What is a review article? | Assignment
Added on 2022-08-17
23 Pages22834 Words10 Views
REVIEW
published: 15 August 2017
doi: 10.3389/fmicb.2017.01566
Frontiers in Microbiology | www.frontiersin.org 1 August 2017 | Volume 8 | Article 1566
Edited by:
John W. A. Rossen,
University Medical Center Groningen,
Netherlands
Reviewed by:
Ariadnna Cruz-Córdova,
Hospital Infantil de México Federico
Gómez, Mexico
Mirjam Kooistra-Smid,
CERTE, Netherlands
*Correspondence:
Massimo E. Maffei
massimo.maffei@unito.it
Specialty section:
This article was submitted to
Infectious Diseases,
a section of the journal
Frontiers in Microbiology
Received: 15 May 2017
Accepted: 02 August 2017
Published: 15 August 2017
Citation:
Terlizzi ME, Gribaudo G and Maffei ME
(2017) UroPathogenic Escherichia coli
(UPEC) Infections: Virulence Factors,
Bladder Responses, Antibiotic, and
Non-antibiotic Antimicrobial
Strategies. Front. Microbiol. 8:1566.
doi: 10.3389/fmicb.2017.01566
UroPathogenic Escherichia coli
(UPEC) Infections: Virulence Factors,
Bladder Responses, Antibiotic, and
Non-antibiotic Antimicrobial
Strategies
Maria E. Terlizzi, Giorgio Gribaudo and Massimo E. Maffei *
Department of Life Sciences and Systems Biology, University of Turin, Torino, Italy
Urinary tract infections (UTIs) are one of the most common pathological conditions in both
community and hospital settings. It has been estimated that about 150 million people
worldwide develop UTI each year, with high social costs in terms of hospitalizations and
medical expenses. Among the common uropathogens associated to UTIs development,
UroPathogenic Escherichia coli (UPEC) is the primary cause. UPEC strains possess
a plethora of both structural (as fimbriae, pili, curli, flagella) and secreted (toxins,
iron-acquisition systems) virulence factors that contribute to their capacity to cause
disease, although the ability to adhere to host epithelial cells in the urinary tract represents
the most important determinant of pathogenicity. On the opposite side, the bladder
epithelium shows a multifaceted array of host defenses including the urine flow and
the secretion of antimicrobial substances, which represent useful tools to counteract
bacterial infections. The fascinating and intricate dynamics between these players
determine a complex interaction system that needs to be revealed. This review will focus
on the most relevant components of UPEC arsenal of pathogenicity together with the
major host responses to infection, the current approved treatment and the emergence
of resistant UPEC strains, the vaccine strategies, the natural antimicrobial compounds
along with innovative anti-adhesive and prophylactic approaches to prevent UTIs.
Keywords: urinary tract infections, uropathogenic Escherichia coli, bladder, antibiotics, non-antibiotic remedies
URINARY TRACT INFECTIONS (UTIs)
Urinary tract infections (UTIs) are widespread and affect a large proportion of the human
population. About 150 million people worldwide develop UTI each year, with high social costs
(Flores-Mireles et al., 2015). It is estimated that 40% of women develop at least one UTI during
their lifetime (Micali et al., 2014) and that 11% of women over 18 years have an episode of UTI per
year (Foxman and Brown, 2003; Foxman, 2014). With roughly eleven-million cases reported in the
sole U.S. each year, the costs are estimated $5 billion annually (Figure 1) (Foxman, 2014).
The UTI refers to the presence of a certain number of bacteria in the urine (generally > 105/ml)
and symptomatic UTIs are classified in order of severity as urosepsis syndrome, pyelonephritis (or
upper UTI, with infection in the kidney) and cystitis (or lower UTI, with bacteria into the bladder;
Foxman, 2014; Smelov et al., 2016). Clinically, UTIs classification comprises either uncomplicated
published: 15 August 2017
doi: 10.3389/fmicb.2017.01566
Frontiers in Microbiology | www.frontiersin.org 1 August 2017 | Volume 8 | Article 1566
Edited by:
John W. A. Rossen,
University Medical Center Groningen,
Netherlands
Reviewed by:
Ariadnna Cruz-Córdova,
Hospital Infantil de México Federico
Gómez, Mexico
Mirjam Kooistra-Smid,
CERTE, Netherlands
*Correspondence:
Massimo E. Maffei
massimo.maffei@unito.it
Specialty section:
This article was submitted to
Infectious Diseases,
a section of the journal
Frontiers in Microbiology
Received: 15 May 2017
Accepted: 02 August 2017
Published: 15 August 2017
Citation:
Terlizzi ME, Gribaudo G and Maffei ME
(2017) UroPathogenic Escherichia coli
(UPEC) Infections: Virulence Factors,
Bladder Responses, Antibiotic, and
Non-antibiotic Antimicrobial
Strategies. Front. Microbiol. 8:1566.
doi: 10.3389/fmicb.2017.01566
UroPathogenic Escherichia coli
(UPEC) Infections: Virulence Factors,
Bladder Responses, Antibiotic, and
Non-antibiotic Antimicrobial
Strategies
Maria E. Terlizzi, Giorgio Gribaudo and Massimo E. Maffei *
Department of Life Sciences and Systems Biology, University of Turin, Torino, Italy
Urinary tract infections (UTIs) are one of the most common pathological conditions in both
community and hospital settings. It has been estimated that about 150 million people
worldwide develop UTI each year, with high social costs in terms of hospitalizations and
medical expenses. Among the common uropathogens associated to UTIs development,
UroPathogenic Escherichia coli (UPEC) is the primary cause. UPEC strains possess
a plethora of both structural (as fimbriae, pili, curli, flagella) and secreted (toxins,
iron-acquisition systems) virulence factors that contribute to their capacity to cause
disease, although the ability to adhere to host epithelial cells in the urinary tract represents
the most important determinant of pathogenicity. On the opposite side, the bladder
epithelium shows a multifaceted array of host defenses including the urine flow and
the secretion of antimicrobial substances, which represent useful tools to counteract
bacterial infections. The fascinating and intricate dynamics between these players
determine a complex interaction system that needs to be revealed. This review will focus
on the most relevant components of UPEC arsenal of pathogenicity together with the
major host responses to infection, the current approved treatment and the emergence
of resistant UPEC strains, the vaccine strategies, the natural antimicrobial compounds
along with innovative anti-adhesive and prophylactic approaches to prevent UTIs.
Keywords: urinary tract infections, uropathogenic Escherichia coli, bladder, antibiotics, non-antibiotic remedies
URINARY TRACT INFECTIONS (UTIs)
Urinary tract infections (UTIs) are widespread and affect a large proportion of the human
population. About 150 million people worldwide develop UTI each year, with high social costs
(Flores-Mireles et al., 2015). It is estimated that 40% of women develop at least one UTI during
their lifetime (Micali et al., 2014) and that 11% of women over 18 years have an episode of UTI per
year (Foxman and Brown, 2003; Foxman, 2014). With roughly eleven-million cases reported in the
sole U.S. each year, the costs are estimated $5 billion annually (Figure 1) (Foxman, 2014).
The UTI refers to the presence of a certain number of bacteria in the urine (generally > 105/ml)
and symptomatic UTIs are classified in order of severity as urosepsis syndrome, pyelonephritis (or
upper UTI, with infection in the kidney) and cystitis (or lower UTI, with bacteria into the bladder;
Foxman, 2014; Smelov et al., 2016). Clinically, UTIs classification comprises either uncomplicated

Terlizzi et al. Uropathogenic Escherichia coli Infections
or complicated cases, depending on the presence of structural or
neurological urinary tract abnormalities (Zacché and Giarenis,
2016). The ORENUC system classifies the risk factors according
to the phenotype (Johansen et al., 2011): O, no known risk
factors; R, risk of recurrent UTIs without a more severe
outcome; E, extraurogenital risk factors; N, relevant nephropathic
diseases; U, urologic resolvable (transient) risk factors; C,
permanent external urinary catheter and unresolved urologic
risk factors (see also Smelov et al., 2016 for a modified
classification). The susceptibility to develop an UTI phenotype
is related to several factors, as dysfunctions of the urinary tract
and/or genetic mechanisms involved in the innate immune
response control to infections (Koves and Wullt, 2016). In
particular, the innate immune system may respond either
to UPEC patterns (pathogen-associated molecular patterns;
PAMPs) or to molecules derived from damaged or dying
cells (danger/damage-associated molecular patterns; DAMPs).
Pattern recognition receptors (PRRs) recognized these patterns
in specialized immune cells, epithelia, and other tissues (Purves
and Hughes, 2016). Assembling in the cytosol of multimeric
protein complexes (inflammasomes) occurs after sensing PAMPs
or DAMPs structures that can be formed in both upper and lower
urinary tract (Guo et al., 2015). They trigger innate immune
responses through mechanisms depending or not from the
production of proinflammatory cytokines (Purves and Hughes,
2016).
The bacterial cystitis (also called acute cystitis) can occur
in both women and men and some people develop recurrent
infections of the urinary tract (Fiore and Fox, 2014). Three
or more urinary tract infections within 12 months define the
recurring UTI, as well as two or more recurrences within
6 months. The same bacterial species that caused previous
infection is typically responsible for relapses. Approximately
20–30% of adult women with an initial UTI will experience a
recurrence within 3–4 months; whereas, in children, about one
third experiencing a UTI before the age of one, will experience
a recurrence within 3 years, and 18% of them will have a
recurrence within a few months (Nuutinen and Uhari, 2001).
However, these figures are understated; in fact, about 50% of
UTI does not come to medical attention. Recurrent UTIs can
be introduced from different sources and the same or different
UTI-causing strains in the gut are able to (re)inoculate the
bladder. Alternatively, bacteria residing in the bladder epithelium
are able to re-emerge periodically and cause UTI recurrence
(Silverman et al., 2013). In patients suffering from recurrent
UTIs, maintenance is ensured by antibiotic prophylaxis; however,
in some cases UTI needs to be treated by surgery (Tolg and Bagli,
2012). During pregnancy, recurrent UTIs may be frequent and
can cause severe adverse outcomes for the mother and the baby,
including preterm birth. The interventions in this setting can be
pharmacologic (antibiotics) or non-pharmacological (alternative
remedies; Schneeberger et al., 2012). In pre-menopausal women,
sexual activities three or more times a week, the use of
spermicides, new or multiple sexual partners and having suffered
from UTI before age 15 are the main risk factors in UTI
development and recurrence. In menopausal women, systemic
hormonal therapy is not an effective prevention and usually
asymptomatic bacteriuria during this period does not require
treatment (Milart et al., 2013). In women after menopause, the
risk increases mainly by low estrogen levels after-effects, which
are often associated to vaginal atrophy (Arnold et al., 2016).
In women over the age of 61–65 years, half have suffered of
genital-urinary symptoms while 29% had episodes of urinary
incontinence, all symptoms associated with bacteriuria (Raz,
2001).
UROPATHOGENIC ESCHERICHIA COLI
AND ITS VIRULENCE
UPEC is the main cause of community-acquired UTIs (about 80–
90%; Foxman, 2014; Flores-Mireles et al., 2015). Four main UPEC
phylogroups (A, B1, B2, and D) have been identified on the basis
of the occurrence of genomic Pathogenicity Islands (PAI) and the
expression of virulence factors, such as adhesins, toxins, surface
polysaccharides, flagella, and iron-acquisition systems (Bien
et al., 2012). Usually, many of these virulence factors are required
for UPEC to cause UTI (Hannan et al., 2012). However, besides
UPEC, UTI can be caused by Klebsiella pneumoniae (about
7%), Proteus mirabilis (about 5%), and Pseudomonas aeruginosa,
Enterococcus faecalis, Enterobacter cloacae, Streptococcus bovis,
and the fungus Candida albicans (for the remaining percentage;
Parish and Holliday, 2012; Palou et al., 2013; Hof, 2017). During
UTIs, UPEC pathogenesis includes: (a) UPEC colonization of the
periurethral and vaginal areas with colonization of the urethra;
(b) ascending into the bladder lumen and growth as plantktonic
cells in urine; (c) adherence to the surface and interaction with
the bladder epithelium defense system (see below); (d) biofilm
formation; (e) invasion and replication by forming bladder
Intracellular Bacterial Communities (IBCs) where quiescent
intracellular reservoirs (QIRs) form and reside in the underlying
urothelium; (f) kidney colonization and host tissue damage with
increased risk for bacteremia/septicemia.
Replication of bacteria in the IBC can easily reach as many as
105 bacteria per cell; furthermore, bacteria in the IBC undergo
morphological changes, flux out of the infected cell, and go
onto infect neighboring cells (Dhakal et al., 2008; Flores-Mireles
et al., 2015; Spaulding and Hultgren, 2016). The flushing of urine
removes most of the invading bacteria, along with UPEC-filled
exfoliated bladder epithelium cells (BECs; Kaper et al., 2004).
UPEC colonize the bladder using a variety of virulence
factors that therefore play critical roles in UTI pathogenesis.
These include surface structural components, such as
lipopolysaccharide (LPS), polysaccharide capsule, flagella,
outer-membrane vesicles, pili, curli, non-pilus adhesins, outer-
membrane proteins (OMPs), as well as secreted toxins, secretion
systems, and TonB-dependent iron-uptake receptors, including
siderophore receptors (Figure 2). All of these components are
attractive candidates for the development of new drugs and
vaccines (Klemm et al., 2010; Werneburg et al., 2015; O’Brien
et al., 2016).
LPS are molecules with amphipathic properties consisting of
fatty acids lined to an oligosaccharide core, which in turn is
bound to a long polysaccharide chain commonly called O antigen
Frontiers in Microbiology | www.frontiersin.org 2 August 2017 | Volume 8 | Article 1566
or complicated cases, depending on the presence of structural or
neurological urinary tract abnormalities (Zacché and Giarenis,
2016). The ORENUC system classifies the risk factors according
to the phenotype (Johansen et al., 2011): O, no known risk
factors; R, risk of recurrent UTIs without a more severe
outcome; E, extraurogenital risk factors; N, relevant nephropathic
diseases; U, urologic resolvable (transient) risk factors; C,
permanent external urinary catheter and unresolved urologic
risk factors (see also Smelov et al., 2016 for a modified
classification). The susceptibility to develop an UTI phenotype
is related to several factors, as dysfunctions of the urinary tract
and/or genetic mechanisms involved in the innate immune
response control to infections (Koves and Wullt, 2016). In
particular, the innate immune system may respond either
to UPEC patterns (pathogen-associated molecular patterns;
PAMPs) or to molecules derived from damaged or dying
cells (danger/damage-associated molecular patterns; DAMPs).
Pattern recognition receptors (PRRs) recognized these patterns
in specialized immune cells, epithelia, and other tissues (Purves
and Hughes, 2016). Assembling in the cytosol of multimeric
protein complexes (inflammasomes) occurs after sensing PAMPs
or DAMPs structures that can be formed in both upper and lower
urinary tract (Guo et al., 2015). They trigger innate immune
responses through mechanisms depending or not from the
production of proinflammatory cytokines (Purves and Hughes,
2016).
The bacterial cystitis (also called acute cystitis) can occur
in both women and men and some people develop recurrent
infections of the urinary tract (Fiore and Fox, 2014). Three
or more urinary tract infections within 12 months define the
recurring UTI, as well as two or more recurrences within
6 months. The same bacterial species that caused previous
infection is typically responsible for relapses. Approximately
20–30% of adult women with an initial UTI will experience a
recurrence within 3–4 months; whereas, in children, about one
third experiencing a UTI before the age of one, will experience
a recurrence within 3 years, and 18% of them will have a
recurrence within a few months (Nuutinen and Uhari, 2001).
However, these figures are understated; in fact, about 50% of
UTI does not come to medical attention. Recurrent UTIs can
be introduced from different sources and the same or different
UTI-causing strains in the gut are able to (re)inoculate the
bladder. Alternatively, bacteria residing in the bladder epithelium
are able to re-emerge periodically and cause UTI recurrence
(Silverman et al., 2013). In patients suffering from recurrent
UTIs, maintenance is ensured by antibiotic prophylaxis; however,
in some cases UTI needs to be treated by surgery (Tolg and Bagli,
2012). During pregnancy, recurrent UTIs may be frequent and
can cause severe adverse outcomes for the mother and the baby,
including preterm birth. The interventions in this setting can be
pharmacologic (antibiotics) or non-pharmacological (alternative
remedies; Schneeberger et al., 2012). In pre-menopausal women,
sexual activities three or more times a week, the use of
spermicides, new or multiple sexual partners and having suffered
from UTI before age 15 are the main risk factors in UTI
development and recurrence. In menopausal women, systemic
hormonal therapy is not an effective prevention and usually
asymptomatic bacteriuria during this period does not require
treatment (Milart et al., 2013). In women after menopause, the
risk increases mainly by low estrogen levels after-effects, which
are often associated to vaginal atrophy (Arnold et al., 2016).
In women over the age of 61–65 years, half have suffered of
genital-urinary symptoms while 29% had episodes of urinary
incontinence, all symptoms associated with bacteriuria (Raz,
2001).
UROPATHOGENIC ESCHERICHIA COLI
AND ITS VIRULENCE
UPEC is the main cause of community-acquired UTIs (about 80–
90%; Foxman, 2014; Flores-Mireles et al., 2015). Four main UPEC
phylogroups (A, B1, B2, and D) have been identified on the basis
of the occurrence of genomic Pathogenicity Islands (PAI) and the
expression of virulence factors, such as adhesins, toxins, surface
polysaccharides, flagella, and iron-acquisition systems (Bien
et al., 2012). Usually, many of these virulence factors are required
for UPEC to cause UTI (Hannan et al., 2012). However, besides
UPEC, UTI can be caused by Klebsiella pneumoniae (about
7%), Proteus mirabilis (about 5%), and Pseudomonas aeruginosa,
Enterococcus faecalis, Enterobacter cloacae, Streptococcus bovis,
and the fungus Candida albicans (for the remaining percentage;
Parish and Holliday, 2012; Palou et al., 2013; Hof, 2017). During
UTIs, UPEC pathogenesis includes: (a) UPEC colonization of the
periurethral and vaginal areas with colonization of the urethra;
(b) ascending into the bladder lumen and growth as plantktonic
cells in urine; (c) adherence to the surface and interaction with
the bladder epithelium defense system (see below); (d) biofilm
formation; (e) invasion and replication by forming bladder
Intracellular Bacterial Communities (IBCs) where quiescent
intracellular reservoirs (QIRs) form and reside in the underlying
urothelium; (f) kidney colonization and host tissue damage with
increased risk for bacteremia/septicemia.
Replication of bacteria in the IBC can easily reach as many as
105 bacteria per cell; furthermore, bacteria in the IBC undergo
morphological changes, flux out of the infected cell, and go
onto infect neighboring cells (Dhakal et al., 2008; Flores-Mireles
et al., 2015; Spaulding and Hultgren, 2016). The flushing of urine
removes most of the invading bacteria, along with UPEC-filled
exfoliated bladder epithelium cells (BECs; Kaper et al., 2004).
UPEC colonize the bladder using a variety of virulence
factors that therefore play critical roles in UTI pathogenesis.
These include surface structural components, such as
lipopolysaccharide (LPS), polysaccharide capsule, flagella,
outer-membrane vesicles, pili, curli, non-pilus adhesins, outer-
membrane proteins (OMPs), as well as secreted toxins, secretion
systems, and TonB-dependent iron-uptake receptors, including
siderophore receptors (Figure 2). All of these components are
attractive candidates for the development of new drugs and
vaccines (Klemm et al., 2010; Werneburg et al., 2015; O’Brien
et al., 2016).
LPS are molecules with amphipathic properties consisting of
fatty acids lined to an oligosaccharide core, which in turn is
bound to a long polysaccharide chain commonly called O antigen
Frontiers in Microbiology | www.frontiersin.org 2 August 2017 | Volume 8 | Article 1566

Terlizzi et al. Uropathogenic Escherichia coli Infections
FIGURE 1 | The urinary tract and sites of infection.
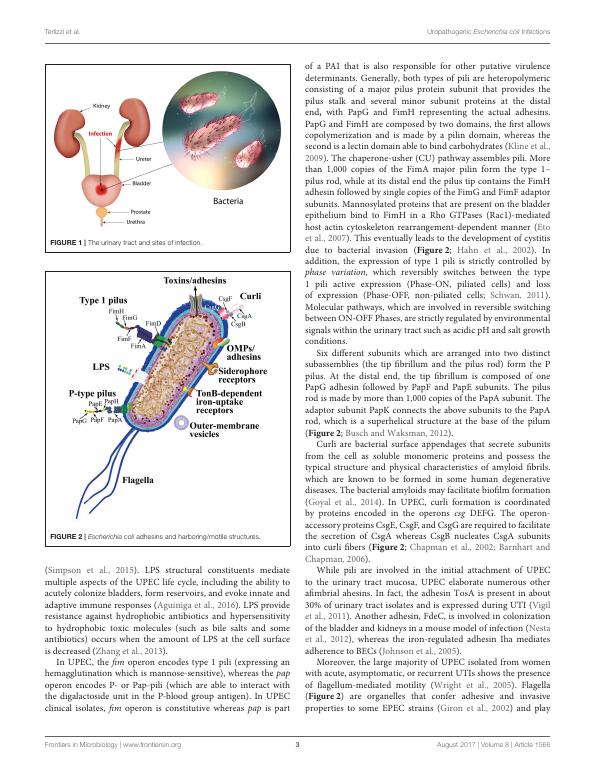
FIGURE 2 | Escherichia coli adhesins and harboring/motile structures.
(Simpson et al., 2015). LPS structural constituents mediate
multiple aspects of the UPEC life cycle, including the ability to
acutely colonize bladders, form reservoirs, and evoke innate and
adaptive immune responses (Aguiniga et al., 2016). LPS provide
resistance against hydrophobic antibiotics and hypersensitivity
to hydrophobic toxic molecules (such as bile salts and some
antibiotics) occurs when the amount of LPS at the cell surface
is decreased (Zhang et al., 2013).
In UPEC, the fim operon encodes type 1 pili (expressing an
hemagglutination which is mannose-sensitive), whereas the pap
operon encodes P- or Pap-pili (which are able to interact with
the digalactoside unit in the P-blood group antigen). In UPEC
clinical isolates, fim operon is constitutive whereas pap is part
of a PAI that is also responsible for other putative virulence
determinants. Generally, both types of pili are heteropolymeric
consisting of a major pilus protein subunit that provides the
pilus stalk and several minor subunit proteins at the distal
end, with PapG and FimH representing the actual adhesins.
PapG and FimH are composed by two domains, the first allows
copolymerization and is made by a pilin domain, whereas the
second is a lectin domain able to bind carbohydrates (Kline et al.,
2009). The chaperone-usher (CU) pathway assembles pili. More
than 1,000 copies of the FimA major pilin form the type 1–
pilus rod, while at its distal end the pilus tip contains the FimH
adhesin followed by single copies of the FimG and FimF adaptor
subunits. Mannosylated proteins that are present on the bladder
epithelium bind to FimH in a Rho GTPases (Rac1)-mediated
host actin cytoskeleton rearrangement-dependent manner (Eto
et al., 2007). This eventually leads to the development of cystitis
due to bacterial invasion (Figure 2; Hahn et al., 2002). In
addition, the expression of type 1 pili is strictly controlled by
phase variation, which reversibly switches between the type
1 pili active expression (Phase-ON, piliated cells) and loss
of expression (Phase-OFF, non-piliated cells; Schwan, 2011).
Molecular pathways, which are involved in reversible switching
between ON-OFF Phases, are strictly regulated by environmental
signals within the urinary tract such as acidic pH and salt growth
conditions.
Six different subunits which are arranged into two distinct
subassemblies (the tip fibrillum and the pilus rod) form the P
pilus. At the distal end, the tip fibrillum is composed of one
PapG adhesin followed by PapF and PapE subunits. The pilus
rod is made by more than 1,000 copies of the PapA subunit. The
adaptor subunit PapK connects the above subunits to the PapA
rod, which is a superhelical structure at the base of the pilum
(Figure 2; Busch and Waksman, 2012).
Curli are bacterial surface appendages that secrete subunits
from the cell as soluble monomeric proteins and possess the
typical structure and physical characteristics of amyloid fibrils.
which are known to be formed in some human degenerative
diseases. The bacterial amyloids may facilitate biofilm formation
(Goyal et al., 2014). In UPEC, curli formation is coordinated
by proteins encoded in the operons csg DEFG. The operon-
accessory proteins CsgE, CsgF, and CsgG are required to facilitate
the secretion of CsgA whereas CsgB nucleates CsgA subunits
into curli fibers (Figure 2; Chapman et al., 2002; Barnhart and
Chapman, 2006).
While pili are involved in the initial attachment of UPEC
to the urinary tract mucosa, UPEC elaborate numerous other
afimbrial ahesins. In fact, the adhesin TosA is present in about
30% of urinary tract isolates and is expressed during UTI (Vigil
et al., 2011). Another adhesin, FdeC, is involved in colonization
of the bladder and kidneys in a mouse model of infection (Nesta
et al., 2012), whereas the iron-regulated adhesin Iha mediates
adherence to BECs (Johnson et al., 2005).
Moreover, the large majority of UPEC isolated from women
with acute, asymptomatic, or recurrent UTIs shows the presence
of flagellum-mediated motility (Wright et al., 2005). Flagella
(Figure 2) are organelles that confer adhesive and invasive
properties to some EPEC strains (Giron et al., 2002) and play
Frontiers in Microbiology | www.frontiersin.org 3 August 2017 | Volume 8 | Article 1566
FIGURE 1 | The urinary tract and sites of infection.
FIGURE 2 | Escherichia coli adhesins and harboring/motile structures.
(Simpson et al., 2015). LPS structural constituents mediate
multiple aspects of the UPEC life cycle, including the ability to
acutely colonize bladders, form reservoirs, and evoke innate and
adaptive immune responses (Aguiniga et al., 2016). LPS provide
resistance against hydrophobic antibiotics and hypersensitivity
to hydrophobic toxic molecules (such as bile salts and some
antibiotics) occurs when the amount of LPS at the cell surface
is decreased (Zhang et al., 2013).
In UPEC, the fim operon encodes type 1 pili (expressing an
hemagglutination which is mannose-sensitive), whereas the pap
operon encodes P- or Pap-pili (which are able to interact with
the digalactoside unit in the P-blood group antigen). In UPEC
clinical isolates, fim operon is constitutive whereas pap is part
of a PAI that is also responsible for other putative virulence
determinants. Generally, both types of pili are heteropolymeric
consisting of a major pilus protein subunit that provides the
pilus stalk and several minor subunit proteins at the distal
end, with PapG and FimH representing the actual adhesins.
PapG and FimH are composed by two domains, the first allows
copolymerization and is made by a pilin domain, whereas the
second is a lectin domain able to bind carbohydrates (Kline et al.,
2009). The chaperone-usher (CU) pathway assembles pili. More
than 1,000 copies of the FimA major pilin form the type 1–
pilus rod, while at its distal end the pilus tip contains the FimH
adhesin followed by single copies of the FimG and FimF adaptor
subunits. Mannosylated proteins that are present on the bladder
epithelium bind to FimH in a Rho GTPases (Rac1)-mediated
host actin cytoskeleton rearrangement-dependent manner (Eto
et al., 2007). This eventually leads to the development of cystitis
due to bacterial invasion (Figure 2; Hahn et al., 2002). In
addition, the expression of type 1 pili is strictly controlled by
phase variation, which reversibly switches between the type
1 pili active expression (Phase-ON, piliated cells) and loss
of expression (Phase-OFF, non-piliated cells; Schwan, 2011).
Molecular pathways, which are involved in reversible switching
between ON-OFF Phases, are strictly regulated by environmental
signals within the urinary tract such as acidic pH and salt growth
conditions.
Six different subunits which are arranged into two distinct
subassemblies (the tip fibrillum and the pilus rod) form the P
pilus. At the distal end, the tip fibrillum is composed of one
PapG adhesin followed by PapF and PapE subunits. The pilus
rod is made by more than 1,000 copies of the PapA subunit. The
adaptor subunit PapK connects the above subunits to the PapA
rod, which is a superhelical structure at the base of the pilum
(Figure 2; Busch and Waksman, 2012).
Curli are bacterial surface appendages that secrete subunits
from the cell as soluble monomeric proteins and possess the
typical structure and physical characteristics of amyloid fibrils.
which are known to be formed in some human degenerative
diseases. The bacterial amyloids may facilitate biofilm formation
(Goyal et al., 2014). In UPEC, curli formation is coordinated
by proteins encoded in the operons csg DEFG. The operon-
accessory proteins CsgE, CsgF, and CsgG are required to facilitate
the secretion of CsgA whereas CsgB nucleates CsgA subunits
into curli fibers (Figure 2; Chapman et al., 2002; Barnhart and
Chapman, 2006).
While pili are involved in the initial attachment of UPEC
to the urinary tract mucosa, UPEC elaborate numerous other
afimbrial ahesins. In fact, the adhesin TosA is present in about
30% of urinary tract isolates and is expressed during UTI (Vigil
et al., 2011). Another adhesin, FdeC, is involved in colonization
of the bladder and kidneys in a mouse model of infection (Nesta
et al., 2012), whereas the iron-regulated adhesin Iha mediates
adherence to BECs (Johnson et al., 2005).
Moreover, the large majority of UPEC isolated from women
with acute, asymptomatic, or recurrent UTIs shows the presence
of flagellum-mediated motility (Wright et al., 2005). Flagella
(Figure 2) are organelles that confer adhesive and invasive
properties to some EPEC strains (Giron et al., 2002) and play
Frontiers in Microbiology | www.frontiersin.org 3 August 2017 | Volume 8 | Article 1566
Terlizzi et al. Uropathogenic Escherichia coli Infections
a key role in the dynamic of biofilms (Pratt and Kolter, 1998).
It was recently reported that during biofilm formation, flagella
play different roles such as adherence, maturation, and dispersal
as shown by gene expression and regulation during the growth
phase (Nakamura et al., 2016).
On the other hand, UPEC toxins play different pathogenetic
roles during infection. The α-hemolysin is in fact associated with
renal damage and scarring, induces Ca2+ oscillations in renal
tubular epithelial cells, thereby potentially enhancing ascension
and colonization of ureters and kidney parenchyma by disrupting
the normal flow of urine. Recently (Nagamatsu et al., 2015),
α-hemolysin was found to induce proinflammatory Caspase-
1/Caspase-4-dependent cell death in bladder epithelial cells,
resulting in cell exfoliation (see below).
UPEC toxins, adhesins, enzymes, and non-protein antigens
like LPS are not released as soluble molecules; rather, they are
associated with outer-membrane vesicles, which bud off the
surface of Gram-negative bacteria during all stages of growth
(Figure 2; Ellis and Kuehn, 2010). The formation of membrane
vesicles is considered a “smart” way to protect bacterial toxins
and an efficient system to deliver them into host cell (Wiles et al.,
2008).
Iron acquisition is a critical requirement for UPEC survival
in an environment that is iron-limited as the urinary tract
(Skaar, 2010). Thus, is not suprising that IBC UPEC show
upregulation of redundant systems for the acquisition of iron
(Reigstad et al., 2007). In this regard, siderophores are small-
molecule iron chelators that are produced by UPEC strains to
scavenge ferric iron (Fe3+), thus UPEC express yersiniabactin,
salmochelin, and aerobactin. Siderophore receptors require the
TonB cytoplasmic membrane-localized complex, a high-affinity
iron acquisition system that allows binding and chelation of iron
at the cell surface to promote its uptake (O’Brien et al., 2016).
However, uroepithelial cells, to prevent bacterial iron
scavenging, upregulate genes for the transferrin receptor and for
lipocalin 2.
Lastly, further UPEC factors associated with colonization have
been linked to the regulation of metabolic pathways mediated by
two-component signaling systems (TCSs). TCSs are main signal
transduction pathways by which bacteria sense and respond
to a wide array of environmental stimuli, including quorum
sensing signals, nutrients, antibiotics. TCSs are composed
by a membrane-bound sensor histidine kinase (HK) and a
cytoplasmic response regulator (RR) that functions by regulating
gene expression (Stock et al., 2000). Among UPEC-associated
TCSs involved in UTI pathogenesis, the BarA/UvrY system has
been described to regulate switching between glycolytic and
gluconeogenic pathways (Tomenius et al., 2006) the EvgS/EvgA
and PhoQ/PhoP systems have been involved in acid resistance
(Eguchi et al., 2011), while the function of KguS/KguR is in the
control of the utilization of α-ketoglutarate. In this way they
facilate the adaptation of UPEC in the urinary tract (Cai et al.,
2013).
The importance of the above described UPEC virulence
factors in UTI pathogenesis has been further supported, in recent
years, by the application of multiple “omics” technologies aimed
at investigating the UPEC genomic diversity, the global gene
expression in different models of infection both in vitro and in
vivo, and to define the occurrence of UPEC-specific proteins
as new candidate therapeutic and vaccine targets (as recently
reviewed by Lo et al., 2017).
Next-generation sequencing (NGS) technologies are
providing rapid low-cost determination of UPEC genomes
useful to monitor outbreaks, epidemiology of emerging strains,
as well as evolution of resistance (Petty et al., 2014; Stoesser
et al., 2016). On the other hand, analysis of different UPEC
genomes and the comparison with the E. coli genomic database
revealed the plasticity of UPEC pan genome, and the presence of
UPEC-specific PAIs genes predicted to encode putative virulence
factors, such as pilus proteins, adhesins, and iron-uptake systems
(Moriel et al., 2016).
Transcriptomics investigations by both microarrays and NGS-
based RNA sequencing (RNA-seq), on the other hand, has led to
the identification of virulence and fitness UPEC genes, expressed
during different in vitro and in vivo infection-relevant conditions.
In this regard, RNA-seq-based transcriptome analysis of mouse
macrophages infected in vitro with two UPEC strains, allowed to
identify strain-specific differentially expressed genes associated
to the survival in macrophages, such as those involved in the
responses to oxidative stress, as well as those involved in the
initial adhesion of UPEC to cells, such as multiple flagella genes
(Mavromatis et al., 2015). Moreover, the global gene expression
of different UPEC strains has been investigated by RNA-seq of
urine samples collected from UTI patients. These transcriptomics
studies defined the global transcription profile for UPEC during
UTI, highlighted the high genomic diversity of different UPEC
strains, and confirmed, on a global scale, the expression during
UTI of several genes encoding virulence factors. In fact, it has
been observed the transcription of genes associated with the
UPEC’s adhesion to the uroepithelium (type 1 and P pili),
of genes involved in iron uptake (enterobactin, aerobactin,
yersiniabactin, and salmochelin), of genes encoding toxins
(hemolysins nad cytotoxic factors), as well as those involved in
copper efflux (Bielecki et al., 2014; Subashchandrabose et al.,
2014).
High-resolution liquid chromatograph-mass
spectrometry/mass spectrometry (LC-MS/MS)-based technology
has been applied to identify and characterize the surface
proteome of UPEC isolates and of strains grown in human
urine (Wurpel et al., 2015, 2016). These studies identified several
expressed proteins highly conserved among different strains,
thus representing the core surface proteome of UPEC. UPEC
core surface proteins, such as integral Outer Membrane (OM)
proteins (e.g., OmpA, OmpC, OmpF) and several iron-uptake
proteins, were in fact detected in more than 80% of strains
(Wurpel et al., 2015). Clearly, characterization of those UPEC
surface proteins that are conserved among different strains
and immunogenic is an essential step for identifying potential
vaccine candidates and new therapeutic targets (Cash, 2014).
Moreover, new insights into spatial changes in the UPEC
proteome under experimental conditions mimicking bacterial
growth in the urinary tract, have been provided by MALDI TOF
IMS-based proteome profiling of differentially expressed proteins
within UPEC biofilms. The application of this technique, that
Frontiers in Microbiology | www.frontiersin.org 4 August 2017 | Volume 8 | Article 1566
a key role in the dynamic of biofilms (Pratt and Kolter, 1998).
It was recently reported that during biofilm formation, flagella
play different roles such as adherence, maturation, and dispersal
as shown by gene expression and regulation during the growth
phase (Nakamura et al., 2016).
On the other hand, UPEC toxins play different pathogenetic
roles during infection. The α-hemolysin is in fact associated with
renal damage and scarring, induces Ca2+ oscillations in renal
tubular epithelial cells, thereby potentially enhancing ascension
and colonization of ureters and kidney parenchyma by disrupting
the normal flow of urine. Recently (Nagamatsu et al., 2015),
α-hemolysin was found to induce proinflammatory Caspase-
1/Caspase-4-dependent cell death in bladder epithelial cells,
resulting in cell exfoliation (see below).
UPEC toxins, adhesins, enzymes, and non-protein antigens
like LPS are not released as soluble molecules; rather, they are
associated with outer-membrane vesicles, which bud off the
surface of Gram-negative bacteria during all stages of growth
(Figure 2; Ellis and Kuehn, 2010). The formation of membrane
vesicles is considered a “smart” way to protect bacterial toxins
and an efficient system to deliver them into host cell (Wiles et al.,
2008).
Iron acquisition is a critical requirement for UPEC survival
in an environment that is iron-limited as the urinary tract
(Skaar, 2010). Thus, is not suprising that IBC UPEC show
upregulation of redundant systems for the acquisition of iron
(Reigstad et al., 2007). In this regard, siderophores are small-
molecule iron chelators that are produced by UPEC strains to
scavenge ferric iron (Fe3+), thus UPEC express yersiniabactin,
salmochelin, and aerobactin. Siderophore receptors require the
TonB cytoplasmic membrane-localized complex, a high-affinity
iron acquisition system that allows binding and chelation of iron
at the cell surface to promote its uptake (O’Brien et al., 2016).
However, uroepithelial cells, to prevent bacterial iron
scavenging, upregulate genes for the transferrin receptor and for
lipocalin 2.
Lastly, further UPEC factors associated with colonization have
been linked to the regulation of metabolic pathways mediated by
two-component signaling systems (TCSs). TCSs are main signal
transduction pathways by which bacteria sense and respond
to a wide array of environmental stimuli, including quorum
sensing signals, nutrients, antibiotics. TCSs are composed
by a membrane-bound sensor histidine kinase (HK) and a
cytoplasmic response regulator (RR) that functions by regulating
gene expression (Stock et al., 2000). Among UPEC-associated
TCSs involved in UTI pathogenesis, the BarA/UvrY system has
been described to regulate switching between glycolytic and
gluconeogenic pathways (Tomenius et al., 2006) the EvgS/EvgA
and PhoQ/PhoP systems have been involved in acid resistance
(Eguchi et al., 2011), while the function of KguS/KguR is in the
control of the utilization of α-ketoglutarate. In this way they
facilate the adaptation of UPEC in the urinary tract (Cai et al.,
2013).
The importance of the above described UPEC virulence
factors in UTI pathogenesis has been further supported, in recent
years, by the application of multiple “omics” technologies aimed
at investigating the UPEC genomic diversity, the global gene
expression in different models of infection both in vitro and in
vivo, and to define the occurrence of UPEC-specific proteins
as new candidate therapeutic and vaccine targets (as recently
reviewed by Lo et al., 2017).
Next-generation sequencing (NGS) technologies are
providing rapid low-cost determination of UPEC genomes
useful to monitor outbreaks, epidemiology of emerging strains,
as well as evolution of resistance (Petty et al., 2014; Stoesser
et al., 2016). On the other hand, analysis of different UPEC
genomes and the comparison with the E. coli genomic database
revealed the plasticity of UPEC pan genome, and the presence of
UPEC-specific PAIs genes predicted to encode putative virulence
factors, such as pilus proteins, adhesins, and iron-uptake systems
(Moriel et al., 2016).
Transcriptomics investigations by both microarrays and NGS-
based RNA sequencing (RNA-seq), on the other hand, has led to
the identification of virulence and fitness UPEC genes, expressed
during different in vitro and in vivo infection-relevant conditions.
In this regard, RNA-seq-based transcriptome analysis of mouse
macrophages infected in vitro with two UPEC strains, allowed to
identify strain-specific differentially expressed genes associated
to the survival in macrophages, such as those involved in the
responses to oxidative stress, as well as those involved in the
initial adhesion of UPEC to cells, such as multiple flagella genes
(Mavromatis et al., 2015). Moreover, the global gene expression
of different UPEC strains has been investigated by RNA-seq of
urine samples collected from UTI patients. These transcriptomics
studies defined the global transcription profile for UPEC during
UTI, highlighted the high genomic diversity of different UPEC
strains, and confirmed, on a global scale, the expression during
UTI of several genes encoding virulence factors. In fact, it has
been observed the transcription of genes associated with the
UPEC’s adhesion to the uroepithelium (type 1 and P pili),
of genes involved in iron uptake (enterobactin, aerobactin,
yersiniabactin, and salmochelin), of genes encoding toxins
(hemolysins nad cytotoxic factors), as well as those involved in
copper efflux (Bielecki et al., 2014; Subashchandrabose et al.,
2014).
High-resolution liquid chromatograph-mass
spectrometry/mass spectrometry (LC-MS/MS)-based technology
has been applied to identify and characterize the surface
proteome of UPEC isolates and of strains grown in human
urine (Wurpel et al., 2015, 2016). These studies identified several
expressed proteins highly conserved among different strains,
thus representing the core surface proteome of UPEC. UPEC
core surface proteins, such as integral Outer Membrane (OM)
proteins (e.g., OmpA, OmpC, OmpF) and several iron-uptake
proteins, were in fact detected in more than 80% of strains
(Wurpel et al., 2015). Clearly, characterization of those UPEC
surface proteins that are conserved among different strains
and immunogenic is an essential step for identifying potential
vaccine candidates and new therapeutic targets (Cash, 2014).
Moreover, new insights into spatial changes in the UPEC
proteome under experimental conditions mimicking bacterial
growth in the urinary tract, have been provided by MALDI TOF
IMS-based proteome profiling of differentially expressed proteins
within UPEC biofilms. The application of this technique, that
Frontiers in Microbiology | www.frontiersin.org 4 August 2017 | Volume 8 | Article 1566

Terlizzi et al. Uropathogenic Escherichia coli Infections
allows for in situ two-dimensional assessment of protein spatial
distribution and abundance, revealed the occurrence of different
bacterial subpopulations within biofilms: a type-1 pili-expressing
cells localized at the air-exposed region and a curli-equipped
population localized to the underlying air-liquid interface (Floyd
et al., 2015).
Together, all the above mentioned “omics” approaches have
allowed a great deal of new information to be available and
that is enabling a more comprehensive understanding of UPEC’s
pathogenic mechanisms.
THE BLADDER EPITHELIUM SHOWS
SELF-DEFENSE MECHANISMS AGAINST
INVADING BACTERIA
The most commonly targeted site of UTIs is the bladder. The
bladder epithelium possesses powerful barriers and the BECs
show antibacterial activities. Despite their properties, BECs and
the bladder epithelium are often circumvented by UPEC (Wu
et al., 2017). As discussed, the progressive ascending colonization
of bacteria contaminates the urethra and the origin of this
infection is usually from the gut (Kaper et al., 2004). Owing to the
presence of urine, that represents an ideal growth broth, bacteria
proliferate in a relatively short time lapse, while the flushing of
urine during urination removes most of the invading bacteria.
However, bacterial strains are able of binding tightly to BECs
lining the bladder using fimbrial organelles (Duncan et al., 2004;
Chahales and Thanassi, 2015).
The multilayered bladder epithelium is also known as
“transitional epithelium” and it is composed by three layers:
basal cell layer (5–10 μm in diameter), intermediate cell layer
(20 μm in diameter), and superficial apical layer with large
hexagonal cells (diameters of 25–250 μm), which are also termed
“umbrella cells.” A basement membrane lies underneath the basal
epithelium (Figures 3A,F). The umbrella cells play a prominent
role in maintaining a barrier against most substances found in
urine, and show a number of properties, including specialized
membrane lipids, asymmetric unit membrane particles, and a
plasmalemma with stiff plaques. These plaques may cover up
to 90% of the urothelial cell surface, with each plaque being
composed of nearly 1,000 subunits. These subunits are made by
proteins (uroplakins, UPs), which serve as the major receptors for
UPEC adherence to the host cell and are localized within plaques
on the apical membranes of the mature umbrella cells (Veranic
et al., 2004). There is a correlation between the glycosylation
changes in UPs and the different pathological conditions of
the urothelium such UTI and interstitial cystitis (Birder, 2005;
Katnik-Prastowska et al., 2014; Habuka et al., 2015).
The fusiform vesicles (FVs) are unique cytoplasmic organelles
contained in the umbrella cells. FVs deliver preassembled
crystalline arrays of UP proteins to the apical cell surface
of urothelial umbrella cells. Different Rab GTPases function
as regulators of specific steps in membrane traffic pathways
and are localized to the cytosolic face of specific intracellular
membranes. Rab27b, is a small GTPase regulating intracellular
vesicle movement which is expressed at an extraordinary high
level (0.1% of total protein) in urothelium. The Rab27b+ FVs are
involved in the storage of extra membrane which are necessary
when urine accumulates and causes bladder expansion (Wankel
et al., 2016). In order to enter epithelial cells, UPEC coopt the
superficial epithelial cells by expoiting their bladder volume-
regulating properties by stimulating the exocytosis of fusiform
vesicles right where the bacterial attach. The adherent bacteria
are then internalized when these membranes are subsequently
retracted into cells (Figure 3B; Wu et al., 2017). UPEC have
been found to reside within Rab27b/CD63/Caveolin-1-positive
fusiform vesicles (O’Brien et al., 2016). Internalized UPEC
become encased in Rab27b+ fusiform vesicles within the
cytosol of the superficial epithelium (Figure 3B; Bishop et al.,
2007). Replication of internalized UPEC bacteria rapidly occurs,
resulting in the maturation of IBCs, a structure that possesses
biofilm-like properties which is protected from innate defenses
and antibiotics (Justice et al., 2006; Goller and Seed, 2010). Fusion
with lysosomes is thus impaired, because internalized bacteria are
mostly encased in Rab27b+ compartments.
Defense mechanisms of bladder epithelial cells against
intrusion of bacterial include receptors such as toll-like receptors
(e.g., TLR2, TLR4, TLR5, and TLR11) that are able to promptly
recognize intruding bacteria (Larue et al., 2013). After UPEC
encapsulation within RAB27b+ vesicles in BECs, intracellular
UPEC are recognized by TLR4 which increases intracellular
cyclic AMP (cAMP) levels (Figure 3B). This triggers the
exocytosis of RAB27b+ vesicles harboring UPEC and the
intracellular bacterial expulsion back into the bladder lumen
(Figure 3C).
However, some UPEC break the RAB27b+ vacuole and
cannot be expelled into the urine; thus, these bacteria are
targeted by autophagy and delivered into the lysosomes, where
they actively neutralize the pH by reducing their acidicity
and degradative potential (Abraham and Miao, 2015). These
malfunctioning lysosomes are sensed by a lysosomal transient
receptor potential mucolipin 3 Ca2+ channel (TRPML3), which
is localized on the membrane of lysosomes (Miao et al., 2015).
The activation of this Ca2+channel rapidly fluxes out into
the cytosol the Ca2+ stored in the lysosome, which induces
the spontaneous expulsion into the extracellular space of the
defective lysosomes and its contents (Figure 3D).
Pathogen sensing by TLR4 induces the production of
various soluble factors which are secreted by BECs, including
antimicrobial peptides (AMP, such as cathelicidin and β-defensin
1; Sun et al., 2013; Chromek, 2015), antimicrobial proteins [such
as pentraxin 3 (PTX3); (Uzun et al., 2016)] and chemokines [such
as CXC-chemokine ligand 1 (CXCL1) and CC-chemokine ligand
5 (CCR5); Schiwon et al., 2014; Figure 3E]. Attachment to the
urothelium or bacterial lysis are inhibited by these antimicrobial
peptides, which are also induced when bacteria succeed to attach
to the urothelium (Spencer et al., 2014). Moreover, excretion
in the urine of uromodulin, a major high mannose-containing
glycoprotein, exerts a protective effects against UTI by competing
with the binding of UPEC FimH to uroplakin Ia (Pak et al., 2001).
When all these export mechanisms fail to clear the urothelium
from the invading UPEC, BECs activate the last line of defense.
Acute infections are commonly associated with of the exfoliation
Frontiers in Microbiology | www.frontiersin.org 5 August 2017 | Volume 8 | Article 1566
allows for in situ two-dimensional assessment of protein spatial
distribution and abundance, revealed the occurrence of different
bacterial subpopulations within biofilms: a type-1 pili-expressing
cells localized at the air-exposed region and a curli-equipped
population localized to the underlying air-liquid interface (Floyd
et al., 2015).
Together, all the above mentioned “omics” approaches have
allowed a great deal of new information to be available and
that is enabling a more comprehensive understanding of UPEC’s
pathogenic mechanisms.
THE BLADDER EPITHELIUM SHOWS
SELF-DEFENSE MECHANISMS AGAINST
INVADING BACTERIA
The most commonly targeted site of UTIs is the bladder. The
bladder epithelium possesses powerful barriers and the BECs
show antibacterial activities. Despite their properties, BECs and
the bladder epithelium are often circumvented by UPEC (Wu
et al., 2017). As discussed, the progressive ascending colonization
of bacteria contaminates the urethra and the origin of this
infection is usually from the gut (Kaper et al., 2004). Owing to the
presence of urine, that represents an ideal growth broth, bacteria
proliferate in a relatively short time lapse, while the flushing of
urine during urination removes most of the invading bacteria.
However, bacterial strains are able of binding tightly to BECs
lining the bladder using fimbrial organelles (Duncan et al., 2004;
Chahales and Thanassi, 2015).
The multilayered bladder epithelium is also known as
“transitional epithelium” and it is composed by three layers:
basal cell layer (5–10 μm in diameter), intermediate cell layer
(20 μm in diameter), and superficial apical layer with large
hexagonal cells (diameters of 25–250 μm), which are also termed
“umbrella cells.” A basement membrane lies underneath the basal
epithelium (Figures 3A,F). The umbrella cells play a prominent
role in maintaining a barrier against most substances found in
urine, and show a number of properties, including specialized
membrane lipids, asymmetric unit membrane particles, and a
plasmalemma with stiff plaques. These plaques may cover up
to 90% of the urothelial cell surface, with each plaque being
composed of nearly 1,000 subunits. These subunits are made by
proteins (uroplakins, UPs), which serve as the major receptors for
UPEC adherence to the host cell and are localized within plaques
on the apical membranes of the mature umbrella cells (Veranic
et al., 2004). There is a correlation between the glycosylation
changes in UPs and the different pathological conditions of
the urothelium such UTI and interstitial cystitis (Birder, 2005;
Katnik-Prastowska et al., 2014; Habuka et al., 2015).
The fusiform vesicles (FVs) are unique cytoplasmic organelles
contained in the umbrella cells. FVs deliver preassembled
crystalline arrays of UP proteins to the apical cell surface
of urothelial umbrella cells. Different Rab GTPases function
as regulators of specific steps in membrane traffic pathways
and are localized to the cytosolic face of specific intracellular
membranes. Rab27b, is a small GTPase regulating intracellular
vesicle movement which is expressed at an extraordinary high
level (0.1% of total protein) in urothelium. The Rab27b+ FVs are
involved in the storage of extra membrane which are necessary
when urine accumulates and causes bladder expansion (Wankel
et al., 2016). In order to enter epithelial cells, UPEC coopt the
superficial epithelial cells by expoiting their bladder volume-
regulating properties by stimulating the exocytosis of fusiform
vesicles right where the bacterial attach. The adherent bacteria
are then internalized when these membranes are subsequently
retracted into cells (Figure 3B; Wu et al., 2017). UPEC have
been found to reside within Rab27b/CD63/Caveolin-1-positive
fusiform vesicles (O’Brien et al., 2016). Internalized UPEC
become encased in Rab27b+ fusiform vesicles within the
cytosol of the superficial epithelium (Figure 3B; Bishop et al.,
2007). Replication of internalized UPEC bacteria rapidly occurs,
resulting in the maturation of IBCs, a structure that possesses
biofilm-like properties which is protected from innate defenses
and antibiotics (Justice et al., 2006; Goller and Seed, 2010). Fusion
with lysosomes is thus impaired, because internalized bacteria are
mostly encased in Rab27b+ compartments.
Defense mechanisms of bladder epithelial cells against
intrusion of bacterial include receptors such as toll-like receptors
(e.g., TLR2, TLR4, TLR5, and TLR11) that are able to promptly
recognize intruding bacteria (Larue et al., 2013). After UPEC
encapsulation within RAB27b+ vesicles in BECs, intracellular
UPEC are recognized by TLR4 which increases intracellular
cyclic AMP (cAMP) levels (Figure 3B). This triggers the
exocytosis of RAB27b+ vesicles harboring UPEC and the
intracellular bacterial expulsion back into the bladder lumen
(Figure 3C).
However, some UPEC break the RAB27b+ vacuole and
cannot be expelled into the urine; thus, these bacteria are
targeted by autophagy and delivered into the lysosomes, where
they actively neutralize the pH by reducing their acidicity
and degradative potential (Abraham and Miao, 2015). These
malfunctioning lysosomes are sensed by a lysosomal transient
receptor potential mucolipin 3 Ca2+ channel (TRPML3), which
is localized on the membrane of lysosomes (Miao et al., 2015).
The activation of this Ca2+channel rapidly fluxes out into
the cytosol the Ca2+ stored in the lysosome, which induces
the spontaneous expulsion into the extracellular space of the
defective lysosomes and its contents (Figure 3D).
Pathogen sensing by TLR4 induces the production of
various soluble factors which are secreted by BECs, including
antimicrobial peptides (AMP, such as cathelicidin and β-defensin
1; Sun et al., 2013; Chromek, 2015), antimicrobial proteins [such
as pentraxin 3 (PTX3); (Uzun et al., 2016)] and chemokines [such
as CXC-chemokine ligand 1 (CXCL1) and CC-chemokine ligand
5 (CCR5); Schiwon et al., 2014; Figure 3E]. Attachment to the
urothelium or bacterial lysis are inhibited by these antimicrobial
peptides, which are also induced when bacteria succeed to attach
to the urothelium (Spencer et al., 2014). Moreover, excretion
in the urine of uromodulin, a major high mannose-containing
glycoprotein, exerts a protective effects against UTI by competing
with the binding of UPEC FimH to uroplakin Ia (Pak et al., 2001).
When all these export mechanisms fail to clear the urothelium
from the invading UPEC, BECs activate the last line of defense.
Acute infections are commonly associated with of the exfoliation
Frontiers in Microbiology | www.frontiersin.org 5 August 2017 | Volume 8 | Article 1566

Terlizzi et al. Uropathogenic Escherichia coli Infections
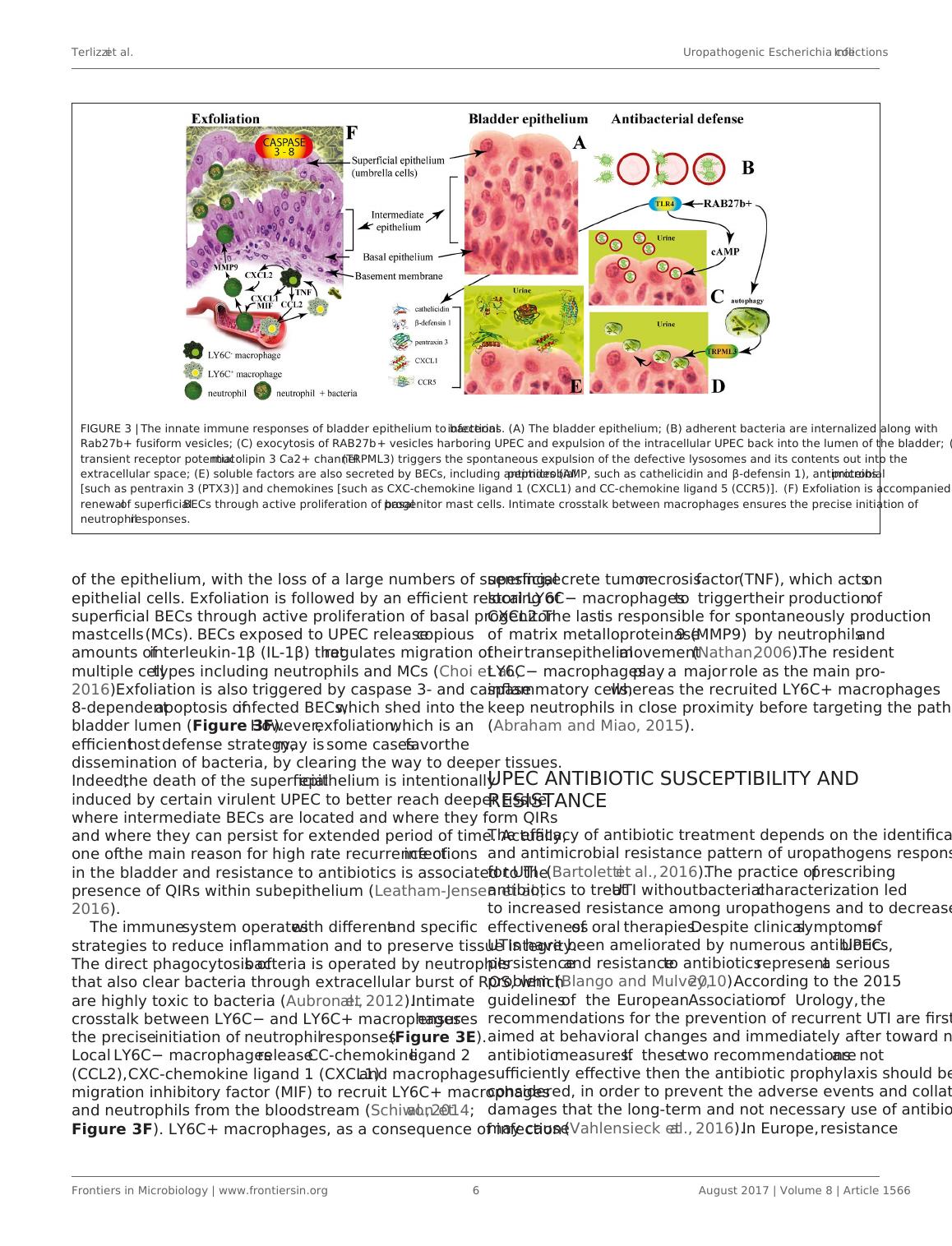
FIGURE 3 | The innate immune responses of bladder epithelium to bacterial infections. (A) The bladder epithelium; (B) adherent bacteria are internalized along with
Rab27b+ fusiform vesicles; (C) exocytosis of RAB27b+ vesicles harboring UPEC and expulsion of the intracellular UPEC back into the lumen of the bladder; (D)
transient receptor potential mucolipin 3 Ca2+ channel (TRPML3) triggers the spontaneous expulsion of the defective lysosomes and its contents out into the
extracellular space; (E) soluble factors are also secreted by BECs, including antimicrobial peptides (AMP, such as cathelicidin and β-defensin 1), antimicrobial proteins
[such as pentraxin 3 (PTX3)] and chemokines [such as CXC-chemokine ligand 1 (CXCL1) and CC-chemokine ligand 5 (CCR5)]. (F) Exfoliation is accompanied by rapid
renewal of superficial BECs through active proliferation of basal progenitor mast cells. Intimate crosstalk between macrophages ensures the precise initiation of
neutrophil responses.
of the epithelium, with the loss of a large numbers of superficial
epithelial cells. Exfoliation is followed by an efficient restoring of
superficial BECs through active proliferation of basal progenitor
mast cells (MCs). BECs exposed to UPEC release copious
amounts of interleukin-1β (IL-1β) that regulates migration of
multiple cell types including neutrophils and MCs (Choi et al.,
2016). Exfoliation is also triggered by caspase 3- and caspase
8-dependent apoptosis of infected BECs, which shed into the
bladder lumen (Figure 3F). However, exfoliation, which is an
efficient host defense strategy, may is some cases favor the
dissemination of bacteria, by clearing the way to deeper tissues.
Indeed, the death of the superficial epithelium is intentionally
induced by certain virulent UPEC to better reach deeper tissue
where intermediate BECs are located and where they form QIRs
and where they can persist for extended period of time. Actually,
one of the main reason for high rate recurrence of infections
in the bladder and resistance to antibiotics is associated to the
presence of QIRs within subepithelium (Leatham-Jensen et al.,
2016).
The immune system operates with different and specific
strategies to reduce inflammation and to preserve tissue integrity.
The direct phagocytosis of bacteria is operated by neutrophils
that also clear bacteria through extracellular burst of ROS, which
are highly toxic to bacteria (Aubron et al., 2012). Intimate
crosstalk between LY6C− and LY6C+ macrophages ensures
the precise initiation of neutrophil responses (Figure 3E).
Local LY6C− macrophages release CC-chemokine ligand 2
(CCL2), CXC-chemokine ligand 1 (CXCL1) and macrophage
migration inhibitory factor (MIF) to recruit LY6C+ macrophages
and neutrophils from the bloodstream (Schiwon et al., 2014;
Figure 3F). LY6C+ macrophages, as a consequence of infection
sensing, secrete tumor necrosis factor (TNF), which acts on
local LY6C− macrophages to trigger their production of
CXCL2. The last is responsible for spontaneously production
of matrix metalloproteinase 9 (MMP9) by neutrophils and
their transepithelial movement (Nathan, 2006). The resident
LY6C− macrophages play a major role as the main pro-
inflammatory cells, whereas the recruited LY6C+ macrophages
keep neutrophils in close proximity before targeting the pathogen
(Abraham and Miao, 2015).
UPEC ANTIBIOTIC SUSCEPTIBILITY AND
RESISTANCE
The efficacy of antibiotic treatment depends on the identification
and antimicrobial resistance pattern of uropathogens responsible
for UTI (Bartoletti et al., 2016). The practice of prescribing
antibiotics to treat UTI without bacterial characterization led
to increased resistance among uropathogens and to decreased
effectiveness of oral therapies. Despite clinical symptoms of
UTIs have been ameliorated by numerous antibiotics, UPEC
persistence and resistance to antibiotics represent a serious
problem (Blango and Mulvey, 2010). According to the 2015
guidelines of the European Association of Urology, the
recommendations for the prevention of recurrent UTI are first
aimed at behavioral changes and immediately after toward non-
antibiotic measures. If these two recommendations are not
sufficiently effective then the antibiotic prophylaxis should be
considered, in order to prevent the adverse events and collateral
damages that the long-term and not necessary use of antibiotics
may cause (Vahlensieck et al., 2016). In Europe, resistance
Frontiers in Microbiology | www.frontiersin.org 6 August 2017 | Volume 8 | Article 1566
FIGURE 3 | The innate immune responses of bladder epithelium to bacterial infections. (A) The bladder epithelium; (B) adherent bacteria are internalized along with
Rab27b+ fusiform vesicles; (C) exocytosis of RAB27b+ vesicles harboring UPEC and expulsion of the intracellular UPEC back into the lumen of the bladder; (D)
transient receptor potential mucolipin 3 Ca2+ channel (TRPML3) triggers the spontaneous expulsion of the defective lysosomes and its contents out into the
extracellular space; (E) soluble factors are also secreted by BECs, including antimicrobial peptides (AMP, such as cathelicidin and β-defensin 1), antimicrobial proteins
[such as pentraxin 3 (PTX3)] and chemokines [such as CXC-chemokine ligand 1 (CXCL1) and CC-chemokine ligand 5 (CCR5)]. (F) Exfoliation is accompanied by rapid
renewal of superficial BECs through active proliferation of basal progenitor mast cells. Intimate crosstalk between macrophages ensures the precise initiation of
neutrophil responses.
of the epithelium, with the loss of a large numbers of superficial
epithelial cells. Exfoliation is followed by an efficient restoring of
superficial BECs through active proliferation of basal progenitor
mast cells (MCs). BECs exposed to UPEC release copious
amounts of interleukin-1β (IL-1β) that regulates migration of
multiple cell types including neutrophils and MCs (Choi et al.,
2016). Exfoliation is also triggered by caspase 3- and caspase
8-dependent apoptosis of infected BECs, which shed into the
bladder lumen (Figure 3F). However, exfoliation, which is an
efficient host defense strategy, may is some cases favor the
dissemination of bacteria, by clearing the way to deeper tissues.
Indeed, the death of the superficial epithelium is intentionally
induced by certain virulent UPEC to better reach deeper tissue
where intermediate BECs are located and where they form QIRs
and where they can persist for extended period of time. Actually,
one of the main reason for high rate recurrence of infections
in the bladder and resistance to antibiotics is associated to the
presence of QIRs within subepithelium (Leatham-Jensen et al.,
2016).
The immune system operates with different and specific
strategies to reduce inflammation and to preserve tissue integrity.
The direct phagocytosis of bacteria is operated by neutrophils
that also clear bacteria through extracellular burst of ROS, which
are highly toxic to bacteria (Aubron et al., 2012). Intimate
crosstalk between LY6C− and LY6C+ macrophages ensures
the precise initiation of neutrophil responses (Figure 3E).
Local LY6C− macrophages release CC-chemokine ligand 2
(CCL2), CXC-chemokine ligand 1 (CXCL1) and macrophage
migration inhibitory factor (MIF) to recruit LY6C+ macrophages
and neutrophils from the bloodstream (Schiwon et al., 2014;
Figure 3F). LY6C+ macrophages, as a consequence of infection
sensing, secrete tumor necrosis factor (TNF), which acts on
local LY6C− macrophages to trigger their production of
CXCL2. The last is responsible for spontaneously production
of matrix metalloproteinase 9 (MMP9) by neutrophils and
their transepithelial movement (Nathan, 2006). The resident
LY6C− macrophages play a major role as the main pro-
inflammatory cells, whereas the recruited LY6C+ macrophages
keep neutrophils in close proximity before targeting the pathogen
(Abraham and Miao, 2015).
UPEC ANTIBIOTIC SUSCEPTIBILITY AND
RESISTANCE
The efficacy of antibiotic treatment depends on the identification
and antimicrobial resistance pattern of uropathogens responsible
for UTI (Bartoletti et al., 2016). The practice of prescribing
antibiotics to treat UTI without bacterial characterization led
to increased resistance among uropathogens and to decreased
effectiveness of oral therapies. Despite clinical symptoms of
UTIs have been ameliorated by numerous antibiotics, UPEC
persistence and resistance to antibiotics represent a serious
problem (Blango and Mulvey, 2010). According to the 2015
guidelines of the European Association of Urology, the
recommendations for the prevention of recurrent UTI are first
aimed at behavioral changes and immediately after toward non-
antibiotic measures. If these two recommendations are not
sufficiently effective then the antibiotic prophylaxis should be
considered, in order to prevent the adverse events and collateral
damages that the long-term and not necessary use of antibiotics
may cause (Vahlensieck et al., 2016). In Europe, resistance
Frontiers in Microbiology | www.frontiersin.org 6 August 2017 | Volume 8 | Article 1566
End of preview
Want to access all the pages? Upload your documents or become a member.
Related Documents
Health Variation 4lg...
|8
|1671
|91
E.coli Pathogens Biofilms In Urinary Tract Infectionslg...
|10
|3152
|87
Case Study Assessment: Pathophysiology, Nursing Priority, and ABG Assessmentlg...
|6
|1461
|51
Urinary Tract Infection: Causes, Symptoms, and Managementlg...
|13
|1736
|1
Pathogenesis and Drug Development Caitlinlg...
|18
|15821
|10
Antimicrobial Medicines and Treatment for E. Coli Infectionlg...
|5
|770
|88
