Biochemistry Report: Enzymes, DNA, and Cell Processes Overview
VerifiedAdded on 2021/01/02
|11
|2387
|184
Report
AI Summary
This report provides a comprehensive overview of biological chemistry, covering essential concepts such as the differences between monomers and polymers, with examples like carbohydrates, lipids, and proteins, and their structural relationship to function. The role of pH and buffers in biological systems is explained, along with the importance and function of enzymes, factors affecting their activity, and the structure of DNA, its role in cell division and protein synthesis, and the relationship between its structure and function. The report emphasizes the significance of these biochemical components in living organisms and their interconnectedness within biological systems. The document is contributed by a student to be published on the website Desklib, a platform which provides all the necessary AI based study tools for students.

Biological Chemistry
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of Contents
INTRODUCTION...........................................................................................................................1
TASK 1............................................................................................................................................1
1.1 Difference Between Polymers and Monomers......................................................................1
2.1 Structure and Function of Carbohydrates, Lipids and Proteins.............................................2
TASK 2............................................................................................................................................4
3.1 pH and Buffer and their involvement in Biological System..................................................4
TASK 3............................................................................................................................................5
4.1 Function and Importance of Enzymes...................................................................................5
5.1 Factors that affects Enzyme Factors......................................................................................6
TASK 4............................................................................................................................................6
6.1 Structure of DNA and its Role in Cell Division and Protein Synthesis and Relation to its
Function.......................................................................................................................................6
CONCLUSION................................................................................................................................8
REFERENCES................................................................................................................................9
INTRODUCTION...........................................................................................................................1
TASK 1............................................................................................................................................1
1.1 Difference Between Polymers and Monomers......................................................................1
2.1 Structure and Function of Carbohydrates, Lipids and Proteins.............................................2
TASK 2............................................................................................................................................4
3.1 pH and Buffer and their involvement in Biological System..................................................4
TASK 3............................................................................................................................................5
4.1 Function and Importance of Enzymes...................................................................................5
5.1 Factors that affects Enzyme Factors......................................................................................6
TASK 4............................................................................................................................................6
6.1 Structure of DNA and its Role in Cell Division and Protein Synthesis and Relation to its
Function.......................................................................................................................................6
CONCLUSION................................................................................................................................8
REFERENCES................................................................................................................................9
INTRODUCTION
Biochemistry sometimes called as Biological Chemistry and it is the study of chemical
processes with in the living organisms. It is related to the molecular biology and through this
molecular mechanism the genetic information is encoded in the DNA which is part of result of
life processes. This report is providing difference between the monomers and polymer molecules
on the base of examples. The structure of carbohydrates, lipids and proteins is related to their
function is explained inn the study. The concepts and importance of pH and buffer, and how they
are involved in the biological system is explained. The importance and functions of enzymes are
given in report. Factors that can affect the enzymes actions are found in report. The structure of
DNA and its role in the cell division and protein synthesis is explained with related to its
function.
TASK 1
1.1 Difference Between Polymers and Monomers
Monomers
Monomer is a molecule that is the unit of polymer and is one of unit part of protein. By
making the different kind of bond these monomers are connected to other monomers to make the
series or chain of monomer which is known as polymer. The monomers can be natural or
synthetic. The Oligomers are the polymers which are consisted of small amount of monomers
sub units. The monomeric protein are the small molecule of protein that are combined to form
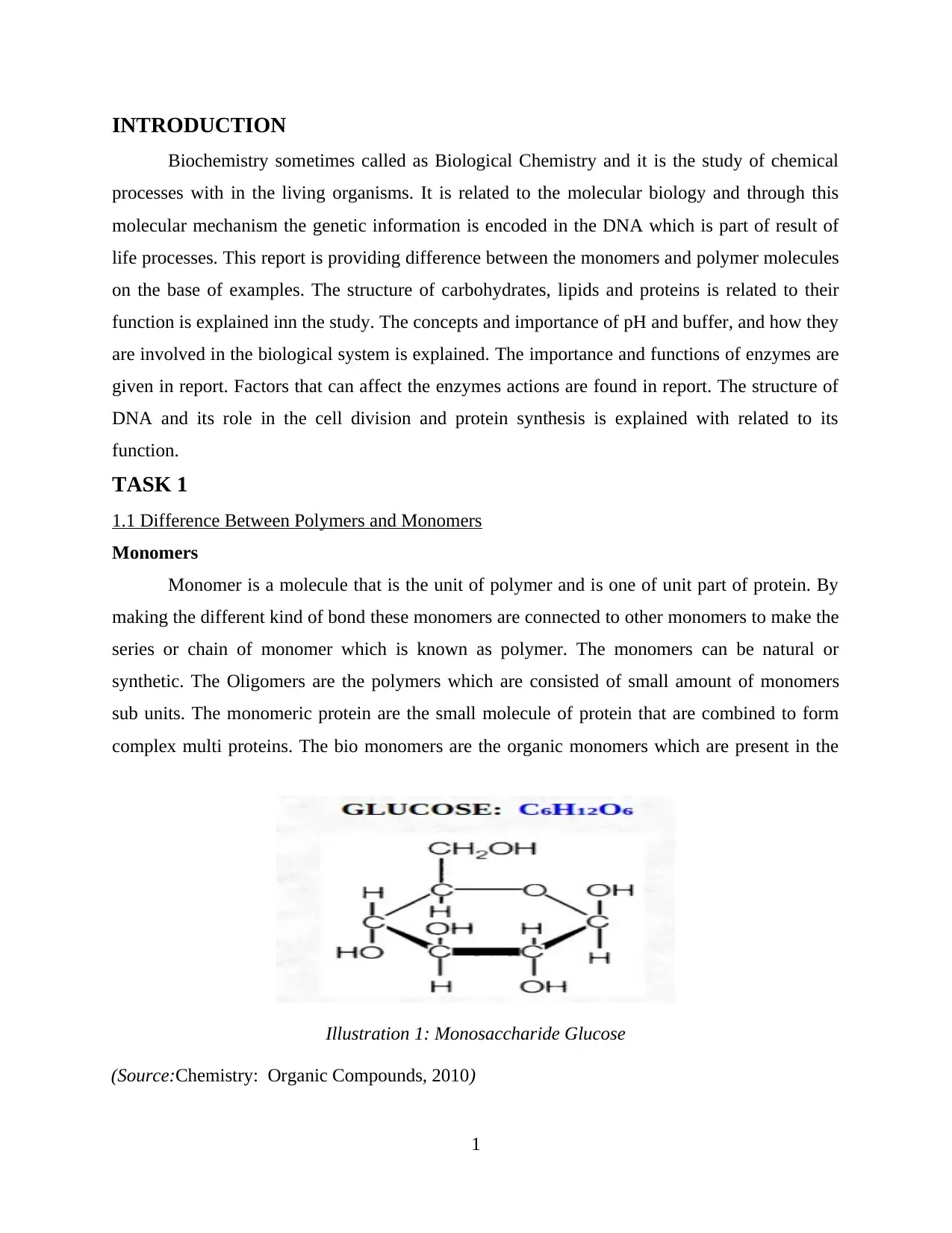
complex multi proteins. The bio monomers are the organic monomers which are present in the
1
Illustration 1: Monosaccharide Glucose
(Source:Chemistry: Organic Compounds, 2010)
Biochemistry sometimes called as Biological Chemistry and it is the study of chemical
processes with in the living organisms. It is related to the molecular biology and through this
molecular mechanism the genetic information is encoded in the DNA which is part of result of
life processes. This report is providing difference between the monomers and polymer molecules
on the base of examples. The structure of carbohydrates, lipids and proteins is related to their
function is explained inn the study. The concepts and importance of pH and buffer, and how they
are involved in the biological system is explained. The importance and functions of enzymes are
given in report. Factors that can affect the enzymes actions are found in report. The structure of
DNA and its role in the cell division and protein synthesis is explained with related to its
function.
TASK 1
1.1 Difference Between Polymers and Monomers
Monomers
Monomer is a molecule that is the unit of polymer and is one of unit part of protein. By
making the different kind of bond these monomers are connected to other monomers to make the
series or chain of monomer which is known as polymer. The monomers can be natural or
synthetic. The Oligomers are the polymers which are consisted of small amount of monomers
sub units. The monomeric protein are the small molecule of protein that are combined to form
complex multi proteins. The bio monomers are the organic monomers which are present in the
1
Illustration 1: Monosaccharide Glucose
(Source:Chemistry: Organic Compounds, 2010)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
living organisms (Wetteland and Liu, 2018). Monomers represents the huge number of
molecules which are differentiated in different groups and that are sugar, Alcohols, amines,
acrylics and epoxides. These are some examples of monomers.
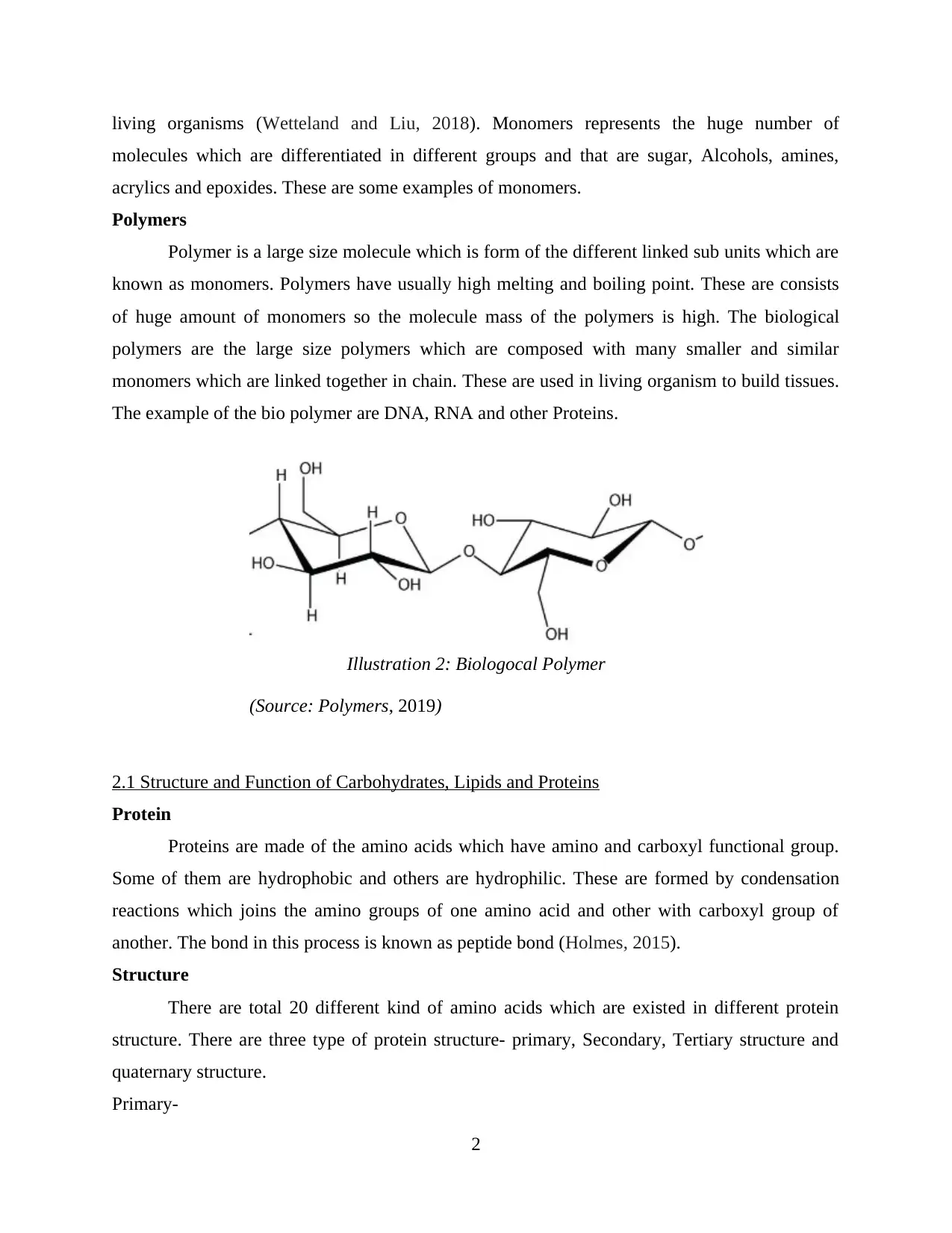
Polymers
Polymer is a large size molecule which is form of the different linked sub units which are
known as monomers. Polymers have usually high melting and boiling point. These are consists
of huge amount of monomers so the molecule mass of the polymers is high. The biological
polymers are the large size polymers which are composed with many smaller and similar
monomers which are linked together in chain. These are used in living organism to build tissues.
The example of the bio polymer are DNA, RNA and other Proteins.
2.1 Structure and Function of Carbohydrates, Lipids and Proteins
Protein
Proteins are made of the amino acids which have amino and carboxyl functional group.
Some of them are hydrophobic and others are hydrophilic. These are formed by condensation
reactions which joins the amino groups of one amino acid and other with carboxyl group of
another. The bond in this process is known as peptide bond (Holmes, 2015).
Structure
There are total 20 different kind of amino acids which are existed in different protein
structure. There are three type of protein structure- primary, Secondary, Tertiary structure and
quaternary structure.
Primary-
2
Illustration 2: Biologocal Polymer
(Source: Polymers, 2019)
molecules which are differentiated in different groups and that are sugar, Alcohols, amines,
acrylics and epoxides. These are some examples of monomers.
Polymers
Polymer is a large size molecule which is form of the different linked sub units which are
known as monomers. Polymers have usually high melting and boiling point. These are consists
of huge amount of monomers so the molecule mass of the polymers is high. The biological
polymers are the large size polymers which are composed with many smaller and similar
monomers which are linked together in chain. These are used in living organism to build tissues.
The example of the bio polymer are DNA, RNA and other Proteins.
2.1 Structure and Function of Carbohydrates, Lipids and Proteins
Protein
Proteins are made of the amino acids which have amino and carboxyl functional group.
Some of them are hydrophobic and others are hydrophilic. These are formed by condensation
reactions which joins the amino groups of one amino acid and other with carboxyl group of
another. The bond in this process is known as peptide bond (Holmes, 2015).
Structure
There are total 20 different kind of amino acids which are existed in different protein
structure. There are three type of protein structure- primary, Secondary, Tertiary structure and
quaternary structure.
Primary-
2
Illustration 2: Biologocal Polymer
(Source: Polymers, 2019)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

It is referred as sequence of amino acids. Ones it forms than it immediately folds and
takes the 3D structure. The shape of protein is depended on amino acid Sequence.
Secondary-
This structure is 3D and form of local segments of protein. Two most common protein
structures are alpha helix and beta sheets.
Tertiary-
These are spherical proteins like enzymes and less random appearing folding of protein
Quaternary-
This type of proteins are comprises two or more polypeptide chains like Haemoglobin is
made of 4-chains.
Functions
1. Structural- make tissues
2. Contractile – muscle like actin and myosin
3. Make antibodies and clotting.
4. Form Hormone like insulin.
5. Make haemoglobin.
Lipids
It is known as a chemical substance which is insoluble in water and insoluble in alcohol,
ether and chloroform (Vinogradov, 2018). Lipids are the important component of living cells.
Lipids are main constituents of plant and animal cells. Cholesterol and triglycerides are kind of
lipids.
3
Illustration 3: Lipid (Cholesterol)
(Source:What is cholesterol? Did you know it is harmless?, 2018)
takes the 3D structure. The shape of protein is depended on amino acid Sequence.
Secondary-
This structure is 3D and form of local segments of protein. Two most common protein
structures are alpha helix and beta sheets.
Tertiary-
These are spherical proteins like enzymes and less random appearing folding of protein
Quaternary-
This type of proteins are comprises two or more polypeptide chains like Haemoglobin is
made of 4-chains.
Functions
1. Structural- make tissues
2. Contractile – muscle like actin and myosin
3. Make antibodies and clotting.
4. Form Hormone like insulin.
5. Make haemoglobin.
Lipids
It is known as a chemical substance which is insoluble in water and insoluble in alcohol,
ether and chloroform (Vinogradov, 2018). Lipids are the important component of living cells.
Lipids are main constituents of plant and animal cells. Cholesterol and triglycerides are kind of
lipids.
3
Illustration 3: Lipid (Cholesterol)
(Source:What is cholesterol? Did you know it is harmless?, 2018)

Structure
The insoluble property of the natural fats and oil is due to the long chained fatty acid
which are highly insoluble in water and this are of hydrophobic. The example of hydrophilic
lipids is Glycerol.
Function
1. Energy storage
2. Membrane Component
3. Insulation
4. Protective coating around cells.
5. Hormones, vitamin, and other metabolites.
Carbohydrates
Carbohydrates are defined as neutral compounds of carbon, hydrogen and oxygen. These
are comes in simple form like sugar and complex such as starches and fiber. The living organism
break down the starches in glucose top feed the cells.
Structure
The bond between the different monomers defines the 3D structure of Carbohydrates.
Different type of carbohydrates have different structure as per individual chemical bond.
Functions
1. Providing energy and regulation of blood glucose.
2. Breakdown fatty acids and prevent the Ketones.
3. Flavour and Sweeteners.
TASK 2
3.1 pH and Buffer and their involvement in Biological System
pH
The pH is a measure of acidity and alkalinity of water soluble substances. The full form
of pH is potential of Hydrogen. The pH value is number from 0 -14 in which 7 is for neutral
point and 1 is considered as most acidic and 14 as most alkalinity. The example of neutral pH 7
is pure water, 1 pH is of gastric acid and 14 of Liquid drain cleaner.
Role of pH in Biological System
All the living things are based on the water based system. This make them more
depended on the aqueous equilibria and specially on the acid-base equilibria. This make pH more
4
The insoluble property of the natural fats and oil is due to the long chained fatty acid
which are highly insoluble in water and this are of hydrophobic. The example of hydrophilic
lipids is Glycerol.
Function
1. Energy storage
2. Membrane Component
3. Insulation
4. Protective coating around cells.
5. Hormones, vitamin, and other metabolites.
Carbohydrates
Carbohydrates are defined as neutral compounds of carbon, hydrogen and oxygen. These
are comes in simple form like sugar and complex such as starches and fiber. The living organism
break down the starches in glucose top feed the cells.
Structure
The bond between the different monomers defines the 3D structure of Carbohydrates.
Different type of carbohydrates have different structure as per individual chemical bond.
Functions
1. Providing energy and regulation of blood glucose.
2. Breakdown fatty acids and prevent the Ketones.
3. Flavour and Sweeteners.
TASK 2
3.1 pH and Buffer and their involvement in Biological System
pH
The pH is a measure of acidity and alkalinity of water soluble substances. The full form
of pH is potential of Hydrogen. The pH value is number from 0 -14 in which 7 is for neutral
point and 1 is considered as most acidic and 14 as most alkalinity. The example of neutral pH 7
is pure water, 1 pH is of gastric acid and 14 of Liquid drain cleaner.
Role of pH in Biological System
All the living things are based on the water based system. This make them more
depended on the aqueous equilibria and specially on the acid-base equilibria. This make pH more
4
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

important in the living organism. The biological element or catalysts are more dependent on the
certain pH level. For example the enzymes only can work as catalyst on certain pH. Enzymes are
the catalyst in different biological reactions. To complete this reaction the particular pH is
required. The pH of body can change the structure of enzymes (Akoh, 2017).
Buffer
The buffer is the solution that can maintain the pH and even if diluted or if any relative
small amount of strong acid or base added in solution. The buffer solution can be made by
mixing weak acid with one of its salt or mixing any base with one of its salt.
Role of Buffer in Biological System
To maintain the certain pH in human body the buffer is needed in the human and animal
body to maintain the optimum state of protonation of enzymes. The buffer solution maintain the
pH of living organism pH near at certain pH. The different pH buffers in the human body are-
Bicarbonate Buffer, Phosphate Buffer, Protein Buffer and Haemoglobin Buffer. The bicarbonate
buffer is used to maintain the pH of blood by making the Co2 in acidic condition and in alkaline
condition the pH buffer is control by excretion of bicarbonate ions through the urine. The
phosphate buffer maintain the pH for environment of all cell. The protein buffer maintain the pH
of carboxylic group. And the haemoglobin buffer maintain the buffer of haemoglobin for the
respiratory system (Jakobek, 2015).
TASK 3
4.1 Function and Importance of Enzymes
Enzymes help to speed up the reaction in the human body. Enzymes bind and alter the
molecules in the different ways. They important in respiration, digesting food, muscle and nerve
system and many other roles. The enzymes are build of protein which is folded in different
shapes. These enzymes are presented in all living organism. Enzymes work as catalyst which are
helpful to speed up the different reactions (Michel, 2018).
In Digestive System
The enzymes break down the complex in small molecules. The proteins are break down
in glucose to provide the fuel to whole body.
DNA Replication
At the time of cell division the DNA need to be copied. Enzymes help to unwinding the
DNA coils and copy the information.
5
certain pH level. For example the enzymes only can work as catalyst on certain pH. Enzymes are
the catalyst in different biological reactions. To complete this reaction the particular pH is
required. The pH of body can change the structure of enzymes (Akoh, 2017).
Buffer
The buffer is the solution that can maintain the pH and even if diluted or if any relative
small amount of strong acid or base added in solution. The buffer solution can be made by
mixing weak acid with one of its salt or mixing any base with one of its salt.
Role of Buffer in Biological System
To maintain the certain pH in human body the buffer is needed in the human and animal
body to maintain the optimum state of protonation of enzymes. The buffer solution maintain the
pH of living organism pH near at certain pH. The different pH buffers in the human body are-
Bicarbonate Buffer, Phosphate Buffer, Protein Buffer and Haemoglobin Buffer. The bicarbonate
buffer is used to maintain the pH of blood by making the Co2 in acidic condition and in alkaline
condition the pH buffer is control by excretion of bicarbonate ions through the urine. The
phosphate buffer maintain the pH for environment of all cell. The protein buffer maintain the pH
of carboxylic group. And the haemoglobin buffer maintain the buffer of haemoglobin for the
respiratory system (Jakobek, 2015).
TASK 3
4.1 Function and Importance of Enzymes
Enzymes help to speed up the reaction in the human body. Enzymes bind and alter the
molecules in the different ways. They important in respiration, digesting food, muscle and nerve
system and many other roles. The enzymes are build of protein which is folded in different
shapes. These enzymes are presented in all living organism. Enzymes work as catalyst which are
helpful to speed up the different reactions (Michel, 2018).
In Digestive System
The enzymes break down the complex in small molecules. The proteins are break down
in glucose to provide the fuel to whole body.
DNA Replication
At the time of cell division the DNA need to be copied. Enzymes help to unwinding the
DNA coils and copy the information.
5
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Liver Enzymes
The different toxins are breakdown in the liver and there are wide range of enzymes in
liver.
5.1 Factors that affects Enzyme Factors
Temperature
Temperature generally speed up the reactions but the high temperature can change the
structure of enzyme that reduce its effectiveness or stop working.
pH Level
The pH level of living organism have certain for the operation of enzymes. Outer this
range the effectiveness of these enzymes slow down. The extreme pH can damage the enzyme.
Enzyme Concentration
The concentration of enzyme can speed up the biological reaction as long as enzymes are
available to bind the reactors and after this the speed of the reaction will slowdown.
Substrate Concentration
In the biological reaction till the substrate is available to make bind the speed of the
reaction will increase and after the no remaining substrate the speed will become slow (Arnold,
2018).
TASK 4
6.1 Structure of DNA and its Role in Cell Division and Protein Synthesis and Relation to its
Function
DNA Structure
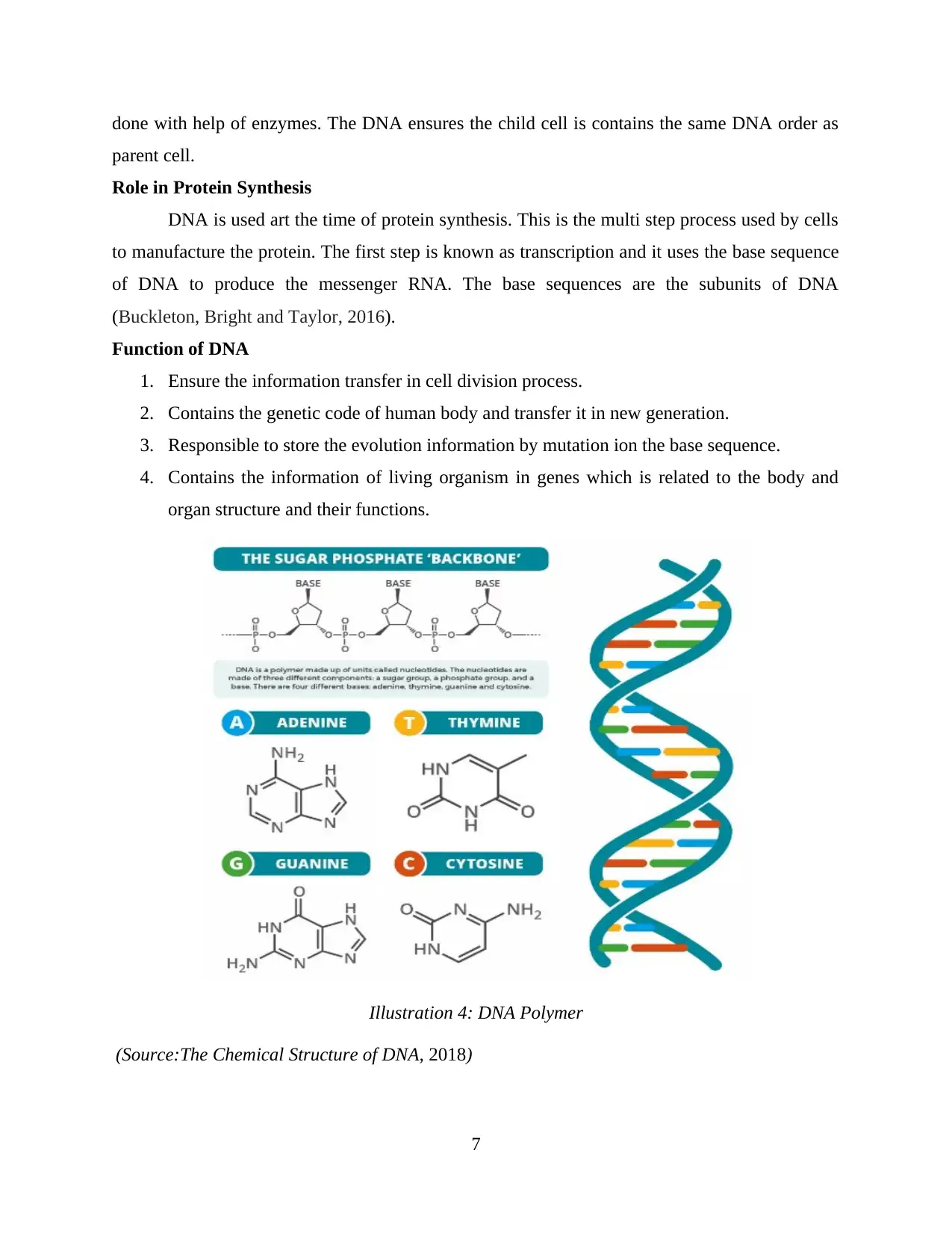
The DNA is made of molecules which are known as Nucleotides. Each nucleotide
contains of phosphate group, sugar group and nitrogen base. The four type nitrogen bases are
Adenine(A), Thymine(T), Guanine(G) and Cytosine(C). The hydrogen bond between this
nitrogen base showed in the picture below. DNA is double helix structure and the order of these
bases is known as DNA instruction and Genetic code. Human has around 3 billions bases in
human DNA. 99 percent of bases are same in all human beings.
Role in cell Division
In the process of the cell division the role of DNA is to save the information for the
longer time. In different stages of cell division the DNA is copied in both child cells and this is
6
The different toxins are breakdown in the liver and there are wide range of enzymes in
liver.
5.1 Factors that affects Enzyme Factors
Temperature
Temperature generally speed up the reactions but the high temperature can change the
structure of enzyme that reduce its effectiveness or stop working.
pH Level
The pH level of living organism have certain for the operation of enzymes. Outer this
range the effectiveness of these enzymes slow down. The extreme pH can damage the enzyme.
Enzyme Concentration
The concentration of enzyme can speed up the biological reaction as long as enzymes are
available to bind the reactors and after this the speed of the reaction will slowdown.
Substrate Concentration
In the biological reaction till the substrate is available to make bind the speed of the
reaction will increase and after the no remaining substrate the speed will become slow (Arnold,
2018).
TASK 4
6.1 Structure of DNA and its Role in Cell Division and Protein Synthesis and Relation to its
Function
DNA Structure
The DNA is made of molecules which are known as Nucleotides. Each nucleotide
contains of phosphate group, sugar group and nitrogen base. The four type nitrogen bases are
Adenine(A), Thymine(T), Guanine(G) and Cytosine(C). The hydrogen bond between this
nitrogen base showed in the picture below. DNA is double helix structure and the order of these
bases is known as DNA instruction and Genetic code. Human has around 3 billions bases in
human DNA. 99 percent of bases are same in all human beings.
Role in cell Division
In the process of the cell division the role of DNA is to save the information for the
longer time. In different stages of cell division the DNA is copied in both child cells and this is
6
done with help of enzymes. The DNA ensures the child cell is contains the same DNA order as
parent cell.
Role in Protein Synthesis
DNA is used art the time of protein synthesis. This is the multi step process used by cells
to manufacture the protein. The first step is known as transcription and it uses the base sequence
of DNA to produce the messenger RNA. The base sequences are the subunits of DNA
(Buckleton, Bright and Taylor, 2016).
Function of DNA
1. Ensure the information transfer in cell division process.
2. Contains the genetic code of human body and transfer it in new generation.
3. Responsible to store the evolution information by mutation ion the base sequence.
4. Contains the information of living organism in genes which is related to the body and
organ structure and their functions.
7
Illustration 4: DNA Polymer
(Source:The Chemical Structure of DNA, 2018)
parent cell.
Role in Protein Synthesis
DNA is used art the time of protein synthesis. This is the multi step process used by cells
to manufacture the protein. The first step is known as transcription and it uses the base sequence
of DNA to produce the messenger RNA. The base sequences are the subunits of DNA
(Buckleton, Bright and Taylor, 2016).
Function of DNA
1. Ensure the information transfer in cell division process.
2. Contains the genetic code of human body and transfer it in new generation.
3. Responsible to store the evolution information by mutation ion the base sequence.
4. Contains the information of living organism in genes which is related to the body and
organ structure and their functions.
7
Illustration 4: DNA Polymer
(Source:The Chemical Structure of DNA, 2018)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

CONCLUSION
This report is concluding the role of chemistry in the living organisms. This report is
providing the information of different polymers and monomers. Through this study the structure
of Carbohydrates, lipid and protein has been studied. The functions of enzymes and factors that
affects the action of enzymes has been understood. The role of DNA in cell division and protein
synthesis is studied with functions of DNA.
8
This report is concluding the role of chemistry in the living organisms. This report is
providing the information of different polymers and monomers. Through this study the structure
of Carbohydrates, lipid and protein has been studied. The functions of enzymes and factors that
affects the action of enzymes has been understood. The role of DNA in cell division and protein
synthesis is studied with functions of DNA.
8
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

REFERENCES
Books and Journals
Akoh, C.C., 2017. Food lipids: chemistry, nutrition, and biotechnology. CRC press.
Arnold, L.H., 2018. Enzymes. In Yeast Cell Envelopes Biochemistry Biophysics and
Ultrastructure (pp. 9-54). CRC Press.
Buckleton, J.S., Bright, J.A. and Taylor, D. eds., 2016. Forensic DNA evidence interpretation.
CRC press.
Holmes, E., 2015. The metabolism of living tissues. Cambridge University Press.
Jakobek, L., 2015. Interactions of polyphenols with carbohydrates, lipids and proteins. Food
chemistry. 175. pp.556-567.
Michel, H., 2018. Crystallization of Membrane Proteins: 0. CRC Press.
Vinogradov, A.P., 2018. Memoir II: The Elementary Chemical Composition of Marine
Organisms. Yale Peabody Museum.
Wetteland, C.L. and Liu, H., 2018. Optical and biological properties of polymer‐based
nanocomposites with improved dispersion of ceramic nanoparticles. Journal of Biomedical
Materials Research Part A. 106(10). pp.2692-2707.
Online
Chemistry: Organic Compounds, 2010. [Online]. Available through :
<https://legacy.owensboro.kctcs.edu/gcaplan/anat/Notes/API%20Notes%20D%20organic
%20chem.htm>.
Polymers, 2019. [Online]. Available through : <https://saylordotorg.github.io/text_introductory-
chemistry/s20-06-polymers.html>.
The Chemical Structure of DNA, 2018. [Online]. Available through :
<https://www.compoundchem.com/2015/03/24/dna/>.
What is cholesterol? Did you know it is harmless?, 2018. [Online]. Available through :
<https://www.nutritionmyths.com/what-is-cholesterol-did-you-know-it-is-harmless/>.
9
Books and Journals
Akoh, C.C., 2017. Food lipids: chemistry, nutrition, and biotechnology. CRC press.
Arnold, L.H., 2018. Enzymes. In Yeast Cell Envelopes Biochemistry Biophysics and
Ultrastructure (pp. 9-54). CRC Press.
Buckleton, J.S., Bright, J.A. and Taylor, D. eds., 2016. Forensic DNA evidence interpretation.
CRC press.
Holmes, E., 2015. The metabolism of living tissues. Cambridge University Press.
Jakobek, L., 2015. Interactions of polyphenols with carbohydrates, lipids and proteins. Food
chemistry. 175. pp.556-567.
Michel, H., 2018. Crystallization of Membrane Proteins: 0. CRC Press.
Vinogradov, A.P., 2018. Memoir II: The Elementary Chemical Composition of Marine
Organisms. Yale Peabody Museum.
Wetteland, C.L. and Liu, H., 2018. Optical and biological properties of polymer‐based
nanocomposites with improved dispersion of ceramic nanoparticles. Journal of Biomedical
Materials Research Part A. 106(10). pp.2692-2707.
Online
Chemistry: Organic Compounds, 2010. [Online]. Available through :
<https://legacy.owensboro.kctcs.edu/gcaplan/anat/Notes/API%20Notes%20D%20organic
%20chem.htm>.
Polymers, 2019. [Online]. Available through : <https://saylordotorg.github.io/text_introductory-
chemistry/s20-06-polymers.html>.
The Chemical Structure of DNA, 2018. [Online]. Available through :
<https://www.compoundchem.com/2015/03/24/dna/>.
What is cholesterol? Did you know it is harmless?, 2018. [Online]. Available through :
<https://www.nutritionmyths.com/what-is-cholesterol-did-you-know-it-is-harmless/>.
9
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




