles /kg of solvent
T = 35°C
Moles of NaOH= 0.04332 × 23.9
1000 =1.0353448× 10−3=moles of C 6 H 5COOH ( aq)
solubility in moles per litre=
moles
volume (litre) =1.0353448 ×10−3 × 1000
25 =0.0414 14 M
mass of solvent=volume×density= 25
1000 ×0.9941=0.024 8525 kg
molar solubility=
moles
mass of solvent (Kg)= 1.0353448× 10−3
0.024 8525 =0. 0416596 moles /kg of solvent
T = 45°C
Moles of NaOH=0.04332 × 31.2
1000 =1.35158× 10−3=moles of C 6 H 5COOH (aq)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7Enthalpy of
solution
BJR2016V1
solubility in moles per litre=
moles
volume (litre) =1.35158 ×10−3 × 1000
25 =0.054063 M
mass of solvent=volume×density= 25
1000 ×0.9902=0.024755 kg
molar solubility= moles
mass of solvent ( Kg) = 1.35158× 10−3
0.024755 =0.054598 moles /kg of solvent
T (K) 1/T (K-1 ) c (M) b (mol/kg) ln
b
283 0.00353357 0.0222665 0.022273 -3.8043801
298 0.0033557 0.0343094 0.034409 -3.36943712
308 0.00324675 041414 0.0416596 -3.17975127
318 0.00314465 0.054063 0.054598 -2.90775803
iv. Plot a graph of ln b against 1/T and attach it to this report. Measure
the slope of the graph and use it to determine H
sol using equation (4);
neglect any obvious outlying points. The optimum slope is best determined by a
linear regression (least squares) analysis – this can be done simply using Excel.
Show your calculation for H
sol here:
-4
-3.5
-3
-2.5
-2
-1.5
-1
-0.5
0
f(x) = − 2262.40771932484 x + 4.19624713904429
R² = 0.995847370559982
A graph of ln b against 1/T
1/T (K-1)
ln b
Use the slope error (see next page) to determine an error
value for
H
. Show all working!
H
and its error term must be
reported to the correct number of significant figures etc.
slope =-2262.40772 103.304936
solution
BJR2016V1
solubility in moles per litre=
moles
volume (litre) =1.35158 ×10−3 × 1000
25 =0.054063 M
mass of solvent=volume×density= 25
1000 ×0.9902=0.024755 kg
molar solubility= moles
mass of solvent ( Kg) = 1.35158× 10−3
0.024755 =0.054598 moles /kg of solvent
T (K) 1/T (K-1 ) c (M) b (mol/kg) ln
b
283 0.00353357 0.0222665 0.022273 -3.8043801
298 0.0033557 0.0343094 0.034409 -3.36943712
308 0.00324675 041414 0.0416596 -3.17975127
318 0.00314465 0.054063 0.054598 -2.90775803
iv. Plot a graph of ln b against 1/T and attach it to this report. Measure
the slope of the graph and use it to determine H
sol using equation (4);
neglect any obvious outlying points. The optimum slope is best determined by a
linear regression (least squares) analysis – this can be done simply using Excel.
Show your calculation for H
sol here:
-4
-3.5
-3
-2.5
-2
-1.5
-1
-0.5
0
f(x) = − 2262.40771932484 x + 4.19624713904429
R² = 0.995847370559982
A graph of ln b against 1/T
1/T (K-1)
ln b
Use the slope error (see next page) to determine an error
value for
H
. Show all working!
H
and its error term must be
reported to the correct number of significant figures etc.
slope =-2262.40772 103.304936

8Enthalpy of
solution
BJR2016V1
H
= H
sol = R slope =(-2262.40772 103.304936) =18810.789
858.928894=+18.810789858928894 kJ mol-1
solution
BJR2016V1
H
= H
sol = R slope =(-2262.40772 103.304936) =18810.789
858.928894=+18.810789858928894 kJ mol-1
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9Enthalpy of
solution
BJR2016V1
v. Use the equation of the line you have fitted to your data to calculate the value of ln b
at 298.15 K, and use:
G = RT ln b
to determine the standard free energy of solution, G
sol, at 298.15 K.
lnb=−2262.40772 1
T + 4.1962
But T=298.15,
lnb= (−2262.40772× 1
298.15 )+ 4.1962=−3.39195
G
sol =−−3.39195 ×8.3144598 ×298.15=8408.50208 J mol-1
vi. Use your G
sol and H
sol values to determine the entropy of solution, S
sol,
for the organic acid at 298 K, from the equation: G
= H
TS
.
From G
= H
TS
.
S
∆ H0−∆ G0
T =18810.789−8408.50208
298 =34.907 J/K
solution
BJR2016V1
v. Use the equation of the line you have fitted to your data to calculate the value of ln b
at 298.15 K, and use:
G = RT ln b
to determine the standard free energy of solution, G
sol, at 298.15 K.
lnb=−2262.40772 1
T + 4.1962
But T=298.15,
lnb= (−2262.40772× 1
298.15 )+ 4.1962=−3.39195
G
sol =−−3.39195 ×8.3144598 ×298.15=8408.50208 J mol-1
vi. Use your G
sol and H
sol values to determine the entropy of solution, S
sol,
for the organic acid at 298 K, from the equation: G
= H
TS
.
From G
= H
TS
.
S
∆ H0−∆ G0
T =18810.789−8408.50208
298 =34.907 J/K
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1
0
Enthalpy of
solution
BJR2016V1
4. Discussion questions
i. List the steps in the procedure which you think will introduce the most error
(due to their nature, not due to your mistakes!). Explain why each of the listed
steps creates greater uncertainty in your results.
Titration of the samples with NaOH procedure. Because solubility of benzoic
acid is a reversible process, slight temperature variations will shift the
equilibrium. Inconsistent titre values with as large range as 23.9mL-27.3mL
are thus recorded for the same number of moles of NaOH due the possible
difference in the solubilities.
Taking the two 25-mL samples from the conical flasks in the 10oC, 25oC, 35oC
and 45oC baths. This is because this procedure required collection of the
samples without drawing any crystal. Since dissolving benzoic acid is
reversible process, it apparent that slight variation in temperature will affect
amount its solubility. The recorded temperature at of the solution at the time
the sample were withdrawn might thus not give the exact solubility
characteristics due to the reversible nature of the solubility.
ii. Why does the sign of your value of S
sol, the entropy of solution of
benzoic acid, make sense (think about change in disorder across equation
2)?
Consider
solute(s) ⇌ solute(aq)
For benzoic acid, this process is endothermic, as the temperature increases, dissociation process is
favoured. This process depends on Gibbs free energy given by the equation
ΔG = ΔH -TΔS
At lower temperatures ΔG is more positive. As the temperature increases, ΔG
becomes more negative. TΔS is thus positive because as temperature increases,
forward reaction is favoured and the number of particles of benzoic acid increases
leading to an increase in entropy
iii. Ammonia is more soluble in water at lower temperatures: will its enthalpy of
solution be positive (endothermic) or negative (exothermic)? Explain briefly – you
may sketch roughly what the van’t Hoff plot would look like in this case.
Ammonia is more soluble at lower temperature, consider absolute temperatures
T1<T2. Now 1/T1>1/T2. The solubility per kg of the solvent b1>b2 hence ln b1>lnb2
From the given information, the gradient=
− ( lnb2−lnb 1 )
−( 1
T 2− 1
T 2 ) =+¿
The enthalpy of solution
H
sol = R slope=-H
so=-(exothermic)
0
Enthalpy of
solution
BJR2016V1
4. Discussion questions
i. List the steps in the procedure which you think will introduce the most error
(due to their nature, not due to your mistakes!). Explain why each of the listed
steps creates greater uncertainty in your results.
Titration of the samples with NaOH procedure. Because solubility of benzoic
acid is a reversible process, slight temperature variations will shift the
equilibrium. Inconsistent titre values with as large range as 23.9mL-27.3mL
are thus recorded for the same number of moles of NaOH due the possible
difference in the solubilities.
Taking the two 25-mL samples from the conical flasks in the 10oC, 25oC, 35oC
and 45oC baths. This is because this procedure required collection of the
samples without drawing any crystal. Since dissolving benzoic acid is
reversible process, it apparent that slight variation in temperature will affect
amount its solubility. The recorded temperature at of the solution at the time
the sample were withdrawn might thus not give the exact solubility
characteristics due to the reversible nature of the solubility.
ii. Why does the sign of your value of S
sol, the entropy of solution of
benzoic acid, make sense (think about change in disorder across equation
2)?
Consider
solute(s) ⇌ solute(aq)
For benzoic acid, this process is endothermic, as the temperature increases, dissociation process is
favoured. This process depends on Gibbs free energy given by the equation
ΔG = ΔH -TΔS
At lower temperatures ΔG is more positive. As the temperature increases, ΔG
becomes more negative. TΔS is thus positive because as temperature increases,
forward reaction is favoured and the number of particles of benzoic acid increases
leading to an increase in entropy
iii. Ammonia is more soluble in water at lower temperatures: will its enthalpy of
solution be positive (endothermic) or negative (exothermic)? Explain briefly – you
may sketch roughly what the van’t Hoff plot would look like in this case.
Ammonia is more soluble at lower temperature, consider absolute temperatures
T1<T2. Now 1/T1>1/T2. The solubility per kg of the solvent b1>b2 hence ln b1>lnb2
From the given information, the gradient=
− ( lnb2−lnb 1 )
−( 1
T 2− 1
T 2 ) =+¿
The enthalpy of solution
H
sol = R slope=-H
so=-(exothermic)

1
1
Enthalpy of
solution
BJR2016V1
iv. The enthalpy of solution of benzoic acid is H for the reaction shown in
Equation 2. Can you think of a side reaction (equilibrium) that will occur when
solute(aq) is C6H5COOH(aq)? You can find the equilibrium constant (K) for this side
reaction in an SI Data book; given the value of this constant, what effect do you
think this side reaction has on your value of Hsol?
The pKa for benzoic acid from the SD tables is 4.20. This value describes the acid
dissociation. Generally, a larger value as this shows that this is a weak acid. From the
equilibrium equation, introduction of more solute by the side reaction as predicted by Le
Chatelier principle implies that the equilibrium will shift to the left. This will imply that the
concentration at the solubility per kg of solvent will decline. Since solubility per kg of the
solvent is directly proportional to Hsol, this side reaction will lower Hsol. The effect is however small
due to the weaker strength of the acid.
v. The enthalpy of solution of benzoic acid at infinite dilution is recorded as 28.7
kJ mol-1. Why is this expected to be different from your measured value?
The measured value in this case is 18.810789 858928894 kJ mol-1
The measured value is less than the value the expected value for infinite dilution. This difference can be attributed to
the following reasons: Van’t Hoff’s equation assumes that ΔH and ΔS for benzoic acid do
not vary with temperature. Any variations in these quantities introduces an
error on the evaluated experimental error.
Systematic errors in the experiment including the possible incorrect reading
of the temperatures and titers values as seen by the wide variations in the
titer values could explain the variations between experimental and
theoretical enthalpy.
The calorimetry system in the experiment lacked accuracy and precision since there
were instance of heat loss as the solutions were being transferred within the
volumetric flasks. Insulations was generally poor.
vi. Do you think that Hsol for benzoic acid changes much with temperature?
Explain your answer briefly (look at the slope of your graph).
Hsol for benzoic acid changes substantially with changes in temperature. This
clearly shown be a large gradient of -2262.40772 103.304936(). From the
formula
Hsol = R slope implying that the larger the gradient the greater the
variation of Hsol with temperature.
Appendix A
Variation of water density with temperature.
Temperature
(C)
Density (kg
L-1)
10 0.9997
25 0.9971
35 0.9941
1
Enthalpy of
solution
BJR2016V1
iv. The enthalpy of solution of benzoic acid is H for the reaction shown in
Equation 2. Can you think of a side reaction (equilibrium) that will occur when
solute(aq) is C6H5COOH(aq)? You can find the equilibrium constant (K) for this side
reaction in an SI Data book; given the value of this constant, what effect do you
think this side reaction has on your value of Hsol?
The pKa for benzoic acid from the SD tables is 4.20. This value describes the acid
dissociation. Generally, a larger value as this shows that this is a weak acid. From the
equilibrium equation, introduction of more solute by the side reaction as predicted by Le
Chatelier principle implies that the equilibrium will shift to the left. This will imply that the
concentration at the solubility per kg of solvent will decline. Since solubility per kg of the
solvent is directly proportional to Hsol, this side reaction will lower Hsol. The effect is however small
due to the weaker strength of the acid.
v. The enthalpy of solution of benzoic acid at infinite dilution is recorded as 28.7
kJ mol-1. Why is this expected to be different from your measured value?
The measured value in this case is 18.810789 858928894 kJ mol-1
The measured value is less than the value the expected value for infinite dilution. This difference can be attributed to
the following reasons: Van’t Hoff’s equation assumes that ΔH and ΔS for benzoic acid do
not vary with temperature. Any variations in these quantities introduces an
error on the evaluated experimental error.
Systematic errors in the experiment including the possible incorrect reading
of the temperatures and titers values as seen by the wide variations in the
titer values could explain the variations between experimental and
theoretical enthalpy.
The calorimetry system in the experiment lacked accuracy and precision since there
were instance of heat loss as the solutions were being transferred within the
volumetric flasks. Insulations was generally poor.
vi. Do you think that Hsol for benzoic acid changes much with temperature?
Explain your answer briefly (look at the slope of your graph).
Hsol for benzoic acid changes substantially with changes in temperature. This
clearly shown be a large gradient of -2262.40772 103.304936(). From the
formula
Hsol = R slope implying that the larger the gradient the greater the
variation of Hsol with temperature.
Appendix A
Variation of water density with temperature.
Temperature
(C)
Density (kg
L-1)
10 0.9997
25 0.9971
35 0.9941
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 13
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





