Pathogens Review: Adhesive Pili in UTI Pathogenesis and Drug
VerifiedAdded on 2022/08/17
|18
|15821
|10
Report
AI Summary
This review, authored by Caitlin N. Spaulding and Scott J. Hultgren, delves into the critical role of adhesive pili in the pathogenesis of urinary tract infections (UTIs), a common bacterial infection affecting millions globally. The review highlights the significance of understanding host-pathogen interactions, disease progression, and UTI pathophysiology, particularly focusing on uropathogenic Escherichia coli (UPEC) and Enterococcus species. It explores the chaperone-usher pathway (CUP) pili assembly mechanisms, emphasizing their function in bacterial adhesion to the bladder epithelium and subsequent infection. The authors discuss the limitations of current antibiotic treatments due to rising resistance and recurrence rates, advocating for the development of alternative, non-antibiotic strategies targeting adhesive pili to prevent and treat UTIs and CAUTIs. The review covers the UPEC pathogenic cascade, including bacterial adhesion, invasion, replication, and host immune responses, alongside the role of quiescent intracellular reservoirs (QIRs) in recurrent infections (rUTI). The financial and patient morbidity associated with UTIs, including economic costs and reduced quality of life, underscore the need for innovative therapeutic approaches. This review serves as a valuable resource for understanding the complex interplay of factors in UTI development and for informing the development of novel therapeutic interventions.

pathogens
Review
Adhesive Pili in UTI Pathogenesis and
Drug Development
Caitlin N. Spaulding1 and Scott J. Hultgren1,2,*
1 Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO 63110,
USA; cspaulding@wustl.edu
2 Center for Women’s Infectious Disease Research, Washington University School of Medicine, St. Louis,
MO 63110, USA
* Correspondence: hultgren@wusm.wustl.edu; Tel.: +1-314-362-7059
Academic Editor: Lawrence S. Young
Received: 16 July 2015; Accepted: 7 March 2016; Published: 15 March 2016
Abstract:Urinary tract infections (UTIs) are one of the most common bacterial infections, affecting
150 million people each year worldwide. High recurrence rates and increasing antimicrobial resistance
among uropathogens are making it imperative to develop alternative strategies for the treatment and
prevention of this common infection. In this Review, we discuss how understanding the: (i) molecular
and biophysical basis of host-pathogen interactions; (ii) consequences of the molecular cross-talk at the
host pathogen interface in terms of disease progression; and (iii) pathophysiology of UTIs is leading
to efforts to translate this knowledge into novel therapeutics to treat and prevent these infections.
Keywords:UTI; rUTI; CAUTI; pili; UPEC; chaperone-usher pathway (CUP) pili; Enterococcus; vaccine;
antibiotic-resistance
1. Introduction
Urinary tract infections (UTIs) can be acquired in the community or hospital setting and are one
of the most common bacterial infections that occur, affecting more than 150 million people worldwide
each year [1–3]. UTI is clinically divided into two major infections, characterized by the localization
of the bacteria in the urinary tract, cystitis and pyelonephritis. Cystitis, or lower UTI, is infection of
the bladder.Once in the bladder, bacteria can ascend the ureters and colonize the kidneys, causing
pyelonephritis or upper UTI. While the incidence of pyelonephritis is fairly low (~0.3%–0.6%) it is
particularly dangerous as uncontrolled bacterial infection can spread to the bloodstream,causing
sepsis (which occurs in ~2% of pyelonephritis cases) [4,5]. UTIs are also categorized as uncomplicated
or complicated infections. Complicated UTI occurs in patients with: (i) functional or structural urinary
tract abnormalities;(ii) renal failure;(iii) immunosuppression;(iv) pregnancy;and/or (v) foreign
bodies, such as indwelling catheters, placed within their urinary tract [6–8]. Catheter-associated UTIs
(CAUTI), make up 70%–80% of all complicated UTI and are the most common type of nosocomial
infection [4,9]. CAUTI are of particular concern as they result in high morbidity, increased mortality
and are the most common cause of secondary sepsis in hospital patients.While complicated UTI
affects individuals of both genders, uncomplicated UTI primarily affects otherwise healthy women [10].
Pyelonephritis often occurs in healthy, non-pregnant women but can be categorized as a complicated
UTI because of the potential of developing a blood stream infection. For women, the lifetime risk of
developing an uncomplicated UTI approaches 60% [5]. Of these women who experience an initial
UTI, 20%–30% willgo on to experience a recurrent infection (rUTI) within 4–6 months,despite
receiving appropriate antibiotic therapy [5,11]. To combat rUTI, these women are treated with frequent
antibiotic therapy often to be taken at the time that symptoms arise or immediately following sexual
intercourse [6]. However, a subset of these women will continue to experience rUTI as frequently as
Pathogens 2016, 5, 30; doi:10.3390/pathogens5010030 www.mdpi.com/journal/pathogens
Review
Adhesive Pili in UTI Pathogenesis and
Drug Development
Caitlin N. Spaulding1 and Scott J. Hultgren1,2,*
1 Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO 63110,
USA; cspaulding@wustl.edu
2 Center for Women’s Infectious Disease Research, Washington University School of Medicine, St. Louis,
MO 63110, USA
* Correspondence: hultgren@wusm.wustl.edu; Tel.: +1-314-362-7059
Academic Editor: Lawrence S. Young
Received: 16 July 2015; Accepted: 7 March 2016; Published: 15 March 2016
Abstract:Urinary tract infections (UTIs) are one of the most common bacterial infections, affecting
150 million people each year worldwide. High recurrence rates and increasing antimicrobial resistance
among uropathogens are making it imperative to develop alternative strategies for the treatment and
prevention of this common infection. In this Review, we discuss how understanding the: (i) molecular
and biophysical basis of host-pathogen interactions; (ii) consequences of the molecular cross-talk at the
host pathogen interface in terms of disease progression; and (iii) pathophysiology of UTIs is leading
to efforts to translate this knowledge into novel therapeutics to treat and prevent these infections.
Keywords:UTI; rUTI; CAUTI; pili; UPEC; chaperone-usher pathway (CUP) pili; Enterococcus; vaccine;
antibiotic-resistance
1. Introduction
Urinary tract infections (UTIs) can be acquired in the community or hospital setting and are one
of the most common bacterial infections that occur, affecting more than 150 million people worldwide
each year [1–3]. UTI is clinically divided into two major infections, characterized by the localization
of the bacteria in the urinary tract, cystitis and pyelonephritis. Cystitis, or lower UTI, is infection of
the bladder.Once in the bladder, bacteria can ascend the ureters and colonize the kidneys, causing
pyelonephritis or upper UTI. While the incidence of pyelonephritis is fairly low (~0.3%–0.6%) it is
particularly dangerous as uncontrolled bacterial infection can spread to the bloodstream,causing
sepsis (which occurs in ~2% of pyelonephritis cases) [4,5]. UTIs are also categorized as uncomplicated
or complicated infections. Complicated UTI occurs in patients with: (i) functional or structural urinary
tract abnormalities;(ii) renal failure;(iii) immunosuppression;(iv) pregnancy;and/or (v) foreign
bodies, such as indwelling catheters, placed within their urinary tract [6–8]. Catheter-associated UTIs
(CAUTI), make up 70%–80% of all complicated UTI and are the most common type of nosocomial
infection [4,9]. CAUTI are of particular concern as they result in high morbidity, increased mortality
and are the most common cause of secondary sepsis in hospital patients.While complicated UTI
affects individuals of both genders, uncomplicated UTI primarily affects otherwise healthy women [10].
Pyelonephritis often occurs in healthy, non-pregnant women but can be categorized as a complicated
UTI because of the potential of developing a blood stream infection. For women, the lifetime risk of
developing an uncomplicated UTI approaches 60% [5]. Of these women who experience an initial
UTI, 20%–30% willgo on to experience a recurrent infection (rUTI) within 4–6 months,despite
receiving appropriate antibiotic therapy [5,11]. To combat rUTI, these women are treated with frequent
antibiotic therapy often to be taken at the time that symptoms arise or immediately following sexual
intercourse [6]. However, a subset of these women will continue to experience rUTI as frequently as
Pathogens 2016, 5, 30; doi:10.3390/pathogens5010030 www.mdpi.com/journal/pathogens
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Pathogens 2016, 5, 30 2 of 18
six or more times a year [12]. Due to its prevalence and high rates of recurrence, UTI is associated
with significant economic costs.The financial burden of UTI in the United States,which reflects
both direct medical costs and indirect costs such as lost work output and wages,is an estimated
$5 billion annually [5]. These infections also result in significant patient morbidity resulting in serious
deterioration in quality of life including:pain,discomfort,disruption of daily activities,and few
treatment options other than long-term antibiotic prophylaxis [5,11,13].
While many bacterialorganisms cause UTI,the most common causative agentof both
uncomplicated and complicated UTI is the gram-negative pathogen uropathogenic Escherichia coli
(E. coli) (UPEC). UPEC are responsible for 80%–90% of all uncomplicated UTI and approximately
65% of complicated UTIs [5,14,15]. Gram-positive Enterococcus species are the second leading cause
of complicated UTI (11%) and the third leading cause of uncomplicated UTI (5%) [15]. The source
population of UPEC and Enterococcus that lead to UTI is thought to be the gastrointestinal tract, where
they can reside as either commensal or transient members of the gut microbiota [11,16,17]. When
present in the gut,UPEC or Enterococcus spp.can be shed in the feces,inoculating peri-urethral
or vaginal areas, and are subsequently introduced into the urinary tract during periods of physical
manipulation such as during sexual activity or catheterization (Figure 1A) [18]. Upon entering the
bladder, uropathogens must bind to an available epithelial receptor and/or, if present, abiotic-surface
to establish and maintain colonization. UPEC and enterococcal species both accomplish this through
the expression of distinctive adhesive pili on their surface.After creating a foothold in the bladder,
uropathogens employ a myriad of additional virulence factors to establish bladder colonization in
the face of an active immune response, micturition, and rapid epithelial cell exfoliation. Historically,
antibiotics have been used, very successfully, to treat patients with UTI. However, the rise of single
and multi-drug resistant uropathogens as well as high rates of recurrence in women infected with
both antibiotic sensitive and drug-resistant uropathogens has become a major concern, highlighting
the need to develop alternative strategies to treat patients with UTI and CAUTI. In this review, we
will discuss the role of adhesive pili during UTI or CAUTI. Here we will focus mainly on UTI and
CAUTI caused by UPEC and Enterococcus spp.due to the high prevalence of these pathogens in
community-acquired and nosocomial infections. We will also explore the development of alternative,
non-antibiotic treatment strategies that target adhesive pili in order to prevent UPEC and Enterococcus
spp. from initiating infection and thus causing disease.
six or more times a year [12]. Due to its prevalence and high rates of recurrence, UTI is associated
with significant economic costs.The financial burden of UTI in the United States,which reflects
both direct medical costs and indirect costs such as lost work output and wages,is an estimated
$5 billion annually [5]. These infections also result in significant patient morbidity resulting in serious
deterioration in quality of life including:pain,discomfort,disruption of daily activities,and few
treatment options other than long-term antibiotic prophylaxis [5,11,13].
While many bacterialorganisms cause UTI,the most common causative agentof both
uncomplicated and complicated UTI is the gram-negative pathogen uropathogenic Escherichia coli
(E. coli) (UPEC). UPEC are responsible for 80%–90% of all uncomplicated UTI and approximately
65% of complicated UTIs [5,14,15]. Gram-positive Enterococcus species are the second leading cause
of complicated UTI (11%) and the third leading cause of uncomplicated UTI (5%) [15]. The source
population of UPEC and Enterococcus that lead to UTI is thought to be the gastrointestinal tract, where
they can reside as either commensal or transient members of the gut microbiota [11,16,17]. When
present in the gut,UPEC or Enterococcus spp.can be shed in the feces,inoculating peri-urethral
or vaginal areas, and are subsequently introduced into the urinary tract during periods of physical
manipulation such as during sexual activity or catheterization (Figure 1A) [18]. Upon entering the
bladder, uropathogens must bind to an available epithelial receptor and/or, if present, abiotic-surface
to establish and maintain colonization. UPEC and enterococcal species both accomplish this through
the expression of distinctive adhesive pili on their surface.After creating a foothold in the bladder,
uropathogens employ a myriad of additional virulence factors to establish bladder colonization in
the face of an active immune response, micturition, and rapid epithelial cell exfoliation. Historically,
antibiotics have been used, very successfully, to treat patients with UTI. However, the rise of single
and multi-drug resistant uropathogens as well as high rates of recurrence in women infected with
both antibiotic sensitive and drug-resistant uropathogens has become a major concern, highlighting
the need to develop alternative strategies to treat patients with UTI and CAUTI. In this review, we
will discuss the role of adhesive pili during UTI or CAUTI. Here we will focus mainly on UTI and
CAUTI caused by UPEC and Enterococcus spp.due to the high prevalence of these pathogens in
community-acquired and nosocomial infections. We will also explore the development of alternative,
non-antibiotic treatment strategies that target adhesive pili in order to prevent UPEC and Enterococcus
spp. from initiating infection and thus causing disease.
Pathogens 2016, 5, 30 3 of 18
Pathogens 2016, 5, 30
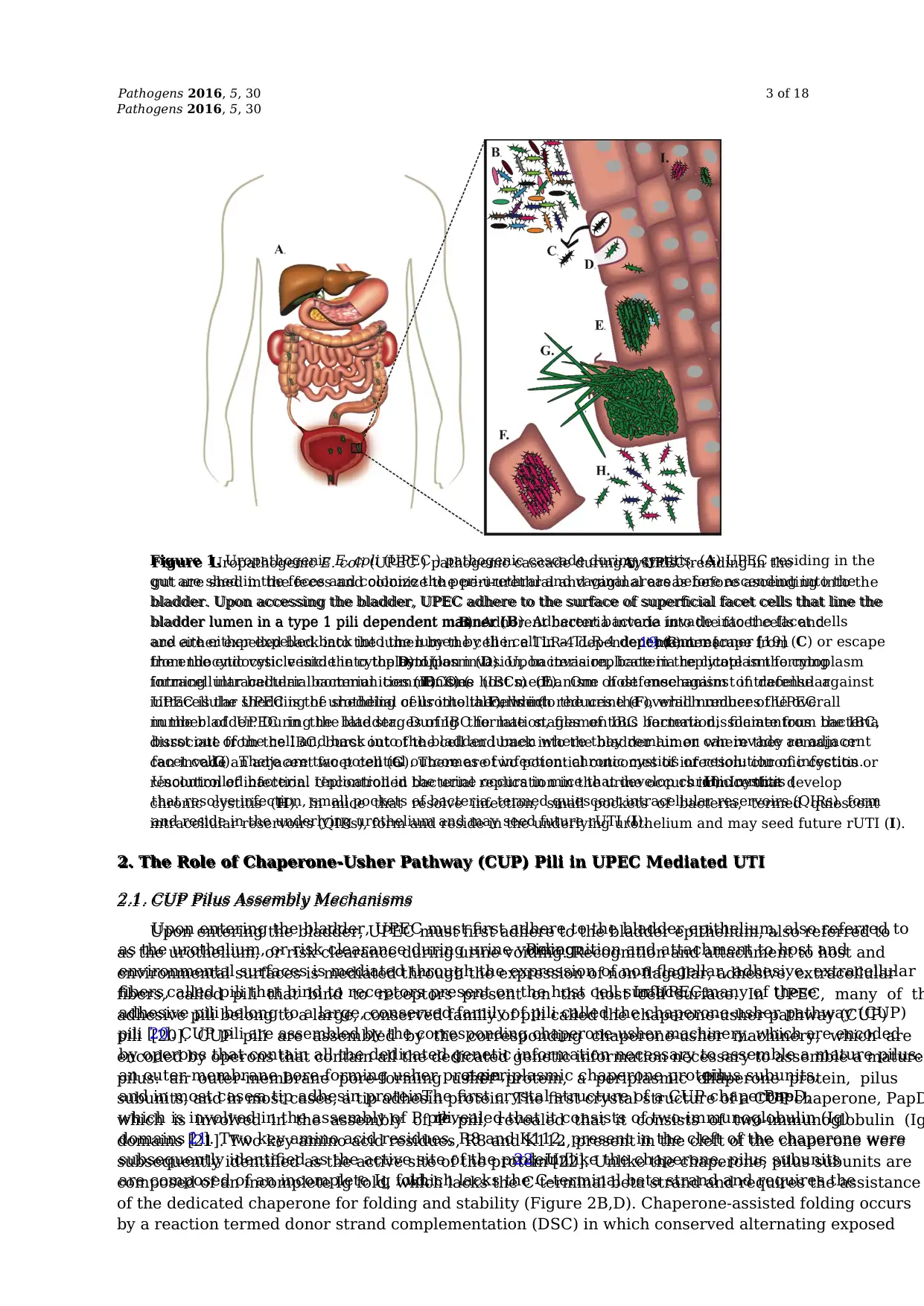
Figure 1. Uropathogenic E. coli (UPEC ) pathogenic cascade during cystitis. (A) UPEC residing in the
gut are shed in the feces and colonize the peri-urethral and vaginal areas before ascending into the
bladder. Upon accessing the bladder, UPEC adhere to the surface of superficial facet cells that line the
bladder lumen in a type 1 pili dependent manner (B). Adherent bacteria invade into the facet cells
and are either expelled back into the lumen by the cell in a TLR-4 dependent manner [19] (C) or escape
from the endocytic vesicle into the cytoplasm (D). Upon invasion, bacteria replicate in the cytoplasm
forming intracellular bacterial communities (IBCs) (E). One host mechanism of defense against
intracellular UPEC is the shedding of urothelial cells into the urine (F), which reduces the overall
number of UPEC in the bladder. During the late stages of IBC formation, filamentous bacteria
dissociate from the IBC, burst out of the cell and back into the bladder lumen where they remain or
can invade an adjacent facet cell (G). There are two potential outcomes of infection: chronic cystitis or
resolution of infection. Uncontrolled bacterial replication in the urine occurs in mice that develop
chronic cystitis (H). In mice that resolve infection, small pockets of bacteria, termed quiescent
intracellular reservoirs (QIRs), form and reside in the underlying urothelium and may seed future rUTI (I).
2. The Role of Chaperone-Usher Pathway (CUP) Pili in UPEC Mediated UTI
2.1. CUP Pilus Assembly Mechanisms
Upon entering the bladder, UPEC must first adhere to the bladder epithelium, also referred to
as the urothelium, or risk clearance during urine voiding. Recognition and attachment to host and
environmental surfaces is mediated through the expression of non-flagellar, adhesive, extracellular
fibers, called pili that bind to receptors present on the host cell surface. In UPEC, many of th
adhesive pili belong to a large, conserved family of pili called the chaperone-usher pathway (CUP)
pili [20]. CUP pili are assembled by the corresponding chaperone-usher machinery, which are
encoded by operons that contain all the dedicated genetic information necessary to assemble a mature
pilus: an outer-membrane pore-forming usher protein, a periplasmic chaperone protein, pilus
subunits, and in most cases, a tip adhesin protein. The first crystal structure of a CUP chaperone, PapD
which is involved in the assembly of P pili, revealed that it consists of two-immunoglobulin (Ig
domains [21]. Two key amino acid residues, R8 and K112, present in the cleft of the chaperone were
subsequently identified as the active site of the protein [22]. Unlike the chaperone, pilus subunits are
composed of an incomplete Ig fold, which lacks the C-terminal beta strand and requires the assistance
of the dedicated chaperone for folding and stability (Figure 2B,D). Chaperone-assisted folding occurs
by a reaction termed donor strand complementation (DSC) in which conserved alternating exposed
Figure 1.Uropathogenic E. coli (UPEC ) pathogenic cascade during cystitis. (A) UPEC residing in the
gut are shed in the feces and colonize the peri-urethral and vaginal areas before ascending into the
bladder. Upon accessing the bladder, UPEC adhere to the surface of superficial facet cells that line the
bladder lumen in a type 1 pili dependent manner (B). Adherent bacteria invade into the facet cells and
are either expelled back into the lumen by the cell in a TLR-4 dependent manner [19] (C) or escape from
the endocytic vesicle into the cytoplasm (D). Upon invasion, bacteria replicate in the cytoplasm forming
intracellular bacterial communities (IBCs) (E). One host mechanism of defense against intracellular
UPEC is the shedding of urothelial cells into the urine (F), which reduces the overall number of UPEC
in the bladder. During the late stages of IBC formation, filamentous bacteria dissociate from the IBC,
burst out of the cell and back into the bladder lumen where they remain or can invade an adjacent
facet cell (G). There are two potential outcomes of infection: chronic cystitis or resolution of infection.
Uncontrolled bacterial replication in the urine occurs in mice that develop chronic cystitis (H). In mice
that resolve infection, small pockets of bacteria, termed quiescent intracellular reservoirs (QIRs), form
and reside in the underlying urothelium and may seed future rUTI (I).
2. The Role of Chaperone-Usher Pathway (CUP) Pili in UPEC Mediated UTI
2.1. CUP Pilus Assembly Mechanisms
Upon entering the bladder, UPEC must first adhere to the bladder epithelium, also referred to
as the urothelium, or risk clearance during urine voiding.Recognition and attachment to host and
environmental surfaces is mediated through the expression of non-flagellar, adhesive, extracellular
fibers,called pili that bind to receptors present on the host cell surface.In UPEC,many of these
adhesive pili belong to a large, conserved family of pili called the chaperone-usher pathway (CUP)
pili [20]. CUP pili are assembled by the corresponding chaperone-usher machinery, which are encoded
by operons that contain all the dedicated genetic information necessary to assemble a mature pilus:
an outer-membrane pore-forming usher protein,a periplasmic chaperone protein,pilus subunits,
and in most cases,a tip adhesin protein.The first crystal structure of a CUP chaperone,PapD,
which is involved in the assembly of P pili,revealed that it consists of two-immunoglobulin (Ig)
domains [21]. Two key amino acid residues, R8 and K112, present in the cleft of the chaperone were
subsequently identified as the active site of the protein [22]. Unlike the chaperone, pilus subunits
are composed of an incomplete Ig fold,which lacks the C-terminal beta strand and requires the
Pathogens 2016, 5, 30
Figure 1. Uropathogenic E. coli (UPEC ) pathogenic cascade during cystitis. (A) UPEC residing in the
gut are shed in the feces and colonize the peri-urethral and vaginal areas before ascending into the
bladder. Upon accessing the bladder, UPEC adhere to the surface of superficial facet cells that line the
bladder lumen in a type 1 pili dependent manner (B). Adherent bacteria invade into the facet cells
and are either expelled back into the lumen by the cell in a TLR-4 dependent manner [19] (C) or escape
from the endocytic vesicle into the cytoplasm (D). Upon invasion, bacteria replicate in the cytoplasm
forming intracellular bacterial communities (IBCs) (E). One host mechanism of defense against
intracellular UPEC is the shedding of urothelial cells into the urine (F), which reduces the overall
number of UPEC in the bladder. During the late stages of IBC formation, filamentous bacteria
dissociate from the IBC, burst out of the cell and back into the bladder lumen where they remain or
can invade an adjacent facet cell (G). There are two potential outcomes of infection: chronic cystitis or
resolution of infection. Uncontrolled bacterial replication in the urine occurs in mice that develop
chronic cystitis (H). In mice that resolve infection, small pockets of bacteria, termed quiescent
intracellular reservoirs (QIRs), form and reside in the underlying urothelium and may seed future rUTI (I).
2. The Role of Chaperone-Usher Pathway (CUP) Pili in UPEC Mediated UTI
2.1. CUP Pilus Assembly Mechanisms
Upon entering the bladder, UPEC must first adhere to the bladder epithelium, also referred to
as the urothelium, or risk clearance during urine voiding. Recognition and attachment to host and
environmental surfaces is mediated through the expression of non-flagellar, adhesive, extracellular
fibers, called pili that bind to receptors present on the host cell surface. In UPEC, many of th
adhesive pili belong to a large, conserved family of pili called the chaperone-usher pathway (CUP)
pili [20]. CUP pili are assembled by the corresponding chaperone-usher machinery, which are
encoded by operons that contain all the dedicated genetic information necessary to assemble a mature
pilus: an outer-membrane pore-forming usher protein, a periplasmic chaperone protein, pilus
subunits, and in most cases, a tip adhesin protein. The first crystal structure of a CUP chaperone, PapD
which is involved in the assembly of P pili, revealed that it consists of two-immunoglobulin (Ig
domains [21]. Two key amino acid residues, R8 and K112, present in the cleft of the chaperone were
subsequently identified as the active site of the protein [22]. Unlike the chaperone, pilus subunits are
composed of an incomplete Ig fold, which lacks the C-terminal beta strand and requires the assistance
of the dedicated chaperone for folding and stability (Figure 2B,D). Chaperone-assisted folding occurs
by a reaction termed donor strand complementation (DSC) in which conserved alternating exposed
Figure 1.Uropathogenic E. coli (UPEC ) pathogenic cascade during cystitis. (A) UPEC residing in the
gut are shed in the feces and colonize the peri-urethral and vaginal areas before ascending into the
bladder. Upon accessing the bladder, UPEC adhere to the surface of superficial facet cells that line the
bladder lumen in a type 1 pili dependent manner (B). Adherent bacteria invade into the facet cells and
are either expelled back into the lumen by the cell in a TLR-4 dependent manner [19] (C) or escape from
the endocytic vesicle into the cytoplasm (D). Upon invasion, bacteria replicate in the cytoplasm forming
intracellular bacterial communities (IBCs) (E). One host mechanism of defense against intracellular
UPEC is the shedding of urothelial cells into the urine (F), which reduces the overall number of UPEC
in the bladder. During the late stages of IBC formation, filamentous bacteria dissociate from the IBC,
burst out of the cell and back into the bladder lumen where they remain or can invade an adjacent
facet cell (G). There are two potential outcomes of infection: chronic cystitis or resolution of infection.
Uncontrolled bacterial replication in the urine occurs in mice that develop chronic cystitis (H). In mice
that resolve infection, small pockets of bacteria, termed quiescent intracellular reservoirs (QIRs), form
and reside in the underlying urothelium and may seed future rUTI (I).
2. The Role of Chaperone-Usher Pathway (CUP) Pili in UPEC Mediated UTI
2.1. CUP Pilus Assembly Mechanisms
Upon entering the bladder, UPEC must first adhere to the bladder epithelium, also referred to
as the urothelium, or risk clearance during urine voiding.Recognition and attachment to host and
environmental surfaces is mediated through the expression of non-flagellar, adhesive, extracellular
fibers,called pili that bind to receptors present on the host cell surface.In UPEC,many of these
adhesive pili belong to a large, conserved family of pili called the chaperone-usher pathway (CUP)
pili [20]. CUP pili are assembled by the corresponding chaperone-usher machinery, which are encoded
by operons that contain all the dedicated genetic information necessary to assemble a mature pilus:
an outer-membrane pore-forming usher protein,a periplasmic chaperone protein,pilus subunits,
and in most cases,a tip adhesin protein.The first crystal structure of a CUP chaperone,PapD,
which is involved in the assembly of P pili,revealed that it consists of two-immunoglobulin (Ig)
domains [21]. Two key amino acid residues, R8 and K112, present in the cleft of the chaperone were
subsequently identified as the active site of the protein [22]. Unlike the chaperone, pilus subunits
are composed of an incomplete Ig fold,which lacks the C-terminal beta strand and requires the
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Pathogens 2016, 5, 30 4 of 18
assistance of the dedicated chaperone for folding and stability (Figure 2B,D).Chaperone-assisted
folding occurs by a reaction termed donor strand complementation (DSC) in which conserved
alternating exposed hydrophobic residues on the chaperone’s G1 strand are buried in complementary
pockets in the pilus subunit, allowing for the completion of the subunits Ig fold (Figure 2C) [23,24].
This interaction allows pilus subunits to fold into a primed, high-energy state in complex with the
chaperone, ultimately allowing the subunits to be targeted to the outer membrane usher (Figure 2E).
The usher, a gated channel, is made up of five functional domains:a 24 stranded transmembrane
β -barrel translocation domain (TD), aβ -sandwich plug (PLUG) that resides in the pore of the TD in the
apo-usher, an N-terminal periplasmic domain (NTD) and two C-terminal periplasmic domains (CTD1
and2) [25–29].The usher catalyzes pilus assembly by driving subunit polymerization in a concerted
reaction termed donor strand exchange (DSE) (Figure 2E,F) [30–33]. Pilus subunits, excluding the
adhesin,encode an N-terminal extension (Nte) comprised of conserved alternating hydrophobic
residues. DSE occurs when the chaperone is displaced and an incoming subunit’s Nte zips into the
previously chaperone-bound groove of a nascently incorporated subunit at the growing terminus of
the pilus. This “zip-in-zip-out mechanism” allows for the final folding of the pilus subunit, such that
every subunit in the pilus completes the Ig fold of its neighbor (Figure 2F).
Pathogens 2016, 5, 30
hydrophobic residues on the chaperone’s G1 strand are buried in complementary pockets in the pilus
subunit, allowing for the completion of the subunits Ig fold (Figure 2C) [23,24]. This interaction allows
pilus subunits to fold into a primed, high-energy state in complex with the chaperone, ultimately
allowing the subunits to be targeted to the outer membrane usher (Figure 2E). The usher, a gated
channel, is made up of five functional domains: a 24 stranded transmembrane β-barrel translocation
domain (TD), a β-sandwich plug (PLUG) that resides in the pore of the TD in the apo-usher, an N-
terminal periplasmic domain (NTD) and two C-terminal periplasmic domains (CTD1 and 2) [25–29].
The usher catalyzes pilus assembly by driving subunit polymerization in a concerted reaction termed
donor strand exchange (DSE) (Figure 2E,F) [30–33]. Pilus subunits, excluding the adhesin, encode an
N-terminal extension (Nte) comprised of conserved alternating hydrophobic residues. DSE occurs
when the chaperone is displaced and an incoming subunit’s Nte zips into the previously chaperone-
bound groove of a nascently incorporated subunit at the growing terminus of the pilus. This “zip-in-
zip-out mechanism” allows for the final folding of the pilus subunit, such that every subunit in the
pilus completes the Ig fold of its neighbor (Figure 2F).
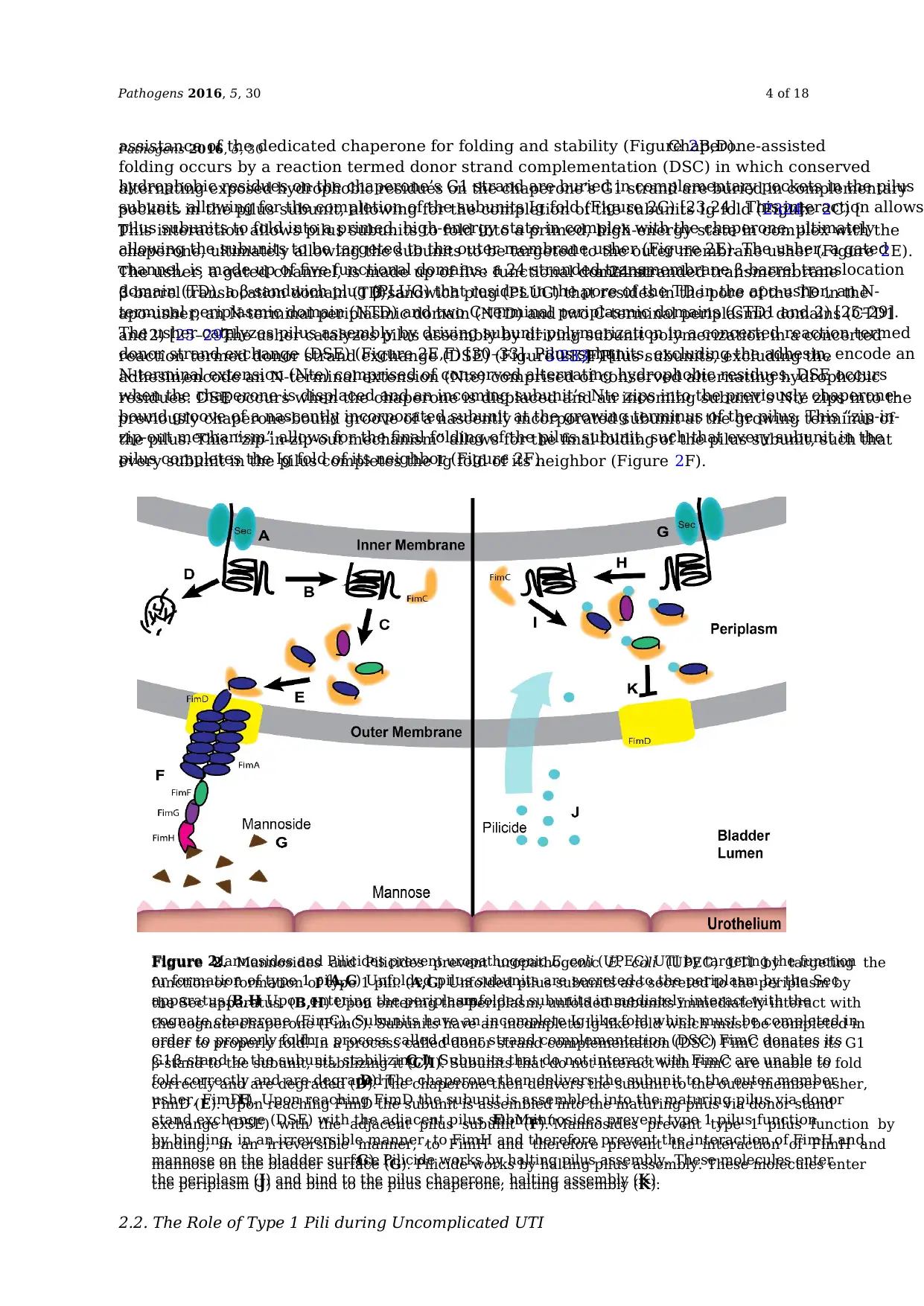
Figure 2. Mannosides and Pilicides prevent uropathogenic E. coli (UPEC) UTI by targeting the
function or formation of type 1 pili. (A,G) Unfolded pilus subunits are secreted to the periplasm by
the Sec apparatus. (B,H) Upon entering the periplasm, unfolded subunits immediately interact with
the cognate chaperone (FimC). Subunits have an incomplete Ig-like fold which must be completed in
order to properly fold. In a process called donor strand complementation (DSC) FimC donates its G1
β-stand to the subunit, stabilizing it (C,I). Subunits that do not interact with FimC are unable to fold
correctly and are degraded (D). The chaperone then delivers the subunit to the outer member usher,
FimD (E). Upon reaching FimD the subunit is assembled into the maturing pilus via donor stand
exchange (DSE) with the adjacent pilus subunit (F). Mannosides prevent type 1 pilus function by
binding, in an irreversible manner, to FimH and therefore prevent the interaction of FimH and
mannose on the bladder surface (G). Pilicide works by halting pilus assembly. These molecules enter
the periplasm (J) and bind to the pilus chaperone, halting assembly (K).
2.2. The Role of Type 1 Pili during Uncomplicated UTI
Figure 2.Mannosides and Pilicides prevent uropathogenic E. coli (UPEC) UTI by targeting the function
or formation of type 1 pili.(A,G) Unfolded pilus subunits are secreted to the periplasm by the Sec
apparatus.(B,H) Upon entering the periplasm,unfolded subunits immediately interact with the
cognate chaperone (FimC). Subunits have an incomplete Ig-like fold which must be completed in
order to properly fold.In a process called donor strand complementation (DSC) FimC donates its
G1β -stand to the subunit, stabilizing it (C,I). Subunits that do not interact with FimC are unable to
fold correctly and are degraded (D). The chaperone then delivers the subunit to the outer member
usher, FimD (E). Upon reaching FimD the subunit is assembled into the maturing pilus via donor
stand exchange (DSE) with the adjacent pilus subunit (F). Mannosides prevent type 1 pilus function
by binding, in an irreversible manner, to FimH and therefore prevent the interaction of FimH and
mannose on the bladder surface (G). Pilicide works by halting pilus assembly. These molecules enter
the periplasm (J) and bind to the pilus chaperone, halting assembly (K).
assistance of the dedicated chaperone for folding and stability (Figure 2B,D).Chaperone-assisted
folding occurs by a reaction termed donor strand complementation (DSC) in which conserved
alternating exposed hydrophobic residues on the chaperone’s G1 strand are buried in complementary
pockets in the pilus subunit, allowing for the completion of the subunits Ig fold (Figure 2C) [23,24].
This interaction allows pilus subunits to fold into a primed, high-energy state in complex with the
chaperone, ultimately allowing the subunits to be targeted to the outer membrane usher (Figure 2E).
The usher, a gated channel, is made up of five functional domains:a 24 stranded transmembrane
β -barrel translocation domain (TD), aβ -sandwich plug (PLUG) that resides in the pore of the TD in the
apo-usher, an N-terminal periplasmic domain (NTD) and two C-terminal periplasmic domains (CTD1
and2) [25–29].The usher catalyzes pilus assembly by driving subunit polymerization in a concerted
reaction termed donor strand exchange (DSE) (Figure 2E,F) [30–33]. Pilus subunits, excluding the
adhesin,encode an N-terminal extension (Nte) comprised of conserved alternating hydrophobic
residues. DSE occurs when the chaperone is displaced and an incoming subunit’s Nte zips into the
previously chaperone-bound groove of a nascently incorporated subunit at the growing terminus of
the pilus. This “zip-in-zip-out mechanism” allows for the final folding of the pilus subunit, such that
every subunit in the pilus completes the Ig fold of its neighbor (Figure 2F).
Pathogens 2016, 5, 30
hydrophobic residues on the chaperone’s G1 strand are buried in complementary pockets in the pilus
subunit, allowing for the completion of the subunits Ig fold (Figure 2C) [23,24]. This interaction allows
pilus subunits to fold into a primed, high-energy state in complex with the chaperone, ultimately
allowing the subunits to be targeted to the outer membrane usher (Figure 2E). The usher, a gated
channel, is made up of five functional domains: a 24 stranded transmembrane β-barrel translocation
domain (TD), a β-sandwich plug (PLUG) that resides in the pore of the TD in the apo-usher, an N-
terminal periplasmic domain (NTD) and two C-terminal periplasmic domains (CTD1 and 2) [25–29].
The usher catalyzes pilus assembly by driving subunit polymerization in a concerted reaction termed
donor strand exchange (DSE) (Figure 2E,F) [30–33]. Pilus subunits, excluding the adhesin, encode an
N-terminal extension (Nte) comprised of conserved alternating hydrophobic residues. DSE occurs
when the chaperone is displaced and an incoming subunit’s Nte zips into the previously chaperone-
bound groove of a nascently incorporated subunit at the growing terminus of the pilus. This “zip-in-
zip-out mechanism” allows for the final folding of the pilus subunit, such that every subunit in the
pilus completes the Ig fold of its neighbor (Figure 2F).
Figure 2. Mannosides and Pilicides prevent uropathogenic E. coli (UPEC) UTI by targeting the
function or formation of type 1 pili. (A,G) Unfolded pilus subunits are secreted to the periplasm by
the Sec apparatus. (B,H) Upon entering the periplasm, unfolded subunits immediately interact with
the cognate chaperone (FimC). Subunits have an incomplete Ig-like fold which must be completed in
order to properly fold. In a process called donor strand complementation (DSC) FimC donates its G1
β-stand to the subunit, stabilizing it (C,I). Subunits that do not interact with FimC are unable to fold
correctly and are degraded (D). The chaperone then delivers the subunit to the outer member usher,
FimD (E). Upon reaching FimD the subunit is assembled into the maturing pilus via donor stand
exchange (DSE) with the adjacent pilus subunit (F). Mannosides prevent type 1 pilus function by
binding, in an irreversible manner, to FimH and therefore prevent the interaction of FimH and
mannose on the bladder surface (G). Pilicide works by halting pilus assembly. These molecules enter
the periplasm (J) and bind to the pilus chaperone, halting assembly (K).
2.2. The Role of Type 1 Pili during Uncomplicated UTI
Figure 2.Mannosides and Pilicides prevent uropathogenic E. coli (UPEC) UTI by targeting the function
or formation of type 1 pili.(A,G) Unfolded pilus subunits are secreted to the periplasm by the Sec
apparatus.(B,H) Upon entering the periplasm,unfolded subunits immediately interact with the
cognate chaperone (FimC). Subunits have an incomplete Ig-like fold which must be completed in
order to properly fold.In a process called donor strand complementation (DSC) FimC donates its
G1β -stand to the subunit, stabilizing it (C,I). Subunits that do not interact with FimC are unable to
fold correctly and are degraded (D). The chaperone then delivers the subunit to the outer member
usher, FimD (E). Upon reaching FimD the subunit is assembled into the maturing pilus via donor
stand exchange (DSE) with the adjacent pilus subunit (F). Mannosides prevent type 1 pilus function
by binding, in an irreversible manner, to FimH and therefore prevent the interaction of FimH and
mannose on the bladder surface (G). Pilicide works by halting pilus assembly. These molecules enter
the periplasm (J) and bind to the pilus chaperone, halting assembly (K).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Pathogens 2016, 5, 30 5 of 18
2.2. The Role of Type 1 Pili during Uncomplicated UTI
A hallmark of the luminalsurface of urothelialcells,also called superficialfacet cells,are
the presence of uroplakin plaques,which consist of 4 uroplakin integral membrane proteins,that
function as a barrier between the toxic contents of the bladder and the underlying urothelium [34,35].
The surface of the uroplakin is studded with mannose [36,37]. The tip adhesin for type 1 pili, FimH,
binds mannose with stereochemical specificity. Therefore, upon entering the bladder, FimH is able to
bind mannosylated residues on the bladder surface, such as those found on uroplakin as well asβ 1-α 3
integrin receptors (Figure 1B) [36,38–40]. FimH mediated interaction with the urothelium induces
a signaling cascade that activates Rho family GTPases and results in actin rearrangement within
urothelial cells, promoting UPEC invasion (Figure 1C,D) [38,41,42]. Studies in mice have revealed
that once inside superficial facet cells, UPEC are able to escape the endocytic vesicle, via unknown
mechanisms, into the cytoplasm where they replicate rapidly forming biofilm-like communities called
intracellular bacterial communities (IBCs) (Figure 1D,E) [43–45]. In mice,type 1 pili are not only
required for cellular adherence and invasion but also for the aggregation of bacteria into an IBC in the
host cell cytoplasm [46]. Mouse studies have shown that invasion is a critical step in UPEC pathogenesis
in naïve mice, allowing the bacteria to rapidly replicate in a niche protected from many innate immune
defense mechanisms and antibiotics.UPEC that cannot invade the urothelium, like those lacking
FimH, are quickly cleared from the bladder, emphasizing the importance of this intracellular stage to
the success of the pathogen [46]. Mature IBCs contain approximately 104 bacteria. Upon maturation,
bacteria within the IBC adopt a filamentous morphology and disperse from the biomass, fluxing out
of the cell and back into the bladder lumen where they can adhere to and/or invade adjacent facet
cells or exposed transitional epithelial cells (Figure 1G,H) [43,44,47,48]. Importantly, evidence of IBCs
and bacterial filaments have been observed in women suffering from acute UTI, one to two days post
self-reported sexual intercourse, but not in healthy controls or infections caused by Gram-positive
organisms, which do not form IBCs [49]. IBCs have also been observed in urine from children with
an acute UTI and their presence was correlated with recurrent UTI, supporting the validity of their
importance in pathogenesis and the ability of the mouse model to recapitulate human disease [ 50].
While the collection of tissue biopsies from women with UTI is generally contraindicated, one
study of tissue biopsies demonstrated the presence of intracellular UPEC [51]. In urine from UTI
patients and/or mice, the expression of type 1 pili has been reported to be variable [52–54]. Several
studies, in mice and humans, have demonstrated that UPEC attached to shed epithelial cells in the
urine express type 1 pili while planktonic UPEC found in the urine tend to be nonpiliated [54,55].
This suggests that the expression of type 1 pili is niche specific, with type 1 pili being expressed by
UPEC that are attached to the epithelial surface.
In addition to type 1 pili, a mosaic of UPEC virulence factors including adhesins, toxins, capsule,
siderophores,and flagella have been identified and characterized [39,56–61]; however the direct
contribution of these putative urovirulence factors has not been exhaustively studied.In a recent
comprehensive genomic analysis of 43 UPEC isolates, we found that “core” genes, defined as genes
shared between all E.coli,constituted approximately 60% of each strain’s genome.Further,we
were unable to find any single set of genes that accurately delineated UPEC from non-UPEC strains
(Schreiber and Hultgren, personal communication). The lack of a strict ‘genetic’ definition of UPEC
indicates that,in most cases,urovirulence in UPEC is more complicated than carriage of a set of
virulence-associated genes that are missing in non-UPEC strains. Instead urovirulence in UPEC strains
may be defined by alternative characteristics, like propensity to certain patterns of gene expression,
as is seen with niche specific expression of type 1 pili in the bladder [54,55], or the carriage of different
alleles of core genes.There are a number of different alleles of FimH, described later in this review,
that alter the affinity of the adhesin for its ligand and may be associated with more virulent strains.
These alternative characteristics may explain how genes that are highly conserved in both UPEC and
non-UPEC strains, like those that encode type 1 pili, contribute to pathogenesis during UTI.
2.2. The Role of Type 1 Pili during Uncomplicated UTI
A hallmark of the luminalsurface of urothelialcells,also called superficialfacet cells,are
the presence of uroplakin plaques,which consist of 4 uroplakin integral membrane proteins,that
function as a barrier between the toxic contents of the bladder and the underlying urothelium [34,35].
The surface of the uroplakin is studded with mannose [36,37]. The tip adhesin for type 1 pili, FimH,
binds mannose with stereochemical specificity. Therefore, upon entering the bladder, FimH is able to
bind mannosylated residues on the bladder surface, such as those found on uroplakin as well asβ 1-α 3
integrin receptors (Figure 1B) [36,38–40]. FimH mediated interaction with the urothelium induces
a signaling cascade that activates Rho family GTPases and results in actin rearrangement within
urothelial cells, promoting UPEC invasion (Figure 1C,D) [38,41,42]. Studies in mice have revealed
that once inside superficial facet cells, UPEC are able to escape the endocytic vesicle, via unknown
mechanisms, into the cytoplasm where they replicate rapidly forming biofilm-like communities called
intracellular bacterial communities (IBCs) (Figure 1D,E) [43–45]. In mice,type 1 pili are not only
required for cellular adherence and invasion but also for the aggregation of bacteria into an IBC in the
host cell cytoplasm [46]. Mouse studies have shown that invasion is a critical step in UPEC pathogenesis
in naïve mice, allowing the bacteria to rapidly replicate in a niche protected from many innate immune
defense mechanisms and antibiotics.UPEC that cannot invade the urothelium, like those lacking
FimH, are quickly cleared from the bladder, emphasizing the importance of this intracellular stage to
the success of the pathogen [46]. Mature IBCs contain approximately 104 bacteria. Upon maturation,
bacteria within the IBC adopt a filamentous morphology and disperse from the biomass, fluxing out
of the cell and back into the bladder lumen where they can adhere to and/or invade adjacent facet
cells or exposed transitional epithelial cells (Figure 1G,H) [43,44,47,48]. Importantly, evidence of IBCs
and bacterial filaments have been observed in women suffering from acute UTI, one to two days post
self-reported sexual intercourse, but not in healthy controls or infections caused by Gram-positive
organisms, which do not form IBCs [49]. IBCs have also been observed in urine from children with
an acute UTI and their presence was correlated with recurrent UTI, supporting the validity of their
importance in pathogenesis and the ability of the mouse model to recapitulate human disease [ 50].
While the collection of tissue biopsies from women with UTI is generally contraindicated, one
study of tissue biopsies demonstrated the presence of intracellular UPEC [51]. In urine from UTI
patients and/or mice, the expression of type 1 pili has been reported to be variable [52–54]. Several
studies, in mice and humans, have demonstrated that UPEC attached to shed epithelial cells in the
urine express type 1 pili while planktonic UPEC found in the urine tend to be nonpiliated [54,55].
This suggests that the expression of type 1 pili is niche specific, with type 1 pili being expressed by
UPEC that are attached to the epithelial surface.
In addition to type 1 pili, a mosaic of UPEC virulence factors including adhesins, toxins, capsule,
siderophores,and flagella have been identified and characterized [39,56–61]; however the direct
contribution of these putative urovirulence factors has not been exhaustively studied.In a recent
comprehensive genomic analysis of 43 UPEC isolates, we found that “core” genes, defined as genes
shared between all E.coli,constituted approximately 60% of each strain’s genome.Further,we
were unable to find any single set of genes that accurately delineated UPEC from non-UPEC strains
(Schreiber and Hultgren, personal communication). The lack of a strict ‘genetic’ definition of UPEC
indicates that,in most cases,urovirulence in UPEC is more complicated than carriage of a set of
virulence-associated genes that are missing in non-UPEC strains. Instead urovirulence in UPEC strains
may be defined by alternative characteristics, like propensity to certain patterns of gene expression,
as is seen with niche specific expression of type 1 pili in the bladder [54,55], or the carriage of different
alleles of core genes.There are a number of different alleles of FimH, described later in this review,
that alter the affinity of the adhesin for its ligand and may be associated with more virulent strains.
These alternative characteristics may explain how genes that are highly conserved in both UPEC and
non-UPEC strains, like those that encode type 1 pili, contribute to pathogenesis during UTI.

Pathogens 2016, 5, 30 6 of 18
2.3. The Host Response to Type 1 Piliated UPEC
The host has evolved several mechanisms to respond to type 1 pilus mediated binding in a manner
that favors bacterial clearance or killing. Caspase-dependent apoptosis and exfoliation of superficial
facet cells is observed within hours of type 1 pili binding and invasion in C57BL/6 mice [39,62,63].
This promotes the clearance of infected cells from the host during micturition (Figure 1F). In the absence
of cell exfoliation,the host can expel UPEC directly from an invaded cell via a TLR-4 dependent
mechanism,reducing the number of successful UPEC invasion events and decreasing the overall
number of IBCs formed during acute infection (Figure 1C) [19]. UPEC interactions with the host also
leads to the robust recruitment of immune cells, mainly neutrophils and inflammatory monocytes,
to the urinary tract as well as the up regulation of pro-inflammatory cytokines which drive the
mobilization of additional immune effectors [64–68].
2.4. The Biophysics of FimH Structure and Function
Several natural allele variants of FimH exist.The residues in the mannose-binding pocket of
FimH are invariant in all sequenced UPEC isolates [37,69], however there is diversity outside of
this pocket, including at a cluster of amino acids that are positively-selected in UPEC strains [69].
Interestingly, mutations in these positively selected residues results in distinct FimH alleles that are
skewed into either elongated or compact conformations, which have been observed in several X-ray
crystal structures of FimH [25,26,70]. The elongated conformation corresponds to the mannose binding
conformation whereas the compact form binds mannose only weakly or not at all [69,71,72]. When
FimH is bound to the FimC chaperone,it adopts an elongated conformation [26] however,upon
its incorporation into the tip of the pilus, it can convert to a compact conformation with an altered
mannose-binding pocket [25,70]. In the FimCH complex, all of the FimH allele variants tested have
a high affinity for mannose.However,when incorporated into a tip-like state,following a DSE
reaction with the FimG adaptor, FimH variants differ greatly in their mannose affinity suggesting
that depending on the allele,FimH is skewed toward either an elongated or compact form after
its incorporation into the tip [73]. Interestingly, UPEC strains containing distinct FimH alleles also
display altered levels of pathogenicity during acute and chronic UTI in C57BL/6 [74] and C3H/HeN
mice [73]. Of particular interest we found that specific mutations in two positively selected residues,
A27 and V163, result in a FimH variant (FimH(A27V/V163A)) that resides within a tip-like complex
predominantly in a high-affinity mannose binding conformation [73]. Surprisingly, this FimH allele is
severely attenuated in a cystitis model [69,73]. These findings support the hypothesis that FimH resides
in an equilibrium of mannose-binding and non-binding conformations, which are governed, in part, by
the identity of select residues that are under positive selection in UPEC isolates. The inter-conversion
between high and low mannose-binding conformations is likely important for tissue-binding and/or
immune evasion, which may begin to explain why the FimH(A27V/V163A) variant, which appears
to be “locked” in a high-affinity elongated state is unable to cause infectionin vivodespite its
high-affinity for mannose.These findings may be related to the important observation made by
others that FimH conformation is influenced by shear force leading to a “catch bond” model of FimH
function [70–72,75,76]. Thus, the ability of FimH to be incorporated into a pilus tip to interconvert into
different conformations affects virulence.
2.5. The Role of P Pili in Pyelonephritis
The ability of UPEC to ascend from the bladder to the upper urinary tract likely involves many
genetic and environmental factors,one of which is thought to be the up-regulation of P pili and
corresponding down-regulation of type 1 pili [77]. P pili, which are encoded by the pap operon, mediate
adhesion of UPEC to kidney tissue resulting in pyelonephritis [61,78–80]. This binding is dependent
on the expression of the P pilus tip adhesin, PapG [56,81]. Three alleles of PapG exist (PapG-I, -II, and
-III) and each allele shows a distinct affinity for a series of Galα1-4Gal containing glycolipid receptors,
2.3. The Host Response to Type 1 Piliated UPEC
The host has evolved several mechanisms to respond to type 1 pilus mediated binding in a manner
that favors bacterial clearance or killing. Caspase-dependent apoptosis and exfoliation of superficial
facet cells is observed within hours of type 1 pili binding and invasion in C57BL/6 mice [39,62,63].
This promotes the clearance of infected cells from the host during micturition (Figure 1F). In the absence
of cell exfoliation,the host can expel UPEC directly from an invaded cell via a TLR-4 dependent
mechanism,reducing the number of successful UPEC invasion events and decreasing the overall
number of IBCs formed during acute infection (Figure 1C) [19]. UPEC interactions with the host also
leads to the robust recruitment of immune cells, mainly neutrophils and inflammatory monocytes,
to the urinary tract as well as the up regulation of pro-inflammatory cytokines which drive the
mobilization of additional immune effectors [64–68].
2.4. The Biophysics of FimH Structure and Function
Several natural allele variants of FimH exist.The residues in the mannose-binding pocket of
FimH are invariant in all sequenced UPEC isolates [37,69], however there is diversity outside of
this pocket, including at a cluster of amino acids that are positively-selected in UPEC strains [69].
Interestingly, mutations in these positively selected residues results in distinct FimH alleles that are
skewed into either elongated or compact conformations, which have been observed in several X-ray
crystal structures of FimH [25,26,70]. The elongated conformation corresponds to the mannose binding
conformation whereas the compact form binds mannose only weakly or not at all [69,71,72]. When
FimH is bound to the FimC chaperone,it adopts an elongated conformation [26] however,upon
its incorporation into the tip of the pilus, it can convert to a compact conformation with an altered
mannose-binding pocket [25,70]. In the FimCH complex, all of the FimH allele variants tested have
a high affinity for mannose.However,when incorporated into a tip-like state,following a DSE
reaction with the FimG adaptor, FimH variants differ greatly in their mannose affinity suggesting
that depending on the allele,FimH is skewed toward either an elongated or compact form after
its incorporation into the tip [73]. Interestingly, UPEC strains containing distinct FimH alleles also
display altered levels of pathogenicity during acute and chronic UTI in C57BL/6 [74] and C3H/HeN
mice [73]. Of particular interest we found that specific mutations in two positively selected residues,
A27 and V163, result in a FimH variant (FimH(A27V/V163A)) that resides within a tip-like complex
predominantly in a high-affinity mannose binding conformation [73]. Surprisingly, this FimH allele is
severely attenuated in a cystitis model [69,73]. These findings support the hypothesis that FimH resides
in an equilibrium of mannose-binding and non-binding conformations, which are governed, in part, by
the identity of select residues that are under positive selection in UPEC isolates. The inter-conversion
between high and low mannose-binding conformations is likely important for tissue-binding and/or
immune evasion, which may begin to explain why the FimH(A27V/V163A) variant, which appears
to be “locked” in a high-affinity elongated state is unable to cause infectionin vivodespite its
high-affinity for mannose.These findings may be related to the important observation made by
others that FimH conformation is influenced by shear force leading to a “catch bond” model of FimH
function [70–72,75,76]. Thus, the ability of FimH to be incorporated into a pilus tip to interconvert into
different conformations affects virulence.
2.5. The Role of P Pili in Pyelonephritis
The ability of UPEC to ascend from the bladder to the upper urinary tract likely involves many
genetic and environmental factors,one of which is thought to be the up-regulation of P pili and
corresponding down-regulation of type 1 pili [77]. P pili, which are encoded by the pap operon, mediate
adhesion of UPEC to kidney tissue resulting in pyelonephritis [61,78–80]. This binding is dependent
on the expression of the P pilus tip adhesin, PapG [56,81]. Three alleles of PapG exist (PapG-I, -II, and
-III) and each allele shows a distinct affinity for a series of Galα1-4Gal containing glycolipid receptors,
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Pathogens 2016, 5, 30 7 of 18
which are expressed to various degrees in the kidneys and ureters of mammals [82]. Human kidneys,
for example, abundantly express the ligands for PapG-II, globoside, and thus are colonized with UPEC
that express PapG-II alleles.Conversely, PapG-III binds strongly to Forssman glycolipid, which is
present in dog but not in human kidneys. Thus, different alleles of PapG mediate host tropisms [83–85].
2.6. Identifying Roles for Additional CUP Pili Types during Disease
A recent analysis of 35 Escherichia spp. genomes and 132 plasmids identified a total of 458 CUP
pili operons, representing 38 distinct CUP pilus types based on usher phylogeny [86,87]. CUP pili
tipped with specific adhesins provides E. coli the ability to bind to distinct ligands with stereo-chemical
specificity. Single UPEC strains were found to carry as many as 16 distinct, intact CUP pilus systems
that likely aid UPEC strains in their colonization of many host niches, including the gut, vagina, urethra,
bladder, and kidneys.Thus, it is likely that the retention of many distinct CUP pilus types reflects
the adaptation to broad environmental and host niches and their function in facilitating the various
tropisms of E. coli [88]. Aside from type 1 and P pili, UPEC strains encode a number of additional CUP
pilus types for which a role in UPEC pathogenesis is minimally defined or completely undetermined.
S pili are known to bind sialosyloligosaccaride residues in host cells and likely mediate infection of
the ureters and kidneys [89]. Clinical isolates containing S pili are often associated with more severe
outcomes in patients with UTI, including: pyelonephritis, sepsis, and meningitis. The Dr pili adhesin
has been shown to bind type IV collagen and decay-accelerating factor (DAF),both of which are
expressed in human kidneys. Dr pili enable UPEC colonization of the mouse renal interstitial basement
membrane [90,91]. The roles for CUP pili, including F1C, F9, Ygi, Yad, and Auf, have been briefly
explored and have been recently reviewed [92–94]. Identifying roles for additional CUP pilus types
will enhance our overall knowledge of UTI pathogenesis and may provide novel drug targets to treat
patients with UTI.
2.7. Outcomes of UTI: Connecting Findings in Humans and Mice
Much of our understanding ofUTI and rUTI pathogenesis comes from studies in mice.
The development of murine models of acute and recurrent cystitis have allowed for many important
advances in our understanding of UTI pathogenesis.However, it is also worth noting that the UTI
mouse models, like all animal models, have limitations. Behavior, genetic, and environment differences
between mice and humans make it challenging to translate all murine findings to humans [95]. Here we
discuss advances in our understanding of UTI pathogenesis based on experiments performed in
relevant mouse models and highlight how these findings have been recapitulated in clinical studies
performed in women with UTI or rUTI.
For women with UTI, several disease outcomes are possible, including: asymptomatic bacteruria
(ABU), acute self-limiting infection,or chronic/recurrentUTI [5]. Placebo studies in women
have shown that approximately 50% of UTIs do not resolve in the absence of effective antibiotic
treatment, implying that cystitis is not always self-limiting. These findings highlight the importance
of clinical intervention to resolve UTI and the developing crisis of emerging multi-drug resistant
uropathogens [96,97]. In naïve mice,there are two major outcomes of UTI:(i) self-limiting acute
infection that resolves within days of the initiation of infection with or without the formation of
quiescent intracellular reservoirs (QIR);or (ii) persistent,high-titer bacteruria concomitant with
high bladder bacterial burden and severe bladder inflammation which, in the absence of antibiotic
intervention,can lastfor the lifetime ofthe animaland is referred to as chronic cystitis [98]
(Figure 1H). The resolution of infection in mice is often accompanied by QIR formation [99] (Figure 1I).
The exfoliation response results in the exposure of the underlying transitional cells, which allows the
subsequent invasion of UPEC into these cells. However, unlike superficial facet cells, the underlying
transitional cells do not support IBC formation; instead UPEC establish QIRs, which are comprised of
8–12 dormant bacteria in Lamp1+ vesicles [99]. The mechanism of QIR formation is unknown, however,
these reservoirs are able to reactivate and release UPEC back into the bladder lumen to initiate a new
which are expressed to various degrees in the kidneys and ureters of mammals [82]. Human kidneys,
for example, abundantly express the ligands for PapG-II, globoside, and thus are colonized with UPEC
that express PapG-II alleles.Conversely, PapG-III binds strongly to Forssman glycolipid, which is
present in dog but not in human kidneys. Thus, different alleles of PapG mediate host tropisms [83–85].
2.6. Identifying Roles for Additional CUP Pili Types during Disease
A recent analysis of 35 Escherichia spp. genomes and 132 plasmids identified a total of 458 CUP
pili operons, representing 38 distinct CUP pilus types based on usher phylogeny [86,87]. CUP pili
tipped with specific adhesins provides E. coli the ability to bind to distinct ligands with stereo-chemical
specificity. Single UPEC strains were found to carry as many as 16 distinct, intact CUP pilus systems
that likely aid UPEC strains in their colonization of many host niches, including the gut, vagina, urethra,
bladder, and kidneys.Thus, it is likely that the retention of many distinct CUP pilus types reflects
the adaptation to broad environmental and host niches and their function in facilitating the various
tropisms of E. coli [88]. Aside from type 1 and P pili, UPEC strains encode a number of additional CUP
pilus types for which a role in UPEC pathogenesis is minimally defined or completely undetermined.
S pili are known to bind sialosyloligosaccaride residues in host cells and likely mediate infection of
the ureters and kidneys [89]. Clinical isolates containing S pili are often associated with more severe
outcomes in patients with UTI, including: pyelonephritis, sepsis, and meningitis. The Dr pili adhesin
has been shown to bind type IV collagen and decay-accelerating factor (DAF),both of which are
expressed in human kidneys. Dr pili enable UPEC colonization of the mouse renal interstitial basement
membrane [90,91]. The roles for CUP pili, including F1C, F9, Ygi, Yad, and Auf, have been briefly
explored and have been recently reviewed [92–94]. Identifying roles for additional CUP pilus types
will enhance our overall knowledge of UTI pathogenesis and may provide novel drug targets to treat
patients with UTI.
2.7. Outcomes of UTI: Connecting Findings in Humans and Mice
Much of our understanding ofUTI and rUTI pathogenesis comes from studies in mice.
The development of murine models of acute and recurrent cystitis have allowed for many important
advances in our understanding of UTI pathogenesis.However, it is also worth noting that the UTI
mouse models, like all animal models, have limitations. Behavior, genetic, and environment differences
between mice and humans make it challenging to translate all murine findings to humans [95]. Here we
discuss advances in our understanding of UTI pathogenesis based on experiments performed in
relevant mouse models and highlight how these findings have been recapitulated in clinical studies
performed in women with UTI or rUTI.
For women with UTI, several disease outcomes are possible, including: asymptomatic bacteruria
(ABU), acute self-limiting infection,or chronic/recurrentUTI [5]. Placebo studies in women
have shown that approximately 50% of UTIs do not resolve in the absence of effective antibiotic
treatment, implying that cystitis is not always self-limiting. These findings highlight the importance
of clinical intervention to resolve UTI and the developing crisis of emerging multi-drug resistant
uropathogens [96,97]. In naïve mice,there are two major outcomes of UTI:(i) self-limiting acute
infection that resolves within days of the initiation of infection with or without the formation of
quiescent intracellular reservoirs (QIR);or (ii) persistent,high-titer bacteruria concomitant with
high bladder bacterial burden and severe bladder inflammation which, in the absence of antibiotic
intervention,can lastfor the lifetime ofthe animaland is referred to as chronic cystitis [98]
(Figure 1H). The resolution of infection in mice is often accompanied by QIR formation [99] (Figure 1I).
The exfoliation response results in the exposure of the underlying transitional cells, which allows the
subsequent invasion of UPEC into these cells. However, unlike superficial facet cells, the underlying
transitional cells do not support IBC formation; instead UPEC establish QIRs, which are comprised of
8–12 dormant bacteria in Lamp1+ vesicles [99]. The mechanism of QIR formation is unknown, however,
these reservoirs are able to reactivate and release UPEC back into the bladder lumen to initiate a new
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Pathogens 2016, 5, 30 8 of 18
infection cycle [99,100]. Interestingly, the fate of disease in mice is determined by whether an acute
host-pathogen checkpoint, which is influenced by the genetic background of the mice, is triggered.
C57BL/6 mice are resistant to chronic cystitis after a single infection;however,they can develop
persistent bacteriuria and chronic cystitis when “superinfected”, by multiple transurethral inoculations
within a 24 hour period [74]. Elevated levels of interleukin-6 (IL-6), keratinocyte cytokine (KC/CXCL1),
and granulocyte colony-stimulating factor (G-CSF) in the serum of C57BL/6 mice prior to the second
infection predicted the development of chronic cystitis [74]. These same cytokines have been found
to precede chronic cystitis in singly infected C3H/HeN mice, which are prone to the development of
chronic cystitis [98]. Superinfection of C3H/HeN mice within a six-hour period doubles the proportion
of mice that developed chronic cystitis.Intracellular bacterialreplication,regulated hemolysin
(HlyA) expression,and caspase 1/11 activation were essential for this increase [74]. The chronic
bladder inflammation that accompanies chronic cystitis includes lymphonodular hyperplasia in
the bladder submucosa and urothelial hyperplasia that results in the loss of superficial facet cells.
Similar histological findings have been observed in humans suffering from persistent bacteriuria and
rUTI [101,102].
Proteomic analysis of chronically infected bladders indicates that chronic inflammation also
results in bladder epithelial remodeling, which may help to explain why mice that experience chronic
cystitis are more susceptible to rUTI upon further bacterial challenge weeks after antibiotic intervention
and resolution of the initial infection [67]. Studies of mice with rUTI may also begin to explain why
a history of UTI is a major risk factor for rUTI in women, highlighting the clinical relevance of this
mouse model. Elevated soluble serum biomarkers that were predictive of rUTI were also detected in
young women with UTI [67]. Interestingly, temperance of the neutrophil response during the first 24 h
of infection, by inhibition of cyclooxygenase-2 (COX 2), protected mice from chronic and recurrent
cystitis [67]. These findings may help to explain the outcome of a small clinical trial that compared
the outcomes of women with UPEC UTI after being given a three-day course of either ciprofloxacin
or ibuprofen.The study found no difference in UTI outcome between these two groups at four or
seven days post treatment [103]. Another study found that symptomatically treating women suffering
from uncomplicated UTI with ibuprofen (without any antibiotics) resolved infection in two-thirds of
the study group [104]. These results indicate that ibuprofen, which works by inhibiting COX 1 and 2,
may act to decrease the severity of UTI in these patients. However, the second study also found that
women in the ibuprofen treated group suffered from more UTI symptoms (abdominal pain, increased
frequency of urination, dysuria) and were more likely to develop pyelonephritis [104].
The development of rUTI is likely a balance between bacterial factors and host genetics. Murine
studies have indicated that the innate immune response is critical for combating UTI, which had
been recapitulated in human clinical studies [105]. Therefore, mutations in the genes involved in this
early immune response can greatly influence the susceptibility of a host to UTI. For example, certain
polymorphisms and altered expression levels of innate immune genes, like Toll-like receptor 4 (TLR4)
and CXCR1, have been associated with less severe symptomatic UTI but an increase in the development
of ABU in women [105]. Alterations in the sequence or expression of other innate immune genes, like
IRF3 and CXCR1, have been associated with increased incidences of acute pyelonephritis [ 105].
3. Pili are Critical for the Establishment of UPEC and Enterococcus Mediated CAUTI
The introduction of a catheter into the urinary tract provides an additional surface on which
bacteria can adhere and establish infection. Uropathogens that are commonly associated with CAUTI,
like those belonging to the Enterococcus genus, also encode a number of distinct adhesive factors and
pili that permit attachment within the host. Of these factors, the endocarditis- and biofilm-associated
(Ebp) pilus has recently been identified to play an essential role in the establishment and persistence of
UTI in a catheterized mouse model [106–110]. Upon catheterization, implanted catheters are coated
with host-derived fibrinogen and are subsequently bound by Ebp expressing Enterococcus faecalis
(E. faecalis),a common enterococcal uropathogen [109]. The Ebp pilus binds fibrinogen via its tip
infection cycle [99,100]. Interestingly, the fate of disease in mice is determined by whether an acute
host-pathogen checkpoint, which is influenced by the genetic background of the mice, is triggered.
C57BL/6 mice are resistant to chronic cystitis after a single infection;however,they can develop
persistent bacteriuria and chronic cystitis when “superinfected”, by multiple transurethral inoculations
within a 24 hour period [74]. Elevated levels of interleukin-6 (IL-6), keratinocyte cytokine (KC/CXCL1),
and granulocyte colony-stimulating factor (G-CSF) in the serum of C57BL/6 mice prior to the second
infection predicted the development of chronic cystitis [74]. These same cytokines have been found
to precede chronic cystitis in singly infected C3H/HeN mice, which are prone to the development of
chronic cystitis [98]. Superinfection of C3H/HeN mice within a six-hour period doubles the proportion
of mice that developed chronic cystitis.Intracellular bacterialreplication,regulated hemolysin
(HlyA) expression,and caspase 1/11 activation were essential for this increase [74]. The chronic
bladder inflammation that accompanies chronic cystitis includes lymphonodular hyperplasia in
the bladder submucosa and urothelial hyperplasia that results in the loss of superficial facet cells.
Similar histological findings have been observed in humans suffering from persistent bacteriuria and
rUTI [101,102].
Proteomic analysis of chronically infected bladders indicates that chronic inflammation also
results in bladder epithelial remodeling, which may help to explain why mice that experience chronic
cystitis are more susceptible to rUTI upon further bacterial challenge weeks after antibiotic intervention
and resolution of the initial infection [67]. Studies of mice with rUTI may also begin to explain why
a history of UTI is a major risk factor for rUTI in women, highlighting the clinical relevance of this
mouse model. Elevated soluble serum biomarkers that were predictive of rUTI were also detected in
young women with UTI [67]. Interestingly, temperance of the neutrophil response during the first 24 h
of infection, by inhibition of cyclooxygenase-2 (COX 2), protected mice from chronic and recurrent
cystitis [67]. These findings may help to explain the outcome of a small clinical trial that compared
the outcomes of women with UPEC UTI after being given a three-day course of either ciprofloxacin
or ibuprofen.The study found no difference in UTI outcome between these two groups at four or
seven days post treatment [103]. Another study found that symptomatically treating women suffering
from uncomplicated UTI with ibuprofen (without any antibiotics) resolved infection in two-thirds of
the study group [104]. These results indicate that ibuprofen, which works by inhibiting COX 1 and 2,
may act to decrease the severity of UTI in these patients. However, the second study also found that
women in the ibuprofen treated group suffered from more UTI symptoms (abdominal pain, increased
frequency of urination, dysuria) and were more likely to develop pyelonephritis [104].
The development of rUTI is likely a balance between bacterial factors and host genetics. Murine
studies have indicated that the innate immune response is critical for combating UTI, which had
been recapitulated in human clinical studies [105]. Therefore, mutations in the genes involved in this
early immune response can greatly influence the susceptibility of a host to UTI. For example, certain
polymorphisms and altered expression levels of innate immune genes, like Toll-like receptor 4 (TLR4)
and CXCR1, have been associated with less severe symptomatic UTI but an increase in the development
of ABU in women [105]. Alterations in the sequence or expression of other innate immune genes, like
IRF3 and CXCR1, have been associated with increased incidences of acute pyelonephritis [ 105].
3. Pili are Critical for the Establishment of UPEC and Enterococcus Mediated CAUTI
The introduction of a catheter into the urinary tract provides an additional surface on which
bacteria can adhere and establish infection. Uropathogens that are commonly associated with CAUTI,
like those belonging to the Enterococcus genus, also encode a number of distinct adhesive factors and
pili that permit attachment within the host. Of these factors, the endocarditis- and biofilm-associated
(Ebp) pilus has recently been identified to play an essential role in the establishment and persistence of
UTI in a catheterized mouse model [106–110]. Upon catheterization, implanted catheters are coated
with host-derived fibrinogen and are subsequently bound by Ebp expressing Enterococcus faecalis
(E. faecalis),a common enterococcal uropathogen [109]. The Ebp pilus binds fibrinogen via its tip

Pathogens 2016, 5, 30 9 of 18
adhesin, EbpA, which contains an N-terminal fibrinogen-binding domain [109]. The mechanism(s) by
which fibrinogen enters the bladder is still under investigation but is likely due to bladder damage
that occurs during the catheterization process and subsequent infection. Mechanical stress induced by
insertion of the catheter into the bladder results in the induction of a robust inflammatory response
and severe bladder edema in humans and mice [107,111–113]. Bladder inflammation,including
increases in serum cytokines IL-1α and IL-6, and edema, is elevated further upon introduction of
E. faecalis into the catheterized mouse [107]. Increases in IL-1α and IL-6 have been previously shown to
stimulate the release of fibrinogen from the liver into the bloodstream as a part of the pro-inflammatory
response and circulating fibrinogen may then leak into the urinary tract via tissue damaged during
catheterization.Once in the bladder,fibrinogen is deposited on the catheter,providing a binding
site for EbpA expressing E. faecalis.Catheterization and the subsequent inflammatory response are
essential in mediating CAUTI, as mice infected with E. faecalis in the absence of a catheter are quickly
cleared from the bladder [107]. Fibrinogen deposited onto the catheter surface not only enhances
E. faecalis biofilm formation but also promotes growth, increasing the severity of the infection [109].
The ability to bind and utilize fibrinogen may be a general feature of infection in gram-positive bacteria,
(as has been shown for Staphylococcus aureus (S.aureus),Staphylococcus epidermidis (S.epidermidis),
and Group A streptococci) and potentially in some fungal pathogens (as was recently shown for
Candida albicans) [114,115]. The Ebp pilus also plays a role in the transition of E. faecalis infection from
the bladder to the kidneys. In E. faecalis, loss of the pilus reduces bacterial colonization of the kidneys
and thus lowers the incidence of pyelonephritis in infected mice [110].
UPEC, the leading cause of complicated UTI, can also use CUP pili to colonize the bladder during
catheterization [15,116]. However, unlike Enterococcal spp., UPEC does not rely on the presence of the
catheter to propagate high levels of bacterial colonization. In a CAUTI mouse model, UPEC undergoes
the same acute pathogenic lifecycle that is observed in cystitis models, including: invasion into the
tissue, IBC formation and maturation, the formation of filaments, and the emergence of filamentous
bacteria back into the bladder lumen [116]. However, in the presence of the catheter, UPEC does appear
to have a more robust extracellular population during acute infection, which is likely due to UPEC
colonization and subsequent biofilm formation on the surface of the catheter [116]. Interestingly, type
1 pili are required for biofilm formation and UPEC colonization of the catheter [116]. These studies
continue to define the importance of type 1 pili as a virulence factor during uncomplicated and
complicated UTI in mice.
4. Development of Pili-Based Alternative Treatments to Treat or Prevent UTI and CAUTI
Pili are potential drug targets due to the critical roles they play in UTI and CAUTI pathogenesis.
Our understanding of the molecular mechanisms by which pili function and impact disease has
permitted the development of novel therapeutics, such as vaccines and small molecule inhibitors,
that target pili and block infection by preventing colonization. While a role for pili during intestinal
colonization by uropathogens is currently unstudied, identifying and targeting pili that do permit
intestinal colonization would provide a unique opportunity to target the source of uropathogens that
lead to downstream infections.
4.1. Vaccines
A number of promising vaccines that target a wide-range of UPEC virulence factors are currently
in development [117–121]. The FimCH vaccine, a subunit vaccine targeting the type 1 pilus adhesin
FimH, is one such example. Significant protection was observed in subcutaneously vaccinated mice
and cynomolgus monkeys given an intra-muscular vaccination, with the protected animals developing
antigen-specific, long-lasting serum IgG antibodies [120,121]. Based on this data, it is presumed that
the vaccine works by stimulating these FimH-specific IgG antibodies that block UPEC colonization of
the bladder. Consistent with this, the polyclonal antibodies derived from vaccinated mice block FimH
function and likely activate immune effector cells, like phagocytes and complement, for clearance
adhesin, EbpA, which contains an N-terminal fibrinogen-binding domain [109]. The mechanism(s) by
which fibrinogen enters the bladder is still under investigation but is likely due to bladder damage
that occurs during the catheterization process and subsequent infection. Mechanical stress induced by
insertion of the catheter into the bladder results in the induction of a robust inflammatory response
and severe bladder edema in humans and mice [107,111–113]. Bladder inflammation,including
increases in serum cytokines IL-1α and IL-6, and edema, is elevated further upon introduction of
E. faecalis into the catheterized mouse [107]. Increases in IL-1α and IL-6 have been previously shown to
stimulate the release of fibrinogen from the liver into the bloodstream as a part of the pro-inflammatory
response and circulating fibrinogen may then leak into the urinary tract via tissue damaged during
catheterization.Once in the bladder,fibrinogen is deposited on the catheter,providing a binding
site for EbpA expressing E. faecalis.Catheterization and the subsequent inflammatory response are
essential in mediating CAUTI, as mice infected with E. faecalis in the absence of a catheter are quickly
cleared from the bladder [107]. Fibrinogen deposited onto the catheter surface not only enhances
E. faecalis biofilm formation but also promotes growth, increasing the severity of the infection [109].
The ability to bind and utilize fibrinogen may be a general feature of infection in gram-positive bacteria,
(as has been shown for Staphylococcus aureus (S.aureus),Staphylococcus epidermidis (S.epidermidis),
and Group A streptococci) and potentially in some fungal pathogens (as was recently shown for
Candida albicans) [114,115]. The Ebp pilus also plays a role in the transition of E. faecalis infection from
the bladder to the kidneys. In E. faecalis, loss of the pilus reduces bacterial colonization of the kidneys
and thus lowers the incidence of pyelonephritis in infected mice [110].
UPEC, the leading cause of complicated UTI, can also use CUP pili to colonize the bladder during
catheterization [15,116]. However, unlike Enterococcal spp., UPEC does not rely on the presence of the
catheter to propagate high levels of bacterial colonization. In a CAUTI mouse model, UPEC undergoes
the same acute pathogenic lifecycle that is observed in cystitis models, including: invasion into the
tissue, IBC formation and maturation, the formation of filaments, and the emergence of filamentous
bacteria back into the bladder lumen [116]. However, in the presence of the catheter, UPEC does appear
to have a more robust extracellular population during acute infection, which is likely due to UPEC
colonization and subsequent biofilm formation on the surface of the catheter [116]. Interestingly, type
1 pili are required for biofilm formation and UPEC colonization of the catheter [116]. These studies
continue to define the importance of type 1 pili as a virulence factor during uncomplicated and
complicated UTI in mice.
4. Development of Pili-Based Alternative Treatments to Treat or Prevent UTI and CAUTI
Pili are potential drug targets due to the critical roles they play in UTI and CAUTI pathogenesis.
Our understanding of the molecular mechanisms by which pili function and impact disease has
permitted the development of novel therapeutics, such as vaccines and small molecule inhibitors,
that target pili and block infection by preventing colonization. While a role for pili during intestinal
colonization by uropathogens is currently unstudied, identifying and targeting pili that do permit
intestinal colonization would provide a unique opportunity to target the source of uropathogens that
lead to downstream infections.
4.1. Vaccines
A number of promising vaccines that target a wide-range of UPEC virulence factors are currently
in development [117–121]. The FimCH vaccine, a subunit vaccine targeting the type 1 pilus adhesin
FimH, is one such example. Significant protection was observed in subcutaneously vaccinated mice
and cynomolgus monkeys given an intra-muscular vaccination, with the protected animals developing
antigen-specific, long-lasting serum IgG antibodies [120,121]. Based on this data, it is presumed that
the vaccine works by stimulating these FimH-specific IgG antibodies that block UPEC colonization of
the bladder. Consistent with this, the polyclonal antibodies derived from vaccinated mice block FimH
function and likely activate immune effector cells, like phagocytes and complement, for clearance
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Pathogens 2016, 5, 30 10 of 18
of the infection [120,121]. The success of this vaccine in animal models highlights its potential as an
alternative treatment to UTI in humans.A FimCH vaccine adjuvanted with PHAD (Avanti Polar
Lipids, Alabaster, AL) is currently completing a Phase 1 human study. This study has enrolled about
67 women with and without a history of rUTI (personal communication, Gary Eldridge).
Other CUP pilus types are the focus of additional vaccine candidates.One such candidate
is the PapDG subunitvaccine. P pili are strongly associated with pyelonephritis and as such
have been designed to target UPEC and prevent complicated upper UTIs [61]. Intraperitoneal
administration of the PapDG vaccine protects animals from infection and elicits a specific IgG antibody
response in cynomolgus monkeys [118,122]. The Dr fimbriae represents another current vaccine target.
Mice vaccinated with purified Dr fimbriae produce high titers of serum antibodies against the fimbriae
but do not have lower colonization rates in either the bladder or kidneys [123].
Recent findings revealed an essential role for the Ebp pilus and its adhesin, EbpA, during E. faecalis
mediated CAUTI in mice. Interestingly, vaccinating mice with EbpA results in significant protection
against subsequent E. faecalis CAUTI compared to unvaccinated mice or mice vaccinated with other
Ebp pilus protein subunits [109]. Vaccinating mice with only the N-terminal portion of EbpA, which
contains the fibrinogen-binding domain, is also sufficient to protect mice from subsequent infection
and may provide a higher level of protection than vaccination with the entire adhesin.
4.2. Small Molecule Inhibitors
The importance ofthe FimH-mannose interaction during infection in mice has prompted
studies that test the effects of oral treatment with D-mannose or mannose-analogs on UTI outcome.
Oral D-mannose treatment has been shown in studies to work as well as antibiotics to prevent rUTI.
One group found that women who received prophylaxis with D-mannose after an initial UTI had
similar rates of rUTI as a group that received antibiotic prophylaxis,indicating that D-mannose
may be useful to prevent rUTI [124]. Another strategy to prevent UPEC pathogenesis has been
the design of orally active, synthetic, small-molecule inhibitors of CUP pilus assembly or function.
One such class of small molecule inhibitors are mannosides. Mannosides are mannose analogs that
were rationally designed to bind within the mannose-binding pocket of FimH with a high affinity and
thus block pilus binding of FimH to host receptors (Figure 2) [125,126]. Studies in mouse models have
demonstrated that mannosides are potent, fast acting and highly efficacious in the treatment of UTI and
CAUTI [116,127–129], highlighting their potential as a novel therapeutic strategy for UTI. Mannoside
treatment is especially promising as a novel antibiotic sparing therapeutic because they are effective
against multi-drug resistant uropathogens [128]. While D-mannose and mannosides both appear to
effectively block FimH-mannose interactions, mannosides have approximately a 1,000,000-fold increase
in potency for inhibiting FimH, making them promising antibiotic-sparing therapeutics [ 125,130].
Another class of compounds are pilicides, which are small, rationally designed 2-pyridinones that
block pilus assembly (Figure 2) [131,132]. Pilicides block assembly by binding to the pilus chaperone,
preventing chaperone-pilus subunit-usher interactions that are fundamental for pilus biogenesis [133].
While pilicides were originally designed to target type 1 pilus assembly, recent studies have found that
pilicide treatment disrupts assembly of at least four-CUP pilus types (type 1-, P-, S-, and Dr- pili) as
well as flagellar motility [134,135]. The ability of pilicides to target multiple pilus types makes it an
extremely valuable treatment option.
5. Conclusions
Complicated and uncomplicated UTI are extremely common,affecting a large portion of the
global population. The high rate of infection and recurrence of UTIs, their monetary cost, their physical
burden, and the increasing occurrence of antibiotic resistant uropathogens emphasize the need for
effective treatments to combat the common causative agents of UTI and CAUTI,like UPEC and
Enterococcus strains.Understanding the molecular mechanisms by which uropathogens are able to
cause disease is the first step to identifying novel drug targets. Adhesive pili, like type 1, P, and Ebp,
of the infection [120,121]. The success of this vaccine in animal models highlights its potential as an
alternative treatment to UTI in humans.A FimCH vaccine adjuvanted with PHAD (Avanti Polar
Lipids, Alabaster, AL) is currently completing a Phase 1 human study. This study has enrolled about
67 women with and without a history of rUTI (personal communication, Gary Eldridge).
Other CUP pilus types are the focus of additional vaccine candidates.One such candidate
is the PapDG subunitvaccine. P pili are strongly associated with pyelonephritis and as such
have been designed to target UPEC and prevent complicated upper UTIs [61]. Intraperitoneal
administration of the PapDG vaccine protects animals from infection and elicits a specific IgG antibody
response in cynomolgus monkeys [118,122]. The Dr fimbriae represents another current vaccine target.
Mice vaccinated with purified Dr fimbriae produce high titers of serum antibodies against the fimbriae
but do not have lower colonization rates in either the bladder or kidneys [123].
Recent findings revealed an essential role for the Ebp pilus and its adhesin, EbpA, during E. faecalis
mediated CAUTI in mice. Interestingly, vaccinating mice with EbpA results in significant protection
against subsequent E. faecalis CAUTI compared to unvaccinated mice or mice vaccinated with other
Ebp pilus protein subunits [109]. Vaccinating mice with only the N-terminal portion of EbpA, which
contains the fibrinogen-binding domain, is also sufficient to protect mice from subsequent infection
and may provide a higher level of protection than vaccination with the entire adhesin.
4.2. Small Molecule Inhibitors
The importance ofthe FimH-mannose interaction during infection in mice has prompted
studies that test the effects of oral treatment with D-mannose or mannose-analogs on UTI outcome.
Oral D-mannose treatment has been shown in studies to work as well as antibiotics to prevent rUTI.
One group found that women who received prophylaxis with D-mannose after an initial UTI had
similar rates of rUTI as a group that received antibiotic prophylaxis,indicating that D-mannose
may be useful to prevent rUTI [124]. Another strategy to prevent UPEC pathogenesis has been
the design of orally active, synthetic, small-molecule inhibitors of CUP pilus assembly or function.
One such class of small molecule inhibitors are mannosides. Mannosides are mannose analogs that
were rationally designed to bind within the mannose-binding pocket of FimH with a high affinity and
thus block pilus binding of FimH to host receptors (Figure 2) [125,126]. Studies in mouse models have
demonstrated that mannosides are potent, fast acting and highly efficacious in the treatment of UTI and
CAUTI [116,127–129], highlighting their potential as a novel therapeutic strategy for UTI. Mannoside
treatment is especially promising as a novel antibiotic sparing therapeutic because they are effective
against multi-drug resistant uropathogens [128]. While D-mannose and mannosides both appear to
effectively block FimH-mannose interactions, mannosides have approximately a 1,000,000-fold increase
in potency for inhibiting FimH, making them promising antibiotic-sparing therapeutics [ 125,130].
Another class of compounds are pilicides, which are small, rationally designed 2-pyridinones that
block pilus assembly (Figure 2) [131,132]. Pilicides block assembly by binding to the pilus chaperone,
preventing chaperone-pilus subunit-usher interactions that are fundamental for pilus biogenesis [133].
While pilicides were originally designed to target type 1 pilus assembly, recent studies have found that
pilicide treatment disrupts assembly of at least four-CUP pilus types (type 1-, P-, S-, and Dr- pili) as
well as flagellar motility [134,135]. The ability of pilicides to target multiple pilus types makes it an
extremely valuable treatment option.
5. Conclusions
Complicated and uncomplicated UTI are extremely common,affecting a large portion of the
global population. The high rate of infection and recurrence of UTIs, their monetary cost, their physical
burden, and the increasing occurrence of antibiotic resistant uropathogens emphasize the need for
effective treatments to combat the common causative agents of UTI and CAUTI,like UPEC and
Enterococcus strains.Understanding the molecular mechanisms by which uropathogens are able to
cause disease is the first step to identifying novel drug targets. Adhesive pili, like type 1, P, and Ebp,
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Pathogens 2016, 5, 30 11 of 18
have been shown to play essential roles in UTI pathogenesis in mouse models and provide potential
drug targets that may greatly reduce or prevent infection in patients.While the mouse models of
uncomplicated UTI and CAUTI have been shown to mimic disease in human patients, the success
of vaccine and small molecular inhibitor therapies against these pili in humans is dependent on
determining the efficacy of these treatments in clinical trials.
Acknowledgments:We thank Karen Dodson, Ana Flores-Mireles, and Henry Schreiber for critical reading of this
manuscript and helpful discussion. This work was supported by the National Institutes of Health and Office of
Research on Women’s Health Specialized Center of Research (DK64540, DK51406, AI48689, AI29549, AI49950 and
AI95542 to Scott J. Hultgren).
AuthorContributions:Caitlin N. Spaulding isresponsible forthe design and contentof this review.
Scott J. Hultgren contributed to the overall organization and content of this work.
Conflicts of Interest:Caitlin Spaulding reported no potentialconflicts ofinterestrelevantto this article.
Scott J. Hultgrenhas intellectual property in the FimH vaccine and ownership interest in Fimbrion Therapeutics,
and may benefit if the company is successful in marketing mannosides.
References
1. Nicolle, L.E. Catheter-related urinary tract infection. Drugs Aging 2005, 22, 627–639. [CrossRef] [PubMed]
2. Stamm, W.E.; Norrby, S.R. Urinary tract infections: disease panorama and challenges. J. Infect. Dis.2001, 183,
1–4. [CrossRef] [PubMed]
3. Harding, G.K.; Ronald, A.R. The management of urinary tract infections: what we have learned in the past
decade. Int. J. Antimicrob. Agents 1994, 4, 83–88. [CrossRef]
4. Foxman,B. The epidemiology of urinary tract infection.Nat. Rev.Urol. 2010, 7, 653–660.[CrossRef]
[PubMed]
5. Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease
burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [CrossRef] [PubMed]
6. Hooton, T.M. Uncomplicated urinary tract infection.N. Engl.J. Med.2012, 366,1028–1037.[CrossRef]
[PubMed]
7. Lichtenberger, P.; Hooton, T.M. Complicated urinary tract infections. Curr. Infect. Dis. Rep.2008, 10, 499–504.
[CrossRef] [PubMed]
8. Levison, M.E.; Kaye, D. Treatment of complicated urinary tract infections with an emphasis on drug-resistant
gram-negative uropathogens. Curr. Infect. Dis. Rep. 2013, 15, 109–115. [CrossRef] [PubMed]
9. Lo, E.; Nicolle, L.E.; Coffin, S.E.; Gould, C.; Maragakis, L.L.; Meddings, J.; Pegues, D.A.; Pettis, A.M.; Saint, S.;
Yokoe, D.S. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals:2014
update. Infect. Control Hosp. Epidemiol. 2014, 35, 32–47. [CrossRef]
10. Foxman, B.; Brown, P. Epidemiology of urinary tract infections: Transmission and risk factors, incidence,
and costs. Infect. Dis. Clin. N. Am. 2003, 17, 227–241. [CrossRef]
11. Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med.
2002, 113, 5S–13S. [CrossRef]
12. Ikaheimo,R.; Siitonen,A.; Heiskanen,T.; Karkkainen,U.; Kuosmanen,P.;Lipponen,P.;Makela,P.H.
Recurrence of urinary tract infection in a primary care setting: Analysis of a 1-year follow-up of 179 women.
Clin. Infect. Dis. 1996, 22, 91–99. [CrossRef] [PubMed]
13. Al-Badr,A.; Al-Shaikh,G. RecurrentUrinary TractInfections Managementin Women: A review.
Sultan Qaboos Univ. Med. J. 2013, 13, 359–367. [CrossRef] [PubMed]
14. Griebling, T.L. Urinary tract infection in women. Urologic Diseases in Amerca; Litwin, M.S., Saigal, C.S., Eds
U.S. Government Printing Office: Washington, DC, USA, 2007; pp. 587–620.
15. Flores-Mireles,A.L.; Walker,J.N.; Caparon,M.; Hultgren,S.J.Urinary tract infections:epidemiology,
mechanims of infection and treatment options. Nat. Rev. Microbiol.2015, 13, 269–284. [CrossRef] [PubMed]
16. Chen, S.L.;Wu, M.;Henderson, J.P.;Hooton, T.M.;Hibbing, M.E.;Hultgren, S.J.;Gordon, J.I. Genomic
diversity and fitness of E.colistrains recovered from the intestinal and urinary tracts of women with
recurrent urinary tract infection. Sci. Transl. Med. 2013. [CrossRef] [PubMed]
have been shown to play essential roles in UTI pathogenesis in mouse models and provide potential
drug targets that may greatly reduce or prevent infection in patients.While the mouse models of
uncomplicated UTI and CAUTI have been shown to mimic disease in human patients, the success
of vaccine and small molecular inhibitor therapies against these pili in humans is dependent on
determining the efficacy of these treatments in clinical trials.
Acknowledgments:We thank Karen Dodson, Ana Flores-Mireles, and Henry Schreiber for critical reading of this
manuscript and helpful discussion. This work was supported by the National Institutes of Health and Office of
Research on Women’s Health Specialized Center of Research (DK64540, DK51406, AI48689, AI29549, AI49950 and
AI95542 to Scott J. Hultgren).
AuthorContributions:Caitlin N. Spaulding isresponsible forthe design and contentof this review.
Scott J. Hultgren contributed to the overall organization and content of this work.
Conflicts of Interest:Caitlin Spaulding reported no potentialconflicts ofinterestrelevantto this article.
Scott J. Hultgrenhas intellectual property in the FimH vaccine and ownership interest in Fimbrion Therapeutics,
and may benefit if the company is successful in marketing mannosides.
References
1. Nicolle, L.E. Catheter-related urinary tract infection. Drugs Aging 2005, 22, 627–639. [CrossRef] [PubMed]
2. Stamm, W.E.; Norrby, S.R. Urinary tract infections: disease panorama and challenges. J. Infect. Dis.2001, 183,
1–4. [CrossRef] [PubMed]
3. Harding, G.K.; Ronald, A.R. The management of urinary tract infections: what we have learned in the past
decade. Int. J. Antimicrob. Agents 1994, 4, 83–88. [CrossRef]
4. Foxman,B. The epidemiology of urinary tract infection.Nat. Rev.Urol. 2010, 7, 653–660.[CrossRef]
[PubMed]
5. Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease
burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [CrossRef] [PubMed]
6. Hooton, T.M. Uncomplicated urinary tract infection.N. Engl.J. Med.2012, 366,1028–1037.[CrossRef]
[PubMed]
7. Lichtenberger, P.; Hooton, T.M. Complicated urinary tract infections. Curr. Infect. Dis. Rep.2008, 10, 499–504.
[CrossRef] [PubMed]
8. Levison, M.E.; Kaye, D. Treatment of complicated urinary tract infections with an emphasis on drug-resistant
gram-negative uropathogens. Curr. Infect. Dis. Rep. 2013, 15, 109–115. [CrossRef] [PubMed]
9. Lo, E.; Nicolle, L.E.; Coffin, S.E.; Gould, C.; Maragakis, L.L.; Meddings, J.; Pegues, D.A.; Pettis, A.M.; Saint, S.;
Yokoe, D.S. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals:2014
update. Infect. Control Hosp. Epidemiol. 2014, 35, 32–47. [CrossRef]
10. Foxman, B.; Brown, P. Epidemiology of urinary tract infections: Transmission and risk factors, incidence,
and costs. Infect. Dis. Clin. N. Am. 2003, 17, 227–241. [CrossRef]
11. Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med.
2002, 113, 5S–13S. [CrossRef]
12. Ikaheimo,R.; Siitonen,A.; Heiskanen,T.; Karkkainen,U.; Kuosmanen,P.;Lipponen,P.;Makela,P.H.
Recurrence of urinary tract infection in a primary care setting: Analysis of a 1-year follow-up of 179 women.
Clin. Infect. Dis. 1996, 22, 91–99. [CrossRef] [PubMed]
13. Al-Badr,A.; Al-Shaikh,G. RecurrentUrinary TractInfections Managementin Women: A review.
Sultan Qaboos Univ. Med. J. 2013, 13, 359–367. [CrossRef] [PubMed]
14. Griebling, T.L. Urinary tract infection in women. Urologic Diseases in Amerca; Litwin, M.S., Saigal, C.S., Eds
U.S. Government Printing Office: Washington, DC, USA, 2007; pp. 587–620.
15. Flores-Mireles,A.L.; Walker,J.N.; Caparon,M.; Hultgren,S.J.Urinary tract infections:epidemiology,
mechanims of infection and treatment options. Nat. Rev. Microbiol.2015, 13, 269–284. [CrossRef] [PubMed]
16. Chen, S.L.;Wu, M.;Henderson, J.P.;Hooton, T.M.;Hibbing, M.E.;Hultgren, S.J.;Gordon, J.I. Genomic
diversity and fitness of E.colistrains recovered from the intestinal and urinary tracts of women with
recurrent urinary tract infection. Sci. Transl. Med. 2013. [CrossRef] [PubMed]

Pathogens 2016, 5, 30 12 of 18
17. Moreno,E.; Andreu,A.; Pigrau,C.; Kuskowski,M.A.; Johnson,J.R.; Prats,G. Relationship between
Escherichia colistrains causing acute cystitis in women and the fecalE. colipopulation ofthe host.
J. Clin. Microbiol. 2008, 46, 2529–2534. [CrossRef] [PubMed]
18. Scholes, D.; Hooton, T.M.; Roberts, P.L.; Stapleton, A.E.; Gupta, K.; Stamm, W.E. Risk factors for recurrent
urinary tract infection in young women. J. Infect. Dis. 2000, 182, 1177–1182. [CrossRef] [PubMed]
19. Song, J.; Li, B.; Grady, R.; Stapleton, A.; Abraham, S. TLR4-mediated expulsion of bacteria from infected
bladder epithelial cells. Proc. Natl. Acad. Soc. USA 2009, 106, 14966–14971. [CrossRef] [PubMed]
20. Busch, A.; Waksman, G. Chaperone-usher pathways: Diversity and pilus assembly mechanism. Philos. Trans.
R. Soc. Lond. B Biol. Sci. 2012, 367, 1112–1122. [CrossRef] [PubMed]
21. Holmgren, A.; Brändén, C. Crystal structure of chaperone protein PapD reveals an immunoglobulin fold.
Nature 1989, 342, 248–251. [CrossRef] [PubMed]
22. Slonim, L.N.; Pinkner, J.S.; Branden, C.I.; Hultgren, S.J. Interactive surface in the PapD chaperone cleft is
conserved in pilus chaperone superfamily and essential in subunit recognition and assembly. EMBO1992,
11, 4747–4756.
23. Sauer, F.G.; Futterer, K.; Pinkner, J.S.; Dodson, K.W.; Hultgren, S.J.; Waksman, G. Structural basis of chaperone
function and pilus biogenesis. Science 1999, 285, 1058–1061. [CrossRef] [PubMed]
24. Barnhart, M.M.; Pinkner, J.S.; Soto, G.E.; Sauer, F.G.; Langermann, S.; Waksman, G.; Frieden, C.; Hultgren, S.J.
PapD-like chaperones provide the missing information for folding of pilin proteins.Proc.Natl. Acad.
Soc. USA 2000, 97, 7709–7714. [CrossRef] [PubMed]
25. Geibel, S.; Procko, E.; Hultgren, S.J.; Baker, D.; Waksman, G. Structural and energetic basis of folded-protein
transport by the FimD usher. Nature 2013, 496, 243–246. [CrossRef] [PubMed]
26. Phan, G.; Remaut, H.; Wang, T.; Allen, W.J.; Pirker, K.F.; Lebedev, A.; Henderson, N.S.; Geibel, S.; Volkan, E.;
Yan, J.; et al. Crystal structure of the FimD usher bound to its cognate FimC-FimH substrate. Nature2011,
474, 49–53. [CrossRef] [PubMed]
27. Remaut, H.; Tang, C.; Henderson, N.S.; Pinkner, J.S.; Wang, T.; Hultgren, S.J.; Thanassi, D.G.; Waksman, G.;
Li, H. Fiber formation across the bacterial outer membrane by the chaperone/usher pathway. Cell2008, 133,
640–652. [CrossRef] [PubMed]
28. Thanassi, D.G.; Stathopoulos, C.; Dodson, K.; Geiger, D.; Hultgren, S.J. Bacterial outer membrane ushers
contain distinct targeting and assembly domains for pilus biogenesis.Bacteriology2002, 184, 6260–6269.
[CrossRef]
29. Nishiyama, M.; Vetsch, M.; Puorger, C.; Jelesarov, I.; Glockshuber, R. Identification and characterization of
the chaperone-subunit complex-binding domain from the type 1 pilus assembly platform FimD. Mol. Biol.
2003, 330, 513–525. [CrossRef]
30. Volkan, E.; Kalas, V.; Pinkner, J.S.; Dodson, K.W.; Henderson, N.S.; Pham, T.; Waksman, G.; Delcour, A.H.;
Thanassi,D.G.; Hultgren,S.J.Molecular basis of usher pore gating in Escherichia colipilus biogenesis.
Proc. Natl. Acad. Soc. USA 2013, 110, 20741–20746. [CrossRef] [PubMed]
31. Sauer, F.G.;Pinkner, J.S.;Waksman, G.;Hultgren, S.J. Chaperone priming of pilus subunits facilitates a
topological transition that drives fiber formation. Cell 2002, 111, 543–551. [CrossRef]
32. Barnhart, M.M.; Sauer, F.G.; Pinkner, J.S.; Hultgren, S.J. Chaperone-subunit-usher interactions required for
donor strand exchange during bacterial pilus assembly. Bacteriology 2003, 1985, 2723–2730. [CrossRef]
33. Remaut, H.; Rose, R.J.; Hannan, T.J.; Hultgren, S.J.; Radford, S.E.; Ashcroft, A.E.; Waksman, G. Donor-strand
exchange in chaperone-assisted pilus assembly proceeds through a concerted beta strand displacement
mechanism. Mol. Cell 2006, 22, 831–842. [CrossRef] [PubMed]
34. Wu, X.R.; Sun, T.T.; Medina, J.J.In vitrobinding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib:
Relation to urinary tract infections. Proc. Natl. Acad. Soc. USA 1996, 93, 9630–9635. [CrossRef]
35. Sun, T.T.; Zhao, H.; Provet, J.; Aebi, U.; Wu, X.R. Formation of asymmetric unit membrane during urothelial
differentiation. Mol. Biol. Rep. 1996, 23, 3–11. [CrossRef] [PubMed]
36. Zhou,G.; Mo, W.J.;Sebbel,P.;Min,G.; Neubert,T.A.;Glockshuber,R.; Wu, X.R.;Sun,T.T.;Kong,X.P.
Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli:Evidence fromin vitroFimH
binding. J. Cell Sci. 2001, 114, 4095–4103. [PubMed]
17. Moreno,E.; Andreu,A.; Pigrau,C.; Kuskowski,M.A.; Johnson,J.R.; Prats,G. Relationship between
Escherichia colistrains causing acute cystitis in women and the fecalE. colipopulation ofthe host.
J. Clin. Microbiol. 2008, 46, 2529–2534. [CrossRef] [PubMed]
18. Scholes, D.; Hooton, T.M.; Roberts, P.L.; Stapleton, A.E.; Gupta, K.; Stamm, W.E. Risk factors for recurrent
urinary tract infection in young women. J. Infect. Dis. 2000, 182, 1177–1182. [CrossRef] [PubMed]
19. Song, J.; Li, B.; Grady, R.; Stapleton, A.; Abraham, S. TLR4-mediated expulsion of bacteria from infected
bladder epithelial cells. Proc. Natl. Acad. Soc. USA 2009, 106, 14966–14971. [CrossRef] [PubMed]
20. Busch, A.; Waksman, G. Chaperone-usher pathways: Diversity and pilus assembly mechanism. Philos. Trans.
R. Soc. Lond. B Biol. Sci. 2012, 367, 1112–1122. [CrossRef] [PubMed]
21. Holmgren, A.; Brändén, C. Crystal structure of chaperone protein PapD reveals an immunoglobulin fold.
Nature 1989, 342, 248–251. [CrossRef] [PubMed]
22. Slonim, L.N.; Pinkner, J.S.; Branden, C.I.; Hultgren, S.J. Interactive surface in the PapD chaperone cleft is
conserved in pilus chaperone superfamily and essential in subunit recognition and assembly. EMBO1992,
11, 4747–4756.
23. Sauer, F.G.; Futterer, K.; Pinkner, J.S.; Dodson, K.W.; Hultgren, S.J.; Waksman, G. Structural basis of chaperone
function and pilus biogenesis. Science 1999, 285, 1058–1061. [CrossRef] [PubMed]
24. Barnhart, M.M.; Pinkner, J.S.; Soto, G.E.; Sauer, F.G.; Langermann, S.; Waksman, G.; Frieden, C.; Hultgren, S.J.
PapD-like chaperones provide the missing information for folding of pilin proteins.Proc.Natl. Acad.
Soc. USA 2000, 97, 7709–7714. [CrossRef] [PubMed]
25. Geibel, S.; Procko, E.; Hultgren, S.J.; Baker, D.; Waksman, G. Structural and energetic basis of folded-protein
transport by the FimD usher. Nature 2013, 496, 243–246. [CrossRef] [PubMed]
26. Phan, G.; Remaut, H.; Wang, T.; Allen, W.J.; Pirker, K.F.; Lebedev, A.; Henderson, N.S.; Geibel, S.; Volkan, E.;
Yan, J.; et al. Crystal structure of the FimD usher bound to its cognate FimC-FimH substrate. Nature2011,
474, 49–53. [CrossRef] [PubMed]
27. Remaut, H.; Tang, C.; Henderson, N.S.; Pinkner, J.S.; Wang, T.; Hultgren, S.J.; Thanassi, D.G.; Waksman, G.;
Li, H. Fiber formation across the bacterial outer membrane by the chaperone/usher pathway. Cell2008, 133,
640–652. [CrossRef] [PubMed]
28. Thanassi, D.G.; Stathopoulos, C.; Dodson, K.; Geiger, D.; Hultgren, S.J. Bacterial outer membrane ushers
contain distinct targeting and assembly domains for pilus biogenesis.Bacteriology2002, 184, 6260–6269.
[CrossRef]
29. Nishiyama, M.; Vetsch, M.; Puorger, C.; Jelesarov, I.; Glockshuber, R. Identification and characterization of
the chaperone-subunit complex-binding domain from the type 1 pilus assembly platform FimD. Mol. Biol.
2003, 330, 513–525. [CrossRef]
30. Volkan, E.; Kalas, V.; Pinkner, J.S.; Dodson, K.W.; Henderson, N.S.; Pham, T.; Waksman, G.; Delcour, A.H.;
Thanassi,D.G.; Hultgren,S.J.Molecular basis of usher pore gating in Escherichia colipilus biogenesis.
Proc. Natl. Acad. Soc. USA 2013, 110, 20741–20746. [CrossRef] [PubMed]
31. Sauer, F.G.;Pinkner, J.S.;Waksman, G.;Hultgren, S.J. Chaperone priming of pilus subunits facilitates a
topological transition that drives fiber formation. Cell 2002, 111, 543–551. [CrossRef]
32. Barnhart, M.M.; Sauer, F.G.; Pinkner, J.S.; Hultgren, S.J. Chaperone-subunit-usher interactions required for
donor strand exchange during bacterial pilus assembly. Bacteriology 2003, 1985, 2723–2730. [CrossRef]
33. Remaut, H.; Rose, R.J.; Hannan, T.J.; Hultgren, S.J.; Radford, S.E.; Ashcroft, A.E.; Waksman, G. Donor-strand
exchange in chaperone-assisted pilus assembly proceeds through a concerted beta strand displacement
mechanism. Mol. Cell 2006, 22, 831–842. [CrossRef] [PubMed]
34. Wu, X.R.; Sun, T.T.; Medina, J.J.In vitrobinding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib:
Relation to urinary tract infections. Proc. Natl. Acad. Soc. USA 1996, 93, 9630–9635. [CrossRef]
35. Sun, T.T.; Zhao, H.; Provet, J.; Aebi, U.; Wu, X.R. Formation of asymmetric unit membrane during urothelial
differentiation. Mol. Biol. Rep. 1996, 23, 3–11. [CrossRef] [PubMed]
36. Zhou,G.; Mo, W.J.;Sebbel,P.;Min,G.; Neubert,T.A.;Glockshuber,R.; Wu, X.R.;Sun,T.T.;Kong,X.P.
Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli:Evidence fromin vitroFimH
binding. J. Cell Sci. 2001, 114, 4095–4103. [PubMed]
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 18
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





